Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Peripheral nerve disorders are common neurological problems caused by dysfunction of peripheral motor, sensory, or autonomic nerves. The causes of neuropathies are disparate and their clinical presentations highly variable. The main causes of neuropathy are entrapments, systemic diseases, inflammatory and autoimmune disorders, inherited disorders, ischemic settings, paraneoplastic conditions, deficiency states, infections, and toxins.
The peripheral nerve is a cable-like structure containing bundles of both unmyelinated and myelinated fibers and their supporting elements. The unmyelinated axons are surrounded only by the plasma membrane of a Schwann cell. The myelinated axons are engulfed by a Schwann cell that wraps around the axons multiple times, thereby insulating the axon with multiple layers of lipid-rich cell membrane. The myelinated axon is surrounded completely by myelin and Schwann cells except at regular gaps called the nodes of Ranvier , which measure approximately 1 μm in adults (see Fig. 64.2 in Chapter 64 , Peripheral Nerve Trauma). The propagation of action potentials from one node of Ranvier to the next (saltatory conduction) is maintained by a thick myelin sheath with low capacitance and high resistance to electric current and by a high concentration of voltage-gated sodium channels at the nodes of Ranvier.
Despite the large number of causes for neuropathy, the pathological reactions of peripheral nerves to various insults remain limited. In general, these pathological processes are divided into four main categories: (1) wallerian degeneration, which is the response to axonal interruption, (2) axonal degeneration or axonopathy, (3) primary neuronal (perikaryal) degeneration or neuronopathy, and (4) segmental demyelination or myelinopathy. The patient’s symptoms, the type and pattern of distribution of signs, and the characteristics of nerve conduction study abnormalities provide information about the underlying pathological changes.
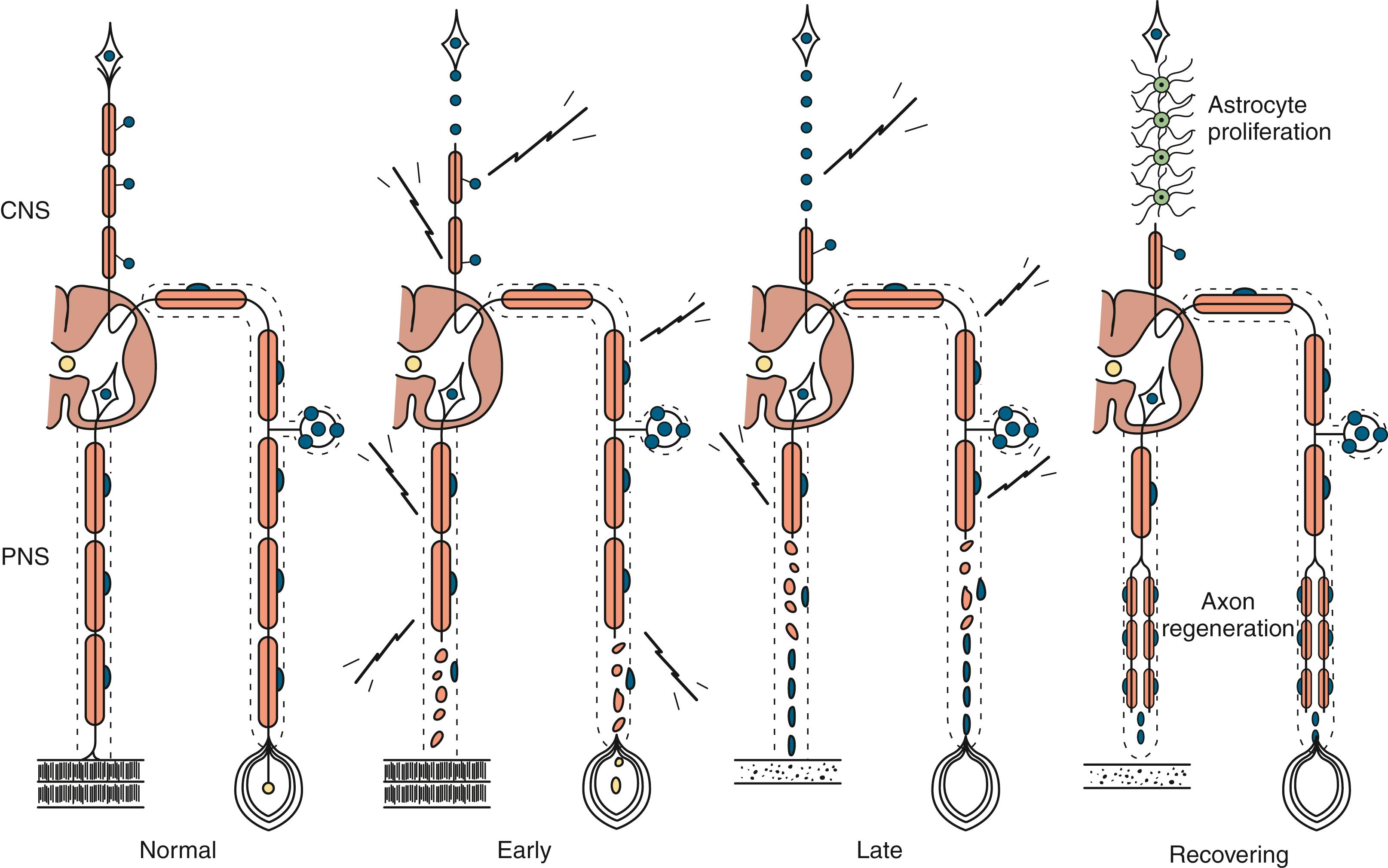
Compression, traction, laceration, thermal, chemical, or ischemic nerve injury that causes interruption of axons leads to wallerian degeneration —that is, distal degeneration of axons and their myelin sheaths. Immediately following injury, motor weakness and sensory loss occur in the distribution of the damaged nerve. On needle electromyography (EMG), there is complete loss of voluntary activity (with a complete lesion) or a decrease in motor unit action potential (MUAP) recruitment (with a partial lesion). However, the axons remain excitable distally since distal conduction failure is not completed until 10–11 days later as the distal nerve trunk becomes progressively unexcitable. On nerve conduction studies, the amplitude of the compound muscle action potential (CMAP), evoked by stimulation distal to the lesion site, begins to decline by the second day after injury and reaches its nadir by the fifth to sixth. For sensory axons, the loss of sensory nerve action potential (SNAP) is delayed by another 2–3 days; distal SNAP remains normal for 5–6 days and then decreases rapidly to reach its nadir by 10–11 days after injury (see Fig. 36.9 in Chapter 36 ). The temporal sequence of wallerian degeneration is length dependent, occurring earlier in shorter than in longer distal nerve stumps. Denervation potentials (fibrillation potentials) are typically seen on needle EMG in some affected muscles (mostly proximal ones) 10–14 days after injury and become full after 3 weeks from acute nerve injury. Axonal interruption initiates secondary morphological changes of the nerve cell body, termed chromatolysis , and the proximal axonal caliber becomes smaller. Regeneration from the proximal stump begins as early as 24 hours following transection but proceeds slowly at a maximal rate of 2–3 mm/day and is often incomplete. Sprouting of intact axons also starts locally in partial lesions, becoming noticeable on needle EMG after 1 month of axonal injury. The quality of recovery depends on the degree of preservation of the Schwann cell/basal lamina tube and the nerve sheath and surrounding tissue, the distance of the site of injury from the cell body, and the patient’s age.
Axonal degeneration (or axonopathy), the most common pathological reaction of peripheral nerve, signifies distal axonal breakdown and is presumably caused by metabolic derangement within neurons or vascular compromise leading to ischemia. Systemic metabolic disorders, toxin exposure, vasculitis, and some inherited neuropathies are the usual causes of axonal degeneration. The myelin sheath breaks down concomitantly with the axon in a process that starts at the most distal part of the nerve fiber and progresses toward the nerve cell body: hence the term dying-back or length-dependent polyneuropathy ( Fig. 106.1 ). A similar sequence of events may occur simultaneously in centrally directed sensory axons, resulting in distal degeneration of rostral dorsal column fibers. The selective length-dependent vulnerability of distal axons could result from failure of the perikaryon to synthesize enzymes or structural proteins, from alterations in axonal transport, or from regional disturbances of energy metabolism. In some axonopathies, alterations in axon caliber, either axonal atrophy or axonal swelling, may precede distal axonal degeneration. Clinically, dying-back polyneuropathy presents with symmetrical distal loss of sensory and motor function in the lower extremities that extends proximally in a graded manner. The result is sensory loss in a stocking-like pattern, distal muscle weakness and atrophy, and loss of distal limb myotatic reflexes. As the polyneuropathy ascends, it affects the hands and distal upper extremities, giving a glove-like sensory loss (hence the term stocking and glove sensory loss ), and hand weakness and atrophy. Axonopathies result in low-amplitude SNAPs and CMAPs, but they affect distal latencies and conduction velocities only slightly. Needle EMG of distal muscles shows acute and/or chronic denervation changes (see Chapter 36 ). Because axonal regeneration proceeds at a maximal rate of 2–3 mm/day, recovery may be delayed and is often incomplete.
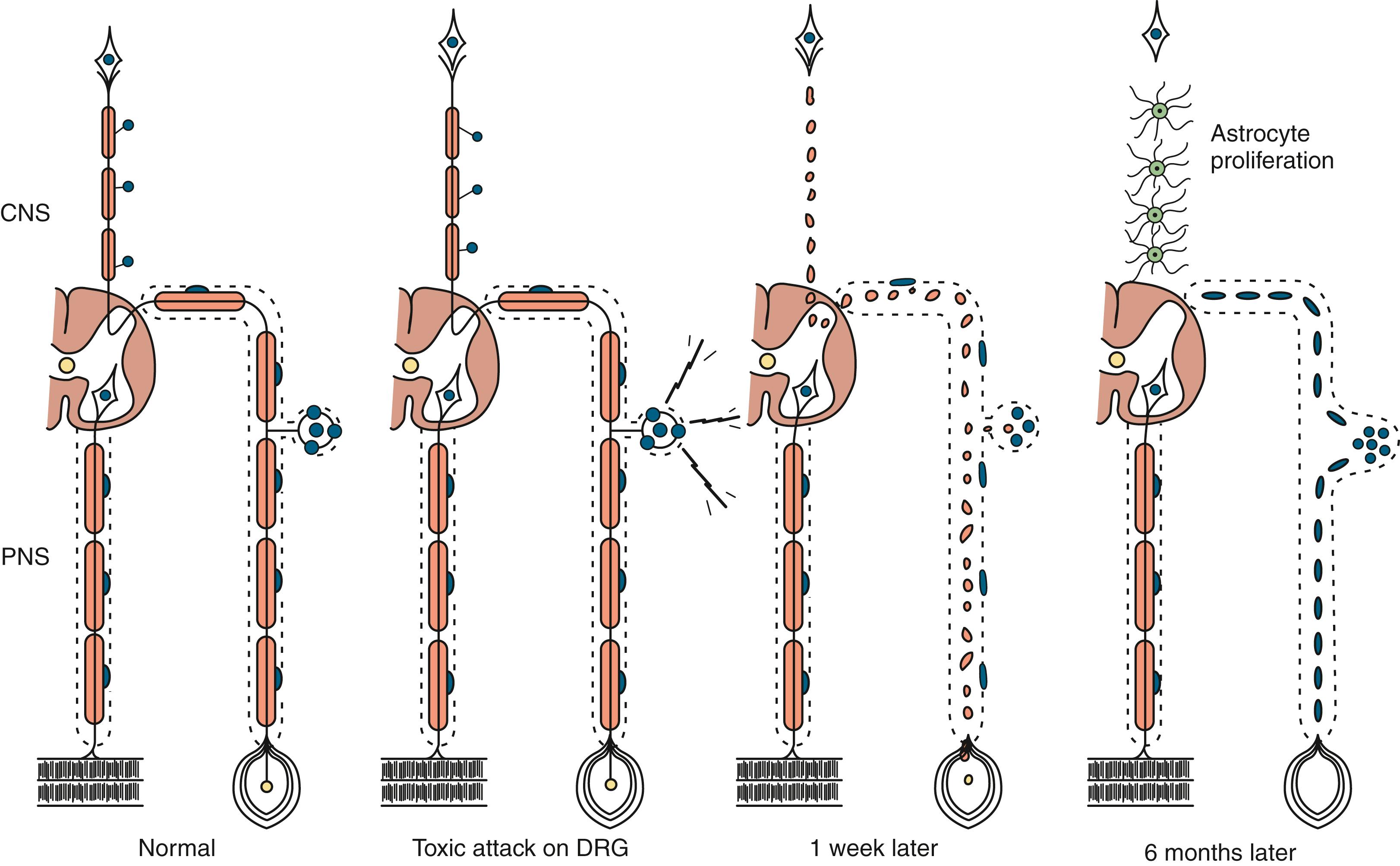
Neuronopathy designates loss of nerve cell bodies with resultant degeneration of their entire peripheral and central axons. Either anterior horn or dorsal root ganglion cells may be affected. Focal weakness without sensory loss occurs when anterior horn cells are affected, as in anterior poliomyelitis or motor neuron disease. Sensory neuronopathy , or dorsal polyganglionopathy , means damage to dorsal root ganglion neurons that results in sensory ataxia, sensory loss, and diffuse areflexia ( Fig. 106.2 ). A number of toxins, such as organic mercury compounds, doxorubicin, and high-dose pyridoxine, or deficiency states, such vitamin E deficiency, produce primary sensory neuronal degeneration. Immune-mediated inflammatory damage of dorsal root ganglion neurons occurs in paraneoplastic sensory neuronopathy (anti-HU syndrome) and Sjögren syndrome ( ). It is often difficult to distinguish between neuronopathies and axonopathies on clinical grounds alone. Once the pathological processes are no longer active, sensory deficits become fixed, and little or no recovery takes place.
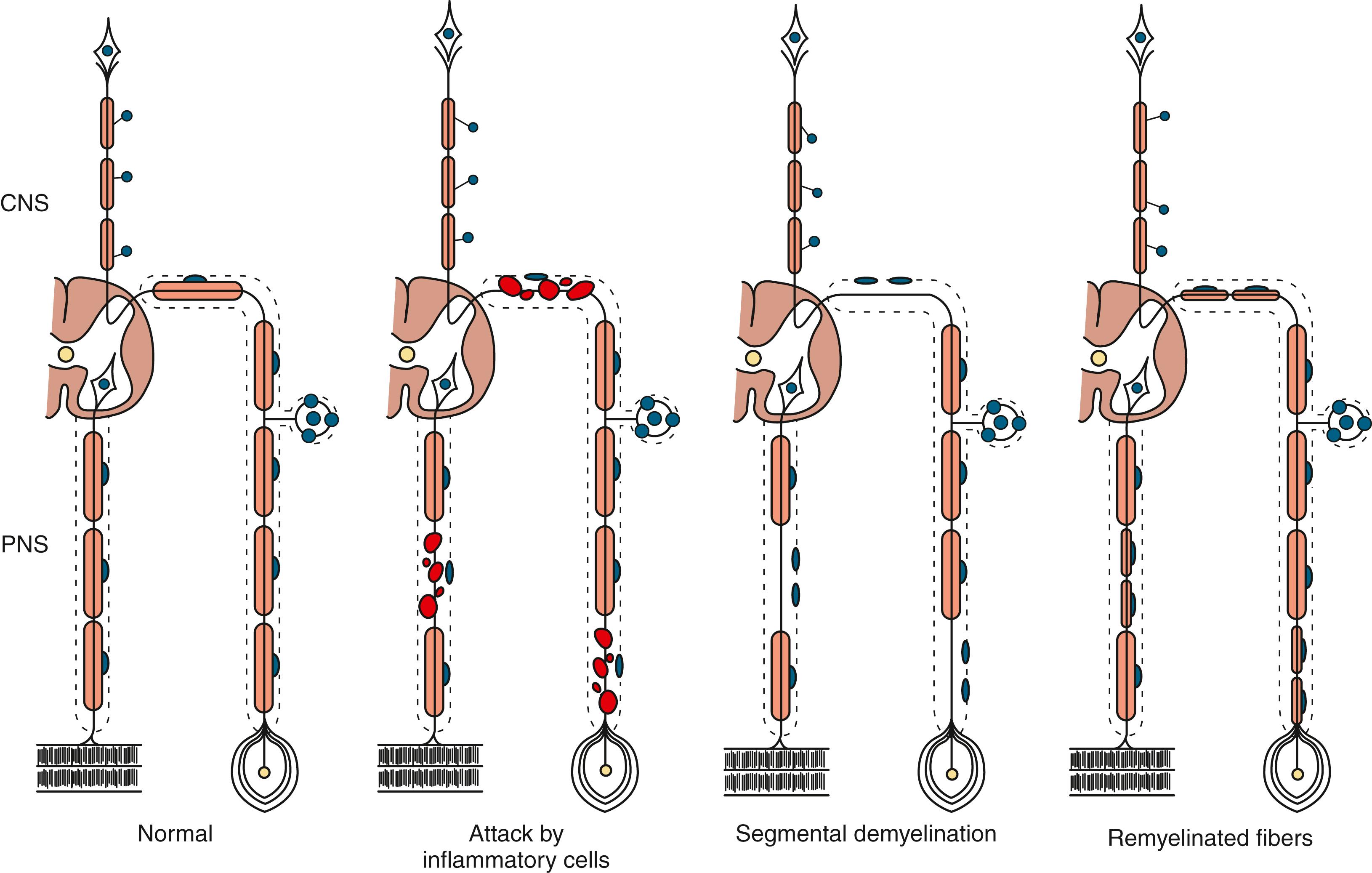
The term segmental demyelination (or myelinopathy ) implies injury of either myelin sheaths or Schwann cells, resulting in breakdown of myelin with sparing of axons ( Fig. 106.3 ). This occurs mechanically by acute nerve compression or chronic nerve entrapment and in immune-mediated demyelinating neuropathies and hereditary disorders of Schwann cell/myelin metabolism. Primary myelin damage may be produced experimentally by myelinotoxic agents such as diphtheria toxin or by acute nerve compression. Remyelination of demyelinated segments usually occurs within weeks. The newly formed remyelinated segments have thinner-than-normal myelin sheaths and internodes of shortened length. Repeated episodes of demyelination and remyelination produce proliferation of multiple layers of Schwann cells around the axon, termed an onion bulb . The physiological consequence of acquired demyelination, such as in inflammatory or compressive demyelination but not hereditary myelinopathies, is conduction block, which results in loss of the ability of the nerve action potential to reach the muscle, thereby producing weakness. Because the axon remains intact, there is little muscle atrophy. Relative sparing of temperature and pinprick sensation in many demyelinating polyneuropathies reflects preserved function of unmyelinated and small-diameter myelinated fibers. Early generalized loss of reflexes, disproportionately mild muscle atrophy in the presence of proximal and distal weakness, neuropathic tremor, and palpably enlarged nerves are all clinical clues that suggest demyelinating polyneuropathy. Nerve conduction studies or analysis of single teased nerve fiber preparations stained with osmium can confirm demyelination. Demyelination is present if motor and sensory nerve conduction velocities (NCVs) are reduced to less than 70% of the lower limits of normal, with relative preservation of CMAP and SNAP amplitudes. The presence of partial motor conduction block, temporal dispersion of CMAPs, and marked prolongation of distal motor and F-wave latencies are all features consistent with acquired demyelination (see Chapter 36 ). Recovery depends on the extent of remyelination, and therefore clinical improvement may occur within weeks. In many demyelinating neuropathies, axonal degeneration may also coexist, as evidenced by some distal limb atrophy and active denervation and reinnervation changes on needle EMG.
There are several patterns of peripheral nerve disease ( Box 106.1 ). Brachial, lumbar, and sacral plexopathy are discussed in Chapter 105 , and radiculopathies are discussed in Chapter 97 .
Mononeuropathy
Plexopathy:
Brachial plexopathy
Lumbar plexopathy
Sacral plexopathy
Radiculopathy:
Cervical radiculopathy
Thoracic radiculopathy
Lumbosacral radiculopathy
Multiple mononeuropathy (mononeuropathy multiplex)
Polyneuropathy:
Symmetrical polyneuropathy
Asymmetrical polyneuropathy
Polyradiculoneuropathy
A mononeuropathy means focal involvement of a single nerve and implies a local process. Direct trauma, compression, entrapment, vascular lesions, and neoplastic compression or infiltration are the most common causes. Electrophysiological studies provide a more precise localization of the lesion than may be possible by clinical examination, distinguish axonal loss from focal segmental demyelination, and sometimes may reveal a more widespread change indicating an underlying generalized polyneuropathy that has made the nerve susceptible to entrapment, as occurs in diabetes mellitus (DM), hypothyroidism, acromegaly, alcoholism, hereditary amyloidosis, and hereditary neuropathy with liability to pressure palsy (HNPP).
Multiple mononeuropathies , or mononeuropathy multiplex , signify simultaneous or sequential damage to multiple noncontiguous nerves. Confluent multiple mononeuropathies may give rise to motor weakness with sensory loss that can simulate a length-dependent peripheral polyneuropathy.
Polyneuropathy is most commonly characterized by symmetrical distal motor and/or sensory deficits that typically have a graded increase in severity distally and distal attenuation of reflexes. The sensory and motor deficits generally follow a length-dependent stocking-glove pattern. Most polyneuropathies are fairly symmetrical, but some are asymmetrical and sometimes the result of a confluent mononeuropathy multiplex. A small number of polyneuropathies (e.g., that associated with acute intermittent porphyria [AIP]) may be predominantly proximal. It is helpful to determine the relative extent of sensory, motor, and autonomic neuron involvement, although most polyneuropathies produce mixed sensorimotor deficits and some degree of autonomic dysfunction.
The “shotgun” approach of ordering several panels of diagnostic tests without an adequate understanding of their significance and usefulness should be avoided. A logical systematic diagnostic approach to peripheral neuropathies consists of a detailed history, comprehensive physical and neurological examinations, a limited set of laboratory studies, and detailed electrodiagnostic (EDX) studies. Additional ancillary testing, such as autonomic testing, skin biopsy, or nerve biopsy, may be considered in special clinical situations. This approach confirms the presence of a peripheral nerve disorder; characterizes the fiber type, pattern, time course, and type of deficit of the peripheral nerve disease; shortens the list of diagnostic and etiological possibilities; and prevents misdiagnoses. Further laboratory or pathological studies to determine a specific diagnosis are sometimes performed based on the findings of the initial evaluation.
The symptoms of peripheral nerve disorders are due to motor, sensory, or autonomic disturbances. The inquiry should seek both negative and positive symptoms. Negative motor symptoms are weakness, atrophy, and walking difficulties. Muscle cramps, fasciculations, myokymia, and tremor are positive motor manifestations . In polyneuropathies, negative motor symptoms include early distal toe and ankle extensor weakness, resulting in tripping on rugs or uneven ground. However, a complaint of difficulty walking in itself does not distinguish muscle weakness from sensory, pyramidal, extrapyramidal, or cerebellar disturbance. If the fingers are weak, patients may complain of difficulty opening jars or turning a key in a lock.
Positive sensory symptoms include prickling, searing, burning, and tight band-like sensations. Paresthesias are unpleasant sensations arising spontaneously without apparent stimulus. The presence of spontaneously reported paresthesias is helpful in distinguishing acquired (>60% of patients) from inherited (<20% of patients) polyneuropathies. Allodynia refers to the perception of nonpainful stimuli as painful, and hyperalgesia is painful hypersensitivity to noxious stimuli. Neuropathic pain, the extreme example of a positive symptom, is a cardinal feature of many neuropathies. Neuropathic pain often has a deep, burning, or drawing character that may be associated with jabbing or shooting pains that typically increase at night or during periods of rest.
Negative sensory manifestations include loss or reduction of pain, temperature, or touch sensation. Imbalance and gait disturbance are common negative sensory symptoms of polyneuropathy, implying loss of proprioception. However, the negative sensory symptoms may be caused by a central myelopathic disorder, including dorsal column dysfunction as occurs with vitamin B 12 deficiency.
Symptoms of autonomic dysfunction are helpful in directing attention toward specific neuropathies that have prominent autonomic symptoms. It is important to ask about orthostatic intolerance (lightheadedness, presyncopal symptoms, or syncope), reduced or excessive sweating, heat intolerance, and bladder, bowel, and sexual dysfunctions. Anorexia, early satiety, nausea, and vomiting are symptoms suggestive of gastroparesis. The degree of autonomic involvement can be documented by noninvasive autonomic function studies (see Chapter 107 ).
Historical information regarding onset, duration, and evolution of symptoms provides important clues to diagnosis. Knowledge about the time course of disease (acute, subacute, or chronic) and the course (monophasic, progressive, or relapsing) narrows diagnostic possibilities. Guillain-Barré syndrome (GBS), acute porphyria, vasculitis, neuralgic amyotrophy, and some forms of toxic neuropathies have acute presentations. A relapsing course is found in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), acute porphyria, Refsum disease, HNPPs, hereditary neuralgic amyotrophy, and repeated episodes of toxin exposure.
In patients with a chronic indolent course over many years, inquiries about similar symptoms and bony deformities (such as pes cavus) in immediate relatives often point to a familial polyneuropathy. Inherited polyneuropathies are a major cause of undiagnosed polyneuropathies, accounting for about 30% of patients referred to tertiary centers for diagnosis. Molecular genetic testing or the clinical and electrophysiological evaluation of relatives of patients with undiagnosed neuropathy may corroborate that the disorder is familial. The presence of constitutional symptoms such as weight loss, malaise, and anorexia suggests an underlying systemic disorder as a cause of the polyneuropathy. Inquiry should be made about preceding or concurrent associated medical conditions (DM, hypothyroidism, chronic renal failure, liver disease, intestinal malabsorption, malignancy, connective tissue diseases, and human immunodeficiency virus [HIV] seropositivity); drug use, including over-the-counter vitamin preparations (vitamin B 6 ); alcohol and dietary habits; and exposure to solvents, pesticides, or heavy metals.
The first step in the examination of patients with neuropathy is to determine the anatomical pattern and localization of the disease process and whether motor, sensory, or autonomic nerves are involved.
In mononeuropathy, the neurological deficit follows the distribution of a single nerve. For example, in a patient with foot drop due to a common fibular (peroneal) nerve lesion, the neurological examination reveals weakness of ankle and toe dorsiflexion and ankle eversion, but ankle inversion, toe flexion, and plantar flexion are normal, since muscles controlling these functions are innervated by the tibial nerve. Similarly, sensory loss is restricted to the lower two-thirds of the lateral leg and dorsum of the foot, but sensation on the sole of the foot is normal.
In multiple mononeuropathies (mononeuropathy multiplex), the neurological findings should point to simultaneous or sequential damage to two or more noncontiguous peripheral nerves. Confluent multiple mononeuropathies, such as with involvement of the fibular and tibial nerves or median and ulnar nerves, may give rise to motor weakness with sensory loss that can simulate a length-dependent peripheral polyneuropathy. EDX studies ascertain whether the primary pathological process is axonal degeneration or segmental demyelination ( Box 106.2 ). Approximately two-thirds of patients with multiple mononeuropathies display a picture of axonal damage. Ischemia caused by systemic or nonsystemic vasculitis or microangiopathy in DM should be considered. Other less common causes are disorders affecting interstitial structures of nerve—namely, infectious, granulomatous, leukemic, or neoplastic infiltration—including leprosy and sarcoidosis. In the event focal demyelination or motor conduction blocks lead to multiple mononeuropathies, multifocal acquired demyelinating sensory and motor neuropathy (MADSAM, Lewis-Sumner syndrome), multifocal motor neuropathy (MMN), or HNPP should be considered.
Vasculitis (systemic, nonsystemic)
Diabetes mellitus
Sarcoidosis
Leprosy (Hansen disease)
Human immunodeficiency virus 1 infection
Multifocal motor neuropathy
Multifocal acquired demyelinating sensory and motor neuropathy (MADSAM, Lewis-Sumner syndrome)
Multiple compressive neuropathies (hypothyroidism, diabetes)
Hereditary neuropathy with liability to pressure palsies (HNPPs)
In polyneuropathy, the sensory deficits generally follow a length-dependent stocking-glove pattern. By the time sensory disturbances of the longest nerves in the body (lower limbs) have reached the level of the knees, paresthesias are usually noted in the distribution of the second-longest nerves (i.e., those in the upper limbs) at the tips of the fingers. When sensory impairment reaches the midthigh, involvement of the third-longest nerves, the anterior intercostal and lumbar segmental nerves, gives rise to a tent-shaped area of hypoesthesia on the anterior chest and abdomen. Involvement of the recurrent laryngeal nerves may occur at this stage, with hoarseness. Motor weakness follows a dying-back pattern and usually is greater in extensor foot muscles than in corresponding flexors. Hence, heel walking is affected earlier than toe walking in most polyneuropathies. It is helpful to determine the relative extent of sensory, motor, and autonomic fiber involvement, although most polyneuropathies produce mixed sensorimotor deficits and some degree of autonomic dysfunction.
Motor deficits tend to dominate the clinical picture in acute and chronic inflammatory demyelinating polyneuropathies, hereditary motor and sensory neuropathies (Charcot-Marie-Tooth disease), and in neuropathies associated with osteosclerotic myeloma, porphyria, lead toxicity, organophosphate intoxication, and hypoglycemia ( Box 106.3 ). The distribution of weakness provides important information. Asymmetrical weakness without sensory loss suggests a motor neuronopathy such as motor neuron disease or MMN. The facial nerve can be affected in several peripheral nerve disorders ( Box 106.4 ). In most polyneuropathies, the legs are more severely affected than the arms, with several notable exceptions ( Box 106.5 ). Polyradiculoneuropathies cause both proximal and distal muscle weakness. For example, proximal and distal weakness is encountered in acute and chronic inflammatory demyelinating polyradiculoneuropathies, osteosclerotic myeloma, porphyria, and diabetic lumbar radiculoplexopathy (amyotrophy). Nerve root involvement is confirmed by denervation in paraspinal muscles on needle EMG or enhancing roots on gadolinium magnetic resonance imaging (MRI).
Guillain-Barré syndrome
Acute motor axonal neuropathy ∗
∗ Pure motor syndromes with normal sensory nerve action potentials.
Chronic inflammatory demyelinating polyradiculoneuropathy
Multifocal motor neuropathy ∗
Neuropathy with osteosclerotic myeloma
Diabetic lumbar radiculoplexopathy
Charcot-Marie-Tooth disease (hereditary motor sensory neuropathies)
Lead intoxication
Guillain-Barré syndrome
Lyme disease
Sarcoidosis
Chronic inflammatory demyelinating polyradiculoneuropathy (rare)
Human immunodeficiency virus 1 infection
Gelsolin familial amyloid neuropathy (Finnish)
Tangier disease
Multifocal motor neuropathy
Multifocal acquired demyelinating sensory and motor neuropathy (MADSAM, Lewis-Sumner syndrome)
Lead neuropathy ∗
∗ Frequently presents with wrist drop.
Porphyria
Tangier disease
Familial amyloid neuropathy type 2
Hereditary motor neuropathy (uncommon forms)
Autonomic dysfunction of clinical importance is seen in association with specific acute (e.g., GBS) or chronic (e.g., amyloidosis and diabetes) sensorimotor polyneuropathies. Rarely, an autonomic neuropathy may be the exclusive manifestation of a peripheral nerve disorder, without somatic nerve involvement ( Box 106.6 ).
Acute pandysautonomic neuropathy (autoimmune, paraneoplastic)
Guillain-Barré syndrome
Porphyria
Toxic: vincristine, Vacor (rodenticide)
Diabetes mellitus
Amyloid neuropathy (familial and primary)
Paraneoplastic sensory neuronopathy (malignant inflammatory sensory polyganglionopathy)
Human immunodeficiency virus-related autonomic neuropathy
Hereditary sensory and autonomic neuropathy
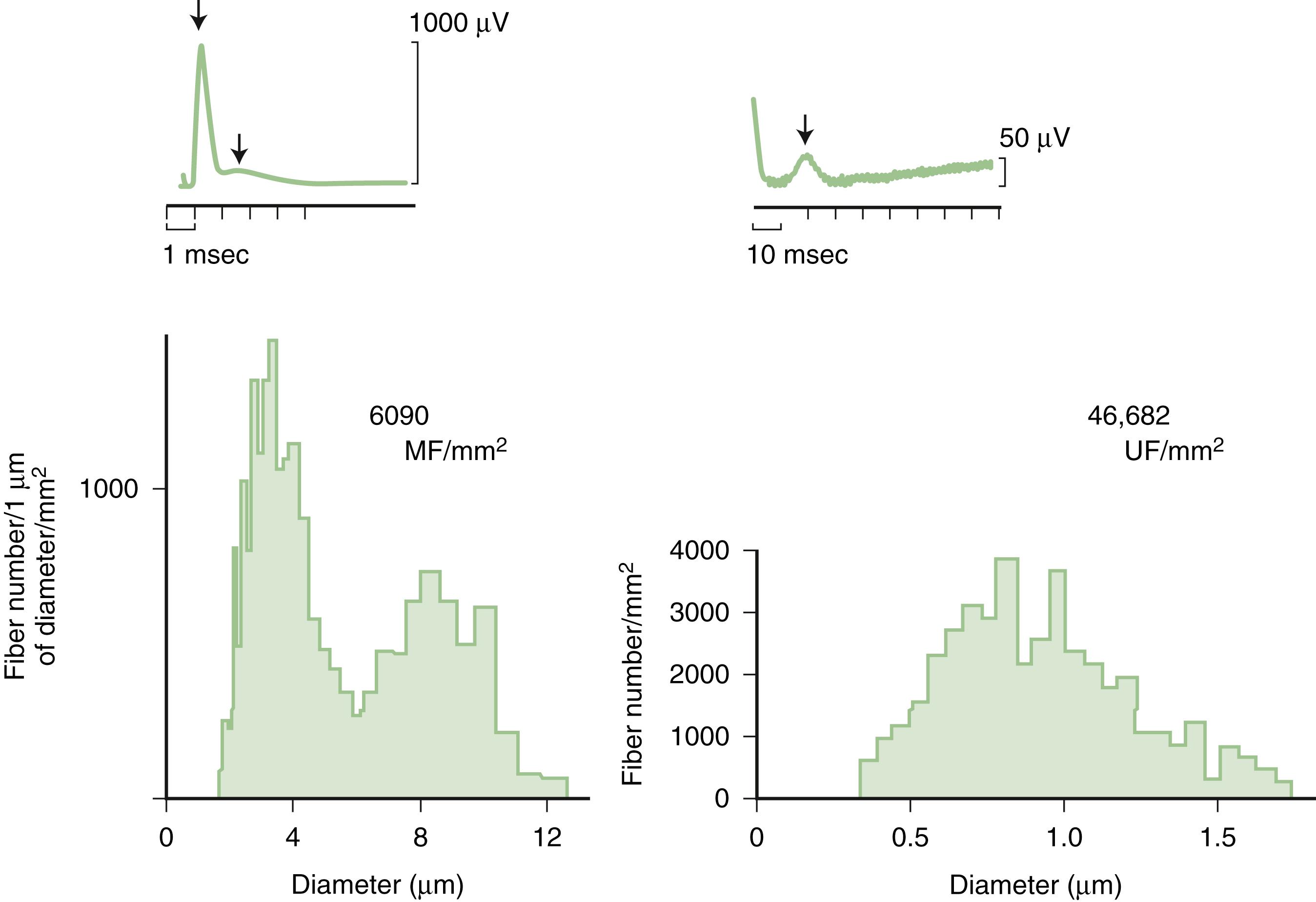
Predominant sensory involvement may be a feature of polyneuropathies caused by diabetes, carcinoma, Sjögren syndrome, dysproteinemia, acquired immunodeficiency syndrome (AIDS), vitamin B 12 deficiency, celiac disease (CD), inherited and idiopathic sensory neuropathies, and intoxications with cisplatin, thalidomide, or pyridoxine. Loss of sensation in peripheral neuropathies often involves all sensory modalities. However, the impairment may be restricted to selective sensory modalities in many situations, which makes it possible to correlate the type of sensory loss with the diameter size of affected afferent fibers ( Fig. 106.4 ). Pain and temperature sensation are mediated by unmyelinated and small myelinated Aδ fibers, whereas vibratory sense, proprioception, and the afferent limb of the tendon reflex are subserved by large myelinated Aα and Aβ fibers. Light touch is mediated by both large and small myelinated fibers. In polyneuropathies preferentially affecting small fibers, diminished pain and temperature sensation predominate, along with spontaneous burning pain, painful dysesthesias, and autonomic dysfunction. There is preservation of tendon reflexes, balance, and motor strength, and hence few abnormal objective neurological signs are found on examination. A pattern of sensory loss that is very characteristic is distal loss of pinprick sensation, above which is a band of hyperalgesia (exaggerated pain from noxious stimuli), with normal sensation above this level. Relatively few disorders cause selective small-fiber neuropathies ( ; Box 106.7 ). Selective large-fiber sensory loss is characterized by areflexia, sensory ataxia, and loss of joint position and vibration sense. Loss of joint position may also manifest as pseudoathetosis (involuntary sinuous movements of fingers and hands when the arms are outstretched and the eyes are closed) and/or a Romberg sign (disproportionate loss of balance with eyes closed compared with eyes open). Striking sensory ataxia, together with pseudoathetosis or asymmetrical truncal or facial sensory loss, directs attention to a primary disorder of sensory neurons or polyganglionopathies. The differential diagnosis of ataxic sensory neuropathies is limited ( Box 106.8 ).
Diabetes mellitus and impaired glucose tolerance
Sjögren (sicca) syndrome
Celiac disease
Amyloid neuropathy (early familial and primary)
Human immunodeficiency virus-associated sensory neuropathy
Hereditary sensory and autonomic neuropathies
Fabry disease
Tangier disease
Cryptogenic small-fiber neuropathy
Sensory neuronopathies (polyganglionopathies):
Paraneoplastic sensory neuronopathy (malignant inflammatory sensory polyganglionopathy):
Sjögren syndrome
Idiopathic
Toxic (cisplatin and analogs, vitamin B 6 excess)
Chronic immune sensory polyradiculopathy
Demyelinating polyradiculoneuropathies:
Guillain-Barré syndrome (Miller-Fisher variant)
Immunoglobulin M monoclonal gammopathy MAG ∗∗
∗∗ Myelin-Associated Glycoprotein.
antibody
CANOMAD: ∗
∗ Chronic ataxic neuropathy with ophthalmoplegia, IgM paraprotein, cold agglutinins, and anti-GD1b disialosyl antibodies.
Tabes dorsalis
Palpation of peripheral nerves is an important though unreliable part of the examination. Hypertrophy of a single nerve trunk suggests either a neoplastic process (e.g., neurofibroma, schwannoma, malignant nerve sheath tumor) or localized perineurial hypertrophic neuropathy. Generalized or multifocal nerve hypertrophy is found in a limited number of peripheral nerve disorders, including leprosy, neurofibromatosis, Charcot-Marie-Tooth (CMT) disease types 1 and 3, acromegaly, Refsum disease, and rarely CIDP.
Certain telltale signs of the skin and its appendages may direct the experienced examiner to a specific diagnosis ( Table 106.1 ): alopecia is seen in thallium poisoning; tightly curled hair in giant axonal neuropathy (GAN); white transverse nail bands, termed Mees lines , in arsenic or thallium intoxications; purpuric skin eruptions of the legs in cryoglobulinemia and some vasculitides; skin hyperpigmentation or hypertrichosis in POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes); telangiectasias over the abdomen and buttocks in Fabry disease; enlarged yellow-orange tonsils in Tangier disease; pes cavus and hammer toes in CMT disease; and overriding toes and ichthyosis in Refsum disease.
| Disease | Skin, Nail, or Hair Manifestations |
|---|---|
| Vasculitis | Purpura, livedo reticularis |
| Cryoglobulinemia | Purpura |
| Fabry disease | Angiokeratomas |
| Leprosy | Skin hypopigmentation |
| Osteosclerotic myeloma (POEMS syndrome) | Skin hyperpigmentation |
| Variegate porphyria | Bullous lesions |
| Refsum disease | Ichthyosis |
| Arsenic or thallium intoxication | Mees lines |
| Thallium poisoning | Alopecia |
| Giant axonal neuropathy | Curled hair |
It is helpful to follow a decision-making pathway based initially on the overall pattern of distribution of deficits, followed by the electrophysiological findings, and finally the clinical course ( Fig. 106.5 ). EDX studies, carefully performed and directed to the particular clinical situation, play a key role in the evaluation by (1) confirming the presence of neuropathy, (2) precisely locating focal nerve lesions, and (3) giving information as to the nature of the underlying nerve pathology ( ; ; ; see Chapter 36 ).
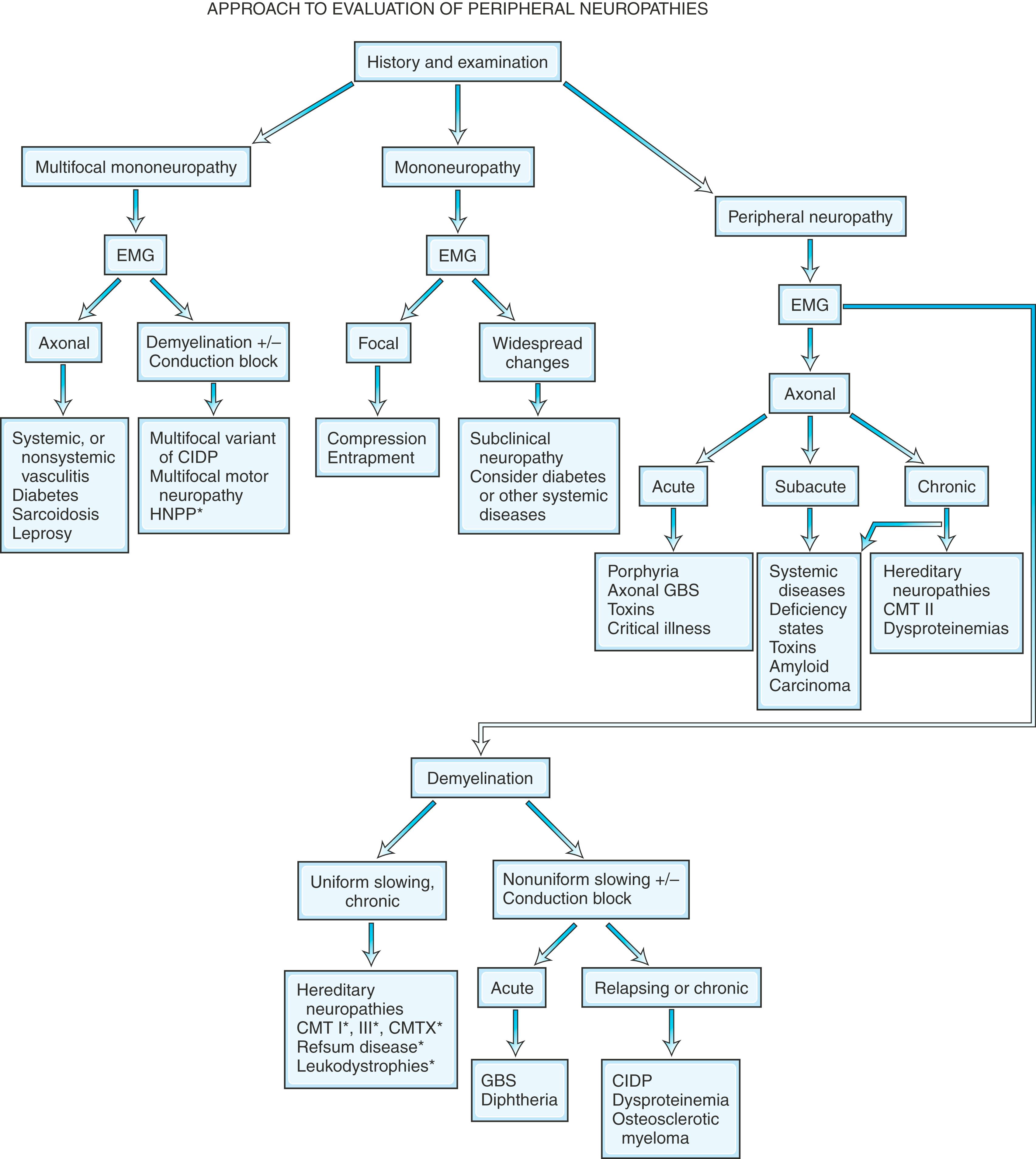
Because routine sensory nerve conduction studies assess only large myelinated fibers, such studies may be entirely normal in selective small-fiber neuropathies. Quantitative sensory testing assessing cold and heat pain thresholds, tests of sudomotor function, and skin biopsy with analysis of intraepidermal nerve fiber density (IENFD) may be helpful in confirming the unmyelinated nerve fiber abnormalities ( ). Since sweating mediated by unmyelinated sympathetic cholinergic fibers is often impaired, the quantitative sudomotor axon reflex (QSART) that evaluates sweating is a highly specific and sensitive method (sensitivity of 80%) to confirm small nerve fiber damage. Quantitative sensory testing assessing both vibratory and thermal detection thresholds has become a useful addition to the bedside sensory examination in controlled clinical trials. Its use in routine clinical practice remains limited because the test is still subjective in that it requires patient cooperation and is time consuming.
High-resolution neuromuscular ultrasound, which is readily available and painless, is increasingly used in the diagnosis of neuromuscular disorders ( ). This technique is an extremely helpful tool in the diagnosis of mononeuropathies, including entrapment neuropathies and traumatic nerve injuries. Focal nerve enlargement, measured as cross-sectional area (CSA), is the most common indicator of nerve entrapment, including carpal tunnel syndrome (CTS) and ulnar neuropathy at the elbow ( ; ). More recent studies have also shown important values of neuromuscular ultrasound in the diagnosis of CIDP and in distinguishing acquired demyelinating polyneuropathies from axonal polyneuropathies. Multifocal enlargement of peripheral nerves at non-entrapment sites and of elements of brachial plexus, measured by CSA, is highly diagnostic. In addition, muscle ultrasound visualizes fasciculations in limb muscles and tongue more readily than needle EMG and increases the sensitivity of diagnosis in patients with suspected advanced life support (ALS) ( ). Muscle ultrasound is also useful in myopathies including inflammatory myopathies and muscular dystrophies by showing increased echogenicity of muscles, and delineating the distribution of muscle involvement, thus helping in diagnosis and selection of muscle biopsy sites ( ; ). It also distinguishes neurogenic from myopathic disorders such as Duchenne muscular dystrophy with severe, homogeneous increase of muscle echo intensity from spinal muscular atrophy with an inhomogeneous increase of echo intensity with severe muscle atrophy ( ).
Skin punch or blister biopsies that demonstrate loss of intraepidermal nerve fibers are alternative methods for documenting small-fiber neuropathy ( ). Only unmyelinated intraepidermal networks of nerve fibers can be demonstrated by immunostaining with the panaxonal marker protein gene product 9.5, studied best with the use of confocal microscopy. Age, gender, and site of skin biopsy have a profound effect on epidermal nerve fiber density. The density of intraepidermal nerve fibers is reduced in skin biopsies obtained from patients with sensory and sensorimotor polyneuropathies including idiopathic, HIV-associated, diabetic polyneuropathies ( ). Skin punch biopsy is most useful in patients with suspected small-fiber neuropathy, when nerve conduction studies are normal. The diagnosis of small-fiber neuropathy is best accomplished when at least two abnormal results are met, including positive clinical findings, quantitative sensory testing, QSART, and skin biopsy ( ). Skin punch biopsy only detects the presence of skin nerve abnormalities and leads to a specific etiological diagnosis only rarely such as in amyloidosis. The skin biopsy also does not permit the study of myelinated fibers unless a thicker biopsy, including dermis, is obtained. Finally, unlike sural nerve biopsy, the interstitial pathological processes of the nerves cannot be studied.
Nerve biopsy (other than for the diagnosis of vasculitis and neoplasia) should be performed only in centers with established experience with the surgical procedure, handling of nerve specimens, and pathological technique; otherwise little useful information is likely to be obtained. The sural nerve is selected most commonly for biopsy, because the resultant sensory deficit is restricted to a small area over the heel and dorsolateral aspect of the foot, and because its morphology has been well characterized in health and disease. The superficial fibular (peroneal) nerve is an alternative lower-extremity cutaneous nerve suitable for biopsy and has the advantage of allowing simultaneous biopsy of the peroneus brevis muscle through the same incision. This combined distal nerve and muscle biopsy procedure increases the yield of identifying suspected vasculitis ( ; ). In contrast, adding a proximal muscle (e.g., quadriceps) to a cutaneous nerve biopsy (e.g., sural) does not significantly increase the diagnostic yield compared to nerve biopsy alone ( ). In patients with proximal involvement of the lower limbs, the intermediate cutaneous nerve of the thigh combined with a muscle biopsy can be performed. When the risk of complication is increased from a biopsy of the lower limbs (e.g., in significant distal leg ischemia, edema) or the neuropathy is preferentially more pronounced in the upper limbs, a cutaneous nerve biopsy of superficial radial or one of the antebrachial sensory nerves may be performed. When the imaging studies indicate a plexus or nerve root pathological process (e.g., inflammatory, infiltrative), a fascicular biopsy of the affected nerve by an expert surgeon may provide invaluable information. Nerve biopsy has proved to be particularly informative when techniques such as single teased fiber preparations, semi-thin sections, ultrastructural studies, and morphometry are applied to quantitate the nerve fiber pathology. Nowadays, relatively few disorders remain in which a nerve biopsy is essential for diagnosis ( ; ; Box 106.9 ). In general, nerve biopsy is most frequently diagnostic in suspected peripheral nerve vasculitis, amyloid neuropathy, and leprosy. It is helpful in the recognition of CIDP, hereditary demyelinating polyneuropathies, and some rare axonal polyneuropathies in which distinctive axonal changes occur, such as in giant axonal neuropathy and polyglucosan body disease. The availability of molecular genetic tests for several CMT neuropathies, HNPP, and familial transthyretin amyloidosis has decreased the necessity for nerve biopsy in these conditions.
Charcot-Marie-Tooth disease types 1 and 3
Chronic inflammatory demyelinating polyradiculoneuropathy
Paraproteinemic neuropathy (immunoglobulin M monoclonal gammopathy with anti-myelin-associated glycoprotein antibody)
Nerve biopsy is an invasive procedure and is associated with as high as 15% complication rate—particularly minor wound infections, wound dehiscence, and stump neuromas. Approximately one-third of patients (particularly those without much sensory loss initially) report unpleasant sensory symptoms at the sural nerve biopsy site that are still present 1 year after the biopsy ( ). The area of the original sensory deficit declines by 90% after 18 months because of collateral reinnervation ( ). The complications may be greater if substantial foot ischemia is present or if the patient smokes cigarettes.
The clinical neuropathic patterns and the results of EDX studies guide the experienced clinician to select the most appropriate laboratory tests. A few laboratory tests should be obtained routinely in all patients with peripheral polyneuropathy. These include complete blood cell count (CBC), sedimentation rate, C-reactive protein, renal functions, fasting blood sugar, hemoglobin A 1C , thyroid studies, vitamin B 12 level, and serum protein electrophoresis with immunofixation electrophoresis. It is important to screen for monoclonal proteins in all patients with chronic undiagnosed polyneuropathy, particularly those older than 60 years, because 10% of such patients have a monoclonal gammopathy. Cerebrospinal fluid (CSF) examination is helpful in the evaluation of suspected demyelinating neuropathies and polyradiculopathies related to meningeal carcinomatosis or lymphomatosis.
Several serum autoantibodies with reactivity to various components of peripheral nerve have been associated with peripheral neuropathy syndromes, and reference laboratories offer panels of nerve antibodies for sensory, sensorimotor, and motor neuropathies. It must be emphasized that the clinical relevance of most autoantibodies has not been established for patient treatment, and their use is not cost-effective ( ). Those of greatest clinical utility are listed in Table 106.2 ( ). An ever-increasing number of molecular genetic tests for inherited neuropathies are available at reference laboratories (see Hereditary Neuropathies, later).
| Autoantibody | Disease (% Positive) |
|---|---|
| Antibodies Against Gangliosides | |
| GM1 (polyclonal IgM) | Multifocal motor neuropathy (70%) |
| GM1, GD1a (polyclonal IgG) | Guillain-Barré syndrome (30%) |
| GQ1b (polyclonal IgG) | Miller-Fisher variant of Guillain-Barré syndrome (>90%) |
| GD1b (monoclonal IgM) | Chronic ataxic neuropathy with ophthalmoplegia, IgM paraprotein, cold agglutinins and anti-GD1b disialosyl antibodies, CANOMAD |
| Antibodies Against Glycoproteins | |
| Myelin-associated glycoprotein (MAG; monoclonal IgM) | Distal acquired demyelinating sensory (DADS) neuropathy or IgM neuropathy (50%–70%) |
| Antibodies Against RNA-Binding Proteins | |
| Anti-Hu, antineuronal nuclear antibody 1 (ANNA1) | Malignant inflammatory polyganglionopathy: that is, paraneoplastic sensory neuronopathy (>95%) |
In patients with initially undiagnosed peripheral neuropathy referred to specialized centers, a definite diagnosis can be made in more than 75% of cases. Inherited neuropathies, CIDP, and neuropathies associated with other systemic diseases accounted for most diagnoses. The improved diagnostic rate resulted in large measure from detailed clinical, EDX, and laboratory evaluations and study of relatives of patients with undiagnosed neuropathy.
Mononeuropathy is defined as a disorder of a single peripheral nerve. This may result from compression, traction, laceration, thermal, or chemical injury. The damage may involve one or more structural components of the peripheral nerve, while the pathophysiological responses to peripheral nerve lesions include axon loss, demyelination, or a combination of both.
Peripheral nerve injuries are classified based on functional status of the nerve and histological findings. Seddon divided peripheral nerve injury into three classes: neurapraxia, axonotmesis, and neurotmesis. This classification remains popular, particularly among surgeons, because of its correlation to outcome ( ). Later, revised the classification into five degrees that have better prognostic implications.
Neurapraxia, or first-degree nerve injury, usually results from brief or mild compression on the nerve that distorts the myelin, resulting in segmental demyelination but leaving the axons intact. The nerve conducts normally distal to but not across the lesion, resulting in conduction block, which is the electrophysiological correlate of neurapraxia. With this type of injury, recovery is usually complete following remyelination that occurs within 1–3 months if the offending cause (such as a compression) is removed.
Axonotmesis injury is characterized by axonal damage that results in wallerian degeneration; distal to the injury, the axons and their investing myelin sheath degenerate (wallerian degeneration) and the end organs (muscle fibers and sensory receptors) become denervated. Sunderland divided this type of nerve lesion into three further subtypes based on the disruptions of the supporting structures (endoneurium, perineurium, and epineurium).
The axonal loss is associated with intact endoneurial tubes as well as intact perineurium and epineurium. These lesions have fairly good prognosis, since nerve regeneration between the site of nerve injury and the target organs is well guided by the intact endoneurial tubes.
The axons and endoneurium are damaged while leaving the perineurium and epineurium intact. These lesions have fair prognosis and may require surgical intervention, mostly because of axonal misdirection and formation of neuromas.
The axons, endoneurium, and perineurium are disrupted, but the epineurium is intact. These lesions have poor prognosis and often require surgical repair.
Neurotmesis, or fifth-degree nerve injury, is the most severe type of nerve injury. It involves complete disruption of the nerve and all supporting structures. The nerve is transected, with loss of continuity between its proximal and distal stumps.
Entrapment neuropathy is defined as a mononeuropathy caused by focal compression or mechanical distortion of a nerve within a fibrous or fibro-osseous tunnel or less commonly by other structures such as bone, ligament, other connective tissues, blood vessels, or mass lesions. Compression, constriction, angulation, and stretching are important mechanisms that produce nerve injury at certain vulnerable anatomical sites (see Tables 106.3 and 106.5 ). The term entrapment is a useful one in that it implies that compression occurs at particular sites where surgical intervention is often required to release the entrapped nerve, such as in the case of the median nerve at the wrist in moderate to severe CTS. Overuse has been implicated as the cause of entrapment neuropathies in certain occupations, including playing of musical instruments by professional musicians.
| Nerve | Site of Compression | Predisposing Factors | Major Clinical Features |
|---|---|---|---|
| Median | Wrist (carpal tunnel syndrome) | Tenosynovitis, arthritis, repetitive wrist motions | Nocturnal paresthesia, pain, hypesthesia lateral 3 fingers, thenar atrophy |
| Anterior interosseous | Strenuous exercise, trauma, neuralgic amyotrophy | Abnormal pinch sign, normal sensation | |
| Elbow (pronator teres syndrome) | Repetitive elbow motions | Tenderness of pronator teres, no weakness, sensory loss | |
| Ulnar | Elbow (ulnar groove or cubital tunnel syndrome) | Elbow leaning, remote trauma (tardy ulnar), trauma, entrapment | Clawing, Froment sign, sensory loss of fourth and fifth fingers |
| Guyon canal | Mechanics, cyclists, ganglion cyst | Interosseous atrophy, normal sensation of dorsum of fourth and little fingers | |
| Radial | Axilla | Crutches | Wrist drop, triceps involved, sensory loss extending into forearm and sometimes arm |
| Spiral groove | Abnormal sleep postures | Wrist drop, triceps spared, sensory loss of dorsum of hand only | |
| Posterior interosseous | Elbow synovitis, neuralgic amyotrophy | Paresis of finger extensors, radial wrist deviation | |
| Superficial sensory branch (cheiralgia paresthetica) | Wrist bands, hand cuffs | Paresthesias in dorsum of hand | |
| Suprascapular | Suprascapular notch | Blunt trauma, neuralgic amyotrophy | Atrophy of supraspinatus and infraspinatus muscles |
| Axillary | Axilla | Shoulder dislocation or surgery | Weakness of arm abduction |
| Long thoracic | Shoulder | Neuralgic amyotrophy, stretch | Posterior scapular winging |
| Lower trunk of the brachial plexus or T1 roots | Thoracic outlet | Cervical rib, cervical band with enlarged C7 transverse process | Atrophy of intrinsic hand muscles (mostly thenar), paresthesias of medial hand and forearm |
| Nerve | Site of Compression | Predisposing Factors | Major Clinical Features |
|---|---|---|---|
| Sciatic | Sciatic notch | Endometriosis, intramuscular injections | Pain down thigh, flail foot, absent ankle jerk |
| Hip | Fracture, dislocations | Pain down thigh, flail foot, absent ankle jerk | |
| Piriformis muscle | Remote fall on buttock | Pain on sitting only, no weakness | |
| Popliteal fossa | Popliteal Baker cyst | Normal hamstrings | |
| Fibular (Peroneal) | Fibular neck | Weight loss, leg crossing, squatting, intraoperative compression, intraneural ganglion | Foot drop, weak ankle eversion, sensory loss in dorsum of foot |
| Anterior compartment | Muscle edema | Severe pain. foot drop | |
| Tibial | Medial malleolus (tarsal tunnel syndrome) | Ankle fracture, tenosynovitis, high heel shoes | Sensory loss over sole of foot, Tinel sign at flexor retinaculum |
| Femoral | Inguinal ligament | Lithotomy position | Weak knee extension, absent knee jerk |
| Pelvis | Intraoperative compression (retractor) | Weak hip flexion and knee extension, absent knee jerk | |
| Lateral femoral cutaneous | Inguinal ligament (meralgia paresthetica) | Weight gain, diabetes, pregnancy, tight clothing, utility belts | Sensory loss in lateral thigh |
| Ilioinguinal | Abdominal wall | Trauma, surgical incision | Direct hernia, sensory loss in the iliac crest, crural area |
| Obturator | Obturator canal | Tumor, surgery, pelvic fracture | Sensory loss in medial thigh, weak hip adduction |
In chronic entrapment, mechanical distortion of the nerve fibers leads to focal demyelination or, in severe cases, to wallerian degeneration. Morphological studies show a combination of active demyelination, remyelination, wallerian degeneration, and axonal regeneration at the site of entrapment. Endoneurial swelling, collagen proliferation, and thickening of perineurial sheaths accompany the nerve fiber changes. Ischemia is not a significant contributing factor to nerve fiber damage in chronic compression. In contrast, ischemia plays a more significant role in nerve injury associated with acute compression secondary to space-occupying lesions such as hematoma or compartment syndromes.
The characteristic electrophysiological feature of entrapment neuropathy is either short-segment conduction delay (i.e., focal slowing) or conduction block across the site of entrapment (see Chapter 36 ). In severe cases, wallerian degeneration gives rise to denervation and reinnervation in affected muscles. Nerve conduction studies together with needle EMG are essential for diagnosis and reliable documentation of the site and severity of nerve entrapment. Neuromuscular ultrasound has had an increasing role in the diagnosis of entrapment neuropathies, particularly when EDX studies are more difficult (such as in young children) or show only signs of nonlocalizing axonal loss such as with severe ulnar neuropathies ( ). In contrast, plain radiography, computed tomography (CT), and MRI may be of occasional value in identifying rare structural abnormalities, but these imaging procedures are often not necessary for routine diagnosis.
Entrapment neuropathies of the upper extremities are shown in Table 106.3 .
The median nerve, formed from contributions of the lateral cord (C6 and C7 fibers) and medial cord (C8 and T1 fibers) of the brachial plexus, runs into the forearm between the two heads of the pronator teres. It then gives off branches to the pronator teres, flexor carpi radialis, flexor digitorum sublimis, and the palmaris longus muscles, as well as the anterior interosseous nerve. The anterior interosseous nerve is the largest branch of the median nerve and is a pure motor nerve. It arises from the median nerve distal to these motor branches in the upper forearm and innervates the flexor pollicis longus, pronator quadratus, and median part of the flexor digitorum profundus muscles of the index and middle fingers. The median nerve then enters the wrist through the carpal tunnel, formed by the carpal bones and the transverse carpal ligament, the latter forming its roof. Before reaching the wrist, the median nerve gives off the palmar cutaneous sensory branch, which runs subcutaneously (not through the carpal tunnel) to innervate the skin over the thenar eminence. Distal to the carpal tunnel, the median nerve divides into motor and sensory divisions. The motor division innervates the first and second lumbricals and most muscles of the thenar eminence including the opponens pollicis, abductor pollicis brevis, and superficial head of the flexor pollicis brevis. The sensory fibers of the median nerve innervate the skin of the thumb, index, middle, and lateral half of the ring fingers.
CTS is by far the most common entrapment neuropathy seen in clinical practice. CTS prevalence in a primary care population is about 36 per 10,000 people, with an annual incidence of 19 per 10,000 for men and 36 per 10,000 for women ( ). Both the incidence and prevalence of CTS seem to be increasing.
This entrapment occurs in the tunnel through which the median nerve and long finger flexor tendons pass. Because the transverse carpal ligament is an unyielding fibrous structure forming the roof of the tunnel, tenosynovitis or arthritis in this area often produces pressure on the median nerve. The syndrome is frequently bilateral and usually of greater intensity in the dominant hand.
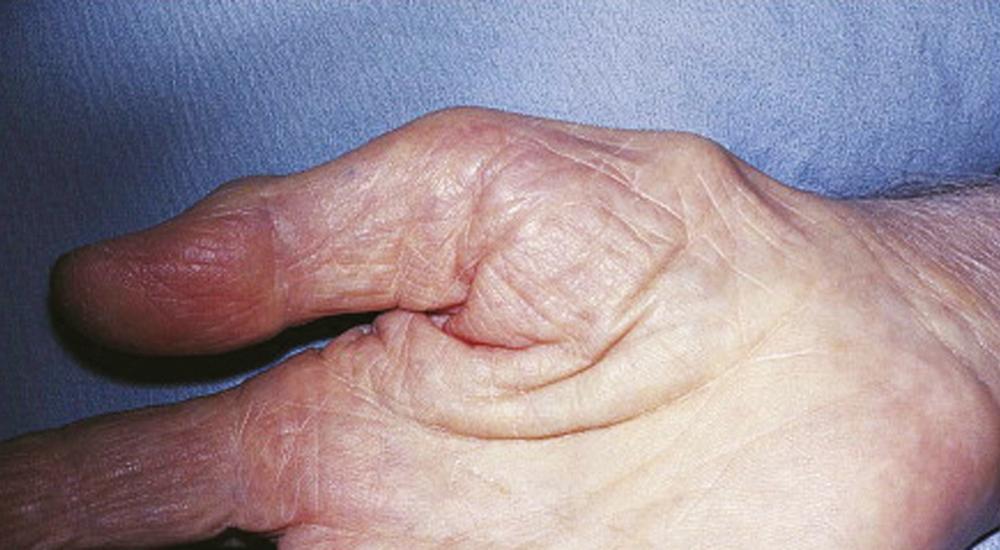
Symptoms consist of nocturnal pain and paresthesias, most often confined to the thumb, index, and middle fingers, but may be reported to involve the entire hand. Patients complain of tingling numbness, and burning sensations, which often awaken them from sleep. Referred pain may radiate to the forearm and even as high as the shoulder ( ). Symptoms are often provoked after excessive use of the hand or wrist or during ordinary activities such as driving or holding a phone, book, or newspaper, in which the wrist is assumed in either a flexed or extended posture. Objective sensory changes may be found in the distribution of the median nerve, most often impaired two-point discrimination, pinprick and light touch sensation, or occasionally hyperesthesia, in the thumb, index, and middle fingers, most evident in finger tips, with sparing of the skin over the thenar eminence. Thenar (abductor pollicis brevis muscle) weakness and atrophy may be present in advanced cases of CTS ( Fig. 106.6 ). Phalen maneuver (flexing the patient’s hand at the wrist for 1 minute) or reversed Phalen maneuver (hyperextension of the wrist for 1 minute) often reproduces the symptoms; it is present in about 80% of patients, and is rarely false positive ( ). A positive Tinel sign , in which percussion of the nerve at the carpal tunnel causes paresthesias in the distribution of the distal distribution of the median nerve, is present in approximately 60% of affected patients but is not as specific for CTS and may be false positive.
Work-related wrist and hand symptoms (repetitive motion injury) from cumulative trauma in the workplace have received increasing attention by the general public in recent years ( ). Although a proportion of these cases have bona fide CTS, longitudinal natural history data suggest that the majority of industrial workers do not develop symptoms of CTS ( ). Symptoms consistent with hand and wrist arthritis in a variety of occupational settings are now recognized as being much more common than CTS ( ). CTS appears to occur in work settings that include repetitive forceful grasping or pinching, awkward positions of the hand and wrist, direct pressure over the carpal tunnel, and the use of handheld vibrating tools. Increased risk for the syndrome has been found in meat packers, garment workers, butchers, grocery checkers, electronic assembly workers, musicians, dental hygienists, and housekeepers. The highest reported incidence of work-related CTS, based on the number of carpal tunnel surgeries performed, was 15% among a group of meat packers. Though using the computer keyboard may aggravate symptoms of CTS, keyboard use does not increase the risk of developing CTS ( ; ; ).
Diseases and conditions that have been found to predispose to the development of CTS include pregnancy, diabetes, obesity, age, rheumatoid arthritis, hypothyroidism, amyloidosis, gout, acromegaly, certain mucopolysaccharidoses, arteriovenous shunts for hemodialysis, old fractures at the wrist, and inflammatory diseases involving tendons or connective tissues at the wrist level ( ). On rare occasions, CTS may be familial, and some patients with CTS have carpal tunnels that are significantly narrower than average.
The most commonly performed EDX tests for CTS are the median nerve sensory and motor conduction studies, which exhibit delayed sensory or motor latencies across the wrist in about 70% of patients. However, these studies are not sensitive enough in the diagnosis of CTS and fail to detect up to a third of patients with CTS, particularly those with mild and early symptoms. Recording the median latency at short distances over the course of the median nerve from palm to wrist and/or comparing this latency with the latency for the ulnar or radial nerve at the same distance (internal comparison nerve conduction studies) increase the sensitivity of these nerve conduction studies ( ; ; Table 106.4 ) .
| Study | Median-Ulnar Palmar Mixed Study | Median-Ulnar Sensory Study to Ring Finger | Median-Ulnar Motor Study to Second Lumbrical Interossei | Median-Radial Sensory Study to Thumb |
|---|---|---|---|---|
| Technique | Palm stimulation of median and ulnar nerves, recording at the wrist | Median and ulnar nerves stimulation at the wrist, recording ring fingers | Median and ulnar nerves stimulation at the wrist, recording second lumbrical and second interossei, respectively (second interosseous space) | Median and radial nerves stimulation at the wrist, recording thumb |
| Abnormal values | Median-ulnar peak latency difference ≥ 0.4 ms | Median-ulnar peak latency difference ≥ 0.4 ms | Median-ulnar onset latency difference ≥ 0.5 ms | Median-radial peak latency difference ≥ 0.4 ms |
Mild CTS must be distinguished from proximal median neuropathies, upper brachial plexopathy, C6 or C7 radiculopathies, and polyneuropathy involving the hands. Occasionally, a transient ischemic attack may mimic the symptoms of CTS.
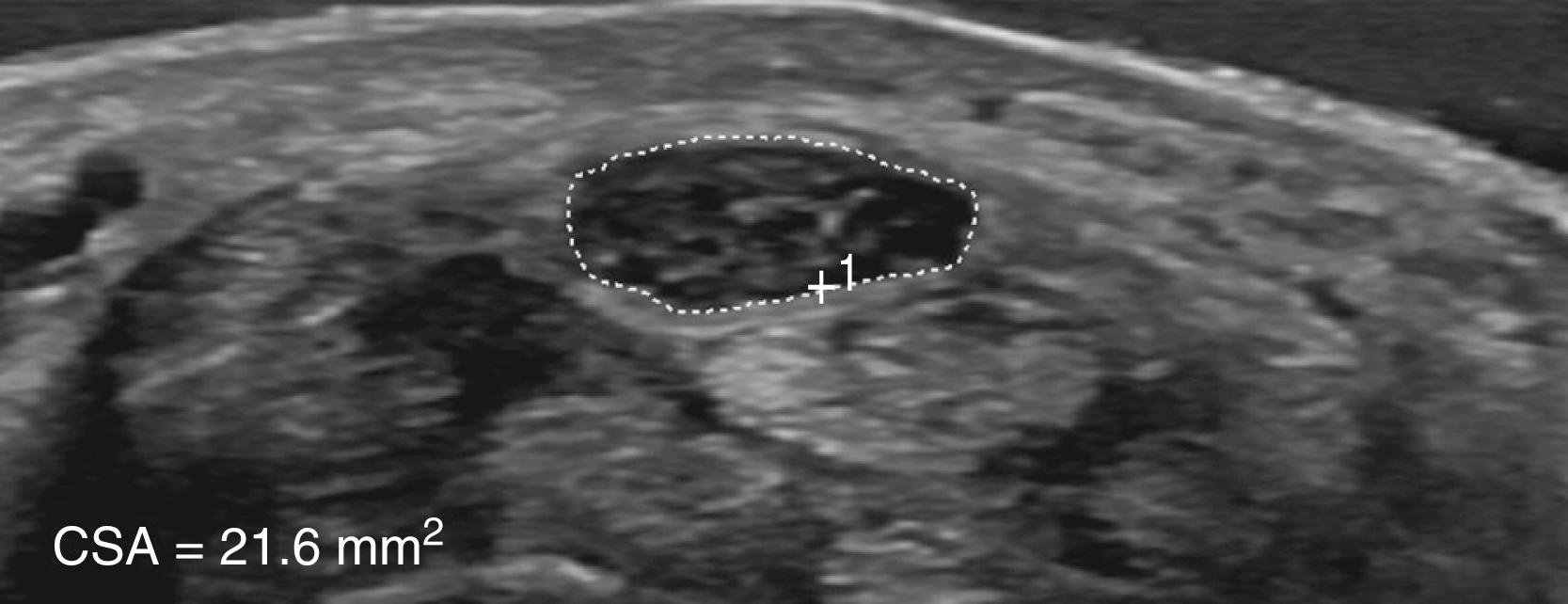
Ultrasound is increasingly used in the diagnosis of CTS. Thickening of the median nerve, best expressed as an increase in the CSA of the median nerve at the carpal tunnel inlet (more than 13 mm; normal <10–13 mm), or flattening of the nerve at the level of the hamate are the best diagnostic criteria ( Fig. 106.7 ) ( ; ). The majority of studies have found that the diagnostic utility of ultrasound and EDX studies are equal ( ; ). MRI is most useful when a space-occupying lesion, such as ganglion, hemangioma, or bony deformity, is suspected in patients with CTS.
In cases with only mild sensory symptoms, treatment with splints in neutral position, nonsteroidal antiinflammatory drugs (NSAIDs), and local corticosteroid injection often suffice ( ). Local corticosteroid injection is slightly better than splinting ( ). Withdrawal of provoking factors is also important. Although nonoperative treatments have been advocated ( ), a comparison of splinting versus surgery suggested that the latter may have a better long-term outcome than the former ( ). Use of a range of devices and appliances to protect the hand against CTS, including gel-padded gloves, has shown little if any improvement in objective measures of nerve function. There is conflicting evidence that NSAIDs, diuretics, laser, and ultrasound are effective. Exercise therapy is not useful ( ). Methylprednisolone injections for CTS significantly relieve symptoms for a few months and reduce the need for surgery, but a significant number of patients will ultimately need surgical treatment ( ). Oral steroids are also effective but are associated with side effects. Severe sensory loss, thenar atrophy, and active denervation on needle EMG of thenar muscles suggest the need for surgical carpal tunnel release. Open surgical sectioning of the volar carpal ligament or fiberoptic techniques are often successful, with more than 90% of patients having prompt resolution of pain and paresthesias ( ; ). Improvement in distal latencies may lag behind the relief of symptoms. Comparing with preoperative values, nerve conduction studies demonstrate improvement in those with moderate abnormalities preoperatively, whereas patients with severe or no abnormalities on baseline nerve conduction studies have poorer results ( ). A correlation between patients seeking workers’ compensation who hire attorneys and poorer operative outcomes has also been reported ( ). Older individuals may not improve as much as younger patients ( ), and factors such as poor mental health, significant alcohol consumption, longer disease duration, and male gender also portend a poorer outcome. Rarely, symptoms persist after operation. Poor surgical results usually are associated with incomplete sectioning of the transverse ligament, surgical damage of the palmar cutaneous branch of the median nerve by an improperly placed skin incision, scarring within the carpal tunnel, or an incorrect preoperative diagnosis. Surgical re-exploration may be required in certain cases with poor response to the initial operation ( ).
Isolated acute involvement of the anterior interosseous nerve is often a presentation of neuralgic amyotrophy (idiopathic brachial plexus neuropathy, Parsonage-Turner syndrome) ( ; ). The majority of these lesions are fascicular lesions of the median nerve in the arm involving the anterior interosseous nerve fascicle selectively ( ). Fascicular torsion of the anterior interosseous fascicle in the lower arm has also been advocated to be due to the high mobility of the anterior interosseous nerve fascicles during elbow flexion leading to torsion injury or inflammation/edema followed by intraneural adhesions ( ). In chronic and indolent lesions, a restricted form of MMN with conduction block should be considered and a careful and detailed electrophysiological study may reveal involvement of other nerves. The anterior interosseous nerve may be externally compressed following anterior elbow dislocations or complex elbow fractures, or rarely by fibrous bands attached to the flexor digitorum superficialis muscle, an anomalous Gantzer muscle which is an accessory portion of the flexor pollicis longus or the flexor digitorum profundus ( ).
Patients often complain of an acute or subacute onset of pain in the forearm or elbow. The patient is unable to flex the distal phalanges of the thumb and index finger, making it impossible to form a circle with those fingers (pinch or O sign) . Sensory and motor nerve conduction studies of the median nerve are usually normal. Needle EMG reveals denervation in muscles innervated by the anterior interosseous nerve, including the flexor pollicis longus, pronator quadratus, and flexor digitorum profundus muscles of the index and middle fingers. Spontaneous recovery usually occurs within 3–12 months, and therefore surgery may not be necessary unless penetrating injury, fracture, or progressive deterioration and weakness are detected. Patients with torsion injury showed good recovery after interfascicular neurolysis.
In the pronator teres syndrome, the median nerve is compressed in the proximal forearm between the two heads of the pronator teres muscle, a fibrous arcade of the flexor digitorum superficialis muscle, or the lacertus fibrosus (a thick fascial band extending from the biceps tendon to the forearm fascia). This extremely rare and controversial entrapment may develop in individuals engaged in repetitive pronating movements of the forearm. Patients usually experience a vague aching pain in the volar aspect of the elbow and forearm, beginning or worsening during activities involving grasping or pronation or both. There is also an insidious onset of paresthesias and numbness of the palm of the hand, mimicking CTS but without the nocturnal symptoms. Resistance to pronation produces pain in the proximal forearm. The pronator teres may be firm and tender on palpation, and the Tinel sign may be elicited over the median nerve in the region of the elbow. Weakness of median-innervated muscles such as the flexor pollicis longus, pronator quadratus, and abductor pollicis brevis (but not of pronator teres) is rarely demonstrated, in contrast to traumatic cases such as following elbow dislocation, forearm fracture, or intracompartmental hemorrhage. Nerve conduction studies in the median nerve are usually normal and do not show the distal median motor and sensory latencies at the wrist that accompany CTS. Needle EMG is also usually normal, with no definite signs of denervation. Injection of corticosteroids into the pronator teres muscle, NSAIDs, and immobilization of the arm with the elbow flexed to 90 degrees and in mild pronation often provide relief of symptoms. Surgery is controversial but may be occasionally necessary, though patients may gain only partial relief.
An often bilateral supracondylar spur of the humerus is present in approximately 1% of normal individuals. This beadlike bony or cartilaginous projection arises from the anteromedial surface of the humerus, located about 5 cm above the medial epicondyle. A fibrous band, the ligament of Struthers, extends from this spur to the medial epicondyle and may rarely compromise the median nerve and the brachial artery above the elbow. Clinical symptoms resemble the pronator teres syndrome, but sometimes the radial pulse diminishes when the forearm is fully extended in supination because of the concomitant entrapment of the brachial artery. Elbow extension causes aggravation of the pain. On needle EMG, there is sometimes denervation in the abductor pollicis brevis, flexor pollicis longus, pronator quadratus, and pronator teres. Involvement of the pronator teres muscle theoretically allows differentiation of the ligament of Struthers syndrome from the pronator teres syndrome. Treatment consists of surgical excision of the spur and ligament.
The ulnar nerve derives its fibers from the C8 and T1 roots via the lower trunk and originates from the medial cord of the brachial plexus, along with the medial brachial and antebrachial cutaneous sensory nerves. The ulnar nerve gives no branches in the arm. At the elbow, the nerve becomes superficial and enters the ulnar groove formed between the medial epicondyle and the olecranon process. Normally, the ulnar nerve remains in the groove, but in some individuals or when there is an unusual degree of physiological cubitus valgus, the nerve may be unduly mobile, tending to slip forward (sublux) over the medial epicondyle when the elbow is flexed. In a small number of individuals, a dense fibrotendinous band and/or an accessory epitrochleoanconeus muscle may be present between the medial epicondyle and the olecranon process. Slightly distal to the groove in the proximal forearm, the ulnar nerve travels under the tendinous arch of the two heads of the flexor carpi ulnaris muscle, known as the humeral-ulnar aponeurosis , which forms the entrance of the cubital tunnel. Muscular branches originate to the flexor carpi ulnaris and the flexor digitorum profundus (ulnar part to the little and ring fingers). The ulnar nerve continues under the flexor carpi ulnaris and then exits in the distal forearm between the deep fascia separating the flexor carpi ulnaris and flexor digitorum profundus. Some 5–8 cm proximal to the wrist, the dorsal ulnar cutaneous sensory branch exits to innervate skin on the dorsal medial hand and the dorsal little and ring fingers. The palmar cutaneous sensory branch originates at the level of the ulnar styloid to supply sensation to the proximal medial palm. The ulnar nerve then enters the wrist through the Guyon canal, which is formed between the pisiform bone and the hook of the hamate and is covered by the volar carpal ligament and the palmaris brevis muscle. Within the Guyon canal, the ulnar nerve divides into its terminal deep palmar and superficial ulnar branches. Because the palmar cutaneous sensory and dorsal cutaneous sensory branches do not pass through the Guyon canal, the deep palmar branch is purely motor and supplies muscular innervation to the hypothenar muscles, the palmar and dorsal interossei, the third and fourth lumbricals, and two muscles in the thenar eminence, the adductor pollicis, and the deep head of the flexor pollicis brevis.
Ulnar mononeuropathy is the second most common entrapment or compression mononeuropathy, although it is considerably less common than CTS. Compression of the ulnar nerve by a thickened, fibrotic flexor carpi ulnaris aponeurosis (humeral-ulnar aponeurosis) at the entrance of the elbow’s cubital tunnel is a common cause of ulnar neuropathy (cubital tunnel syndrome) . Patients with a subluxed ulnar nerve are at high risk for compression at the elbow. Also, prolonged and frequent resting of the flexed elbow on a hard surface such as a desk or armchair may result in external pressure to the nerve (ulnar groove syndrome) . Occupations involving repeated flexion of the elbow may on occasion cause symptoms of ulnar neuropathy. A flexed elbow position increases both the intraneural and extraneural pressure on the nerve. The nerve at the site of repeated compression is associated with fibrous thickening, when a spindle-shaped swelling may be felt. Other possible sources of injury of the ulnar nerve at the elbow include direct compression when the patient uses the arms to raise up in bed following surgical operations ( ) or after periods of prolonged unconsciousness. The ulnar nerve at the elbow may be acutely injured as a result of fracture or dislocation involving the lower end of the humerus and the elbow joint. Occasionally, however, the nerve becomes chronically compressed years after such an injury, which often has led to cubitus valgus deformity (“tardy ulnar palsy”) . The nerve may be damaged by osteophyte outgrowths resulting from arthritis of the elbow joint, by a ganglion or lipoma, by a Charcot elbow, and by the epitrochleoanconeus muscle and/or its dense fibrotendinous band. The ulnar nerve may also be involved in conditions that are known to increase the susceptibility of nerves to compression, such as DM or HNPP. Ulnar neuropathy at the elbow segment may also occur without any apparent cause.
Ulnar nerve lesions at the elbow result in numbness and tingling of the little and ring fingers, with variable degrees of hand weakness. Less commonly, patients present with weakness and wasting with no clear sensory symptoms. There is also variable weakness of the flexor carpi ulnaris and the flexor digitorum profundus of the ring and little fingers (ulnar part). Grip strength is reduced secondary to weakness of the adductor pollicis, flexor pollicis brevis, and palmar and dorsal interosseous muscles. To compensate for adductor pollicis weakness during an attempt to pinch a piece of paper between the thumb and index fingers, the flexor pollicis longus, a median nerve-innervated muscle, becomes involuntarily active and flexes the distal phalanx of the thumb (Froment sign) . Weakness of the interossei muscles results in an inability to forcefully extend the interphalangeal joints, as is necessary in finger-flicking movements. Prominent atrophy of hand muscles ensues and is most noticeable at the first dorsal interosseous muscle. Lumbrical weakness leads to clawing of the fourth and fifth fingers and flexion of the proximal and distal interphalangeal joints, with secondary hyperextension of the metacarpophalangeal joints (benediction posture or ulnar clawing) . Weakness of the third palmar interosseous muscle results in abduction of the little finger, which may get caught when the patient tries to put the hand in a pocket (Wartenberg sign) . In chronic ulnar neuropathies, the weakness and atrophy of small muscles of the hand is always more severe than the weakness and atrophy of the forearm muscles. Sensory loss or hypoesthesia involves the fifth finger, part of the fourth finger, and the hypothenar eminence and includes the dorsum of the hand but does not extend above the wrist level. Pain around the elbow and tenderness of the ulnar nerve with deep palpation is common, but distal hand or finger pain is rare. A Tinel sign at the elbow may be elicited, but this sign as well as provocative tests (flexion compression test, palpating for local ulnar nerve tenderness and nerve thickening) have poor diagnostic values ( ).
Ulnar nerve lesions at the elbow should be distinguished from ulnar nerve lesions at the wrist, lower brachial plexus lesions (lower trunk or medial cord), and C8 radiculopathy. Confirmed sensory loss that extends more than 3 cm above the wrist into the medial forearm and arm, the territories of the medial brachial and antebrachial cutaneous nerves, is inconsistent with an ulnar neuropathy at the elbow and suggests a more proximal lesion of the lower plexus or C8 or T1 roots. Similarly, weakness of median and radial innervated C8 muscles such as the flexor pollicis longus or the long finger extensors points to a plexopathy or radiculopathy.
Compared to evaluating other entrapment neuropathies such as CTS, the EDX studies used to confirm and localize ulnar nerve entrapment at the elbow are more challenging. Localizing ulnar neuropathy at the elbow relies upon the demonstration of focal demyelination across the elbow, namely slowed motor conduction velocity (>10–15 m/sec) or conduction block (localized reduction in CMAP amplitude and area of >20%–30%) or both. Focal slowing or conduction block may be found in the elbow segment in more than 75% of cases ( ). Performing an additional ulnar motor conduction study, recording the first dorsal interosseous muscle in addition to recording the abductor digiti minimi muscle, increases the yield of finding focal slowing or conduction block. In the remaining patients, localization becomes less precise because of predominant axonal loss. To provide the extra nerve length needed during elbow flexion, the ulnar nerve is anatomically redundant in the ulnar groove when the elbow is extended, and this can cause measurement errors. A flexed position of the elbow (70–90 degrees) is preferred to the extended position when doing ulnar motor conduction studies to localize an ulnar lesion at the elbow.
Reference values to determine absolute across-elbow ulnar motor conduction velocity slowing or relative slowing in across-elbow versus forearm conduction velocities have varied depending on study design and population studied. In addition, the optimal distance for measuring ulnar motor NCV around the flexed elbow continues to be debated between 6 and 10 cm ( ). To improve diagnostic accuracy and reduce false-positive and false-negative results, different diagnostic criteria are advocated for patients with different pre-test probability of ulnar neuropathy across the elbow ( ). Patients with high pre-test probability of ulnar neuropathy across the elbow (i.e., with typical clinical symptoms and signs) should have less stringent diagnostic criteria (for example >14 m/sec velocity difference or <47 m/sec velocity), while those with low pre-test probability of ulnar neuropathy across the elbow (i.e., with atypical or inconsistent clinical symptoms and signs) should have more strict diagnostic criteria (such as >23 m/sec velocity difference and <38 m/sec velocity). Short-segment incremental studies (“inching”) by stimulating the ulnar nerve in successive 1- or 2-cm increments across the elbow, looking for either an abrupt drop in amplitude or increase in latency, are useful techniques that help to precisely localize the ulnar nerve lesion ( ; ). Electrophysiological tests are helpful in differentiating between an ulnar neuropathy and a C8 nerve root or brachial plexus lesion. Ulnar sensory nerve conduction sparing points to C8 radiculopathy and normal needle EMG of C8 muscles innervated by the median nerve (e.g., abductor pollicis brevis, flexor pollicis longus) and radial nerve (e.g., extensor indicis proprius) help exclude a C8 root lesion or a lower brachial plexopathy.
High-resolution ultrasonography at the elbow is also useful by accurately detecting enlargement of the ulnar nerve at the elbow ( ). MRI of the elbow may reveal a space-occupying lesion or anomalous structures impinging on the nerve or demonstrate nerve enlargement and increased signal intensity, even in the absence of localizing electrophysiological abnormalities ( ).
Conservative treatment should be attempted in patients with mild or intermittent sensory symptoms or in those with symptoms brought on by occupational causes. Avoidance of repetitive elbow flexion and extension or direct pressure on the elbow may alleviate the symptoms. Elbow protectors are helpful in patients with a history of excessive elbow leaning. Conservative treatment should be continued for at least 3 months before surgery is considered. Several surgical approaches to an ulnar nerve lesion at the elbow are possible, each with its proponents and critics. Techniques include simple release of the flexor carpi ulnaris aponeurosis, anterior transposition of the nerve trunk, and resection of the medial epicondyle. The choice of procedure should be tailored to the specific lesion found at surgery and may be assisted by short-segment incremental electrophysiological studies (“inching”). Transposition of the nerve trunk carries a higher rate of complications than ulnar neurolysis ( ). Depending on the type of surgery and the severity and duration of neuropathy, response to these procedures will vary. Only about 60% of patients, especially those with symptoms of less than 1 year’s duration, benefit from surgery; some experience worsening of symptoms. It appears that those with more thickening of the nerve at the time of diagnosis (as determined by sonography) have a more unfavorable outcome, and those with electrophysiological signs of demyelination across the elbow, specifically significantly greater than 50% conduction block, have a more favorable course ( ; ).
Distal entrapment of the ulnar nerve at the wrist (Guyon canal) or hand is a relatively uncommon condition. Ulnar nerve entrapment in the Guyon canal occurs much less frequently than at the elbow. Aside from direct trauma and laceration, the most common cause is a ganglion cyst. Other usual causes are chronic or repeated external pressure by hand tools, bicycle handlebars, the handles of canes, or excessive push-ups. Compression also may be caused by degenerative wrist joint changes, rheumatoid arthritis, or distal vascular anomalies.
Ulnar nerve entrapment at the wrist may present with a confusing array of sensory and motor symptoms or both, depending on which branches of the nerve are involved. Most cases of ulnar nerve entrapment at the Guyon canal, however, involve solely motor fibers and present with painless unilateral hypothenar and interossei weakness or atrophy. Because the palmar cutaneous and dorsal cutaneous branches leave the ulnar nerve in the distal forearm and do not enter the Guyon canal, sensation in the proximal hypothenar region and the dorsum of the little and ring fingers is not impaired in all cases of ulnar nerve lesions at the wrist or hand. The sensory loss, if present, is confined to the palmar surface of the ulnar-innervated fingers (the little finger and usually the ulnar half of the ring finger) and the distal hypothenar region. Compression at the distal portion of the Guyon canal (also referred to as the pisohamate hiatus ) results in selective involvement of the deep motor branch, with interossei weakness and atrophy and complete or relative sparing of the hypothenar muscles as well as sensation ( ).
The diagnosis is confirmed by EDX studies, often by demonstrating low amplitude (with or without prolonged distal motor latencies) to the first dorsal interosseous or abductor digiti minimi muscles or both, along with denervation of the ulnar-innervated hand muscles that parallels the clinical manifestations. Ulnar SNAP may or may not be abnormal. These EDX studies are also important in excluding an ulnar neuropathy at the elbow. Several features on the EDX examination are inconsistent with an ulnar neuropathy at the wrist: low amplitude or absent dorsal ulnar SNAP, focal slowing or conduction block across the elbow, or denervation of the flexor carpi ulnaris or the flexor digitorum profundus (ulnar portion).
Plain radiograph of the wrist may reveal a fracture of the pisiform or hook of the hamate bone. Neuromuscular ultrasound or MRI is extremely helpful and may demonstrate a structural lesion such as a ganglion cyst. Sources of occupational or recreational trauma should be eliminated. In patients with fractures, ganglia, or mass lesions, surgical intervention is necessary. The prognosis is usually good after surgical decompression with effective reinnervation.
The radial nerve is the largest nerve in the upper extremity. In the arm, lying medial to the humerus, the radial nerve innervates all three heads of the triceps muscle and the anconeus muscle. The nerve passes obliquely behind the humerus and then through the spiral groove, a shallow groove formed deep to the lateral head of the triceps muscle. Before entering the spiral groove in the midarm, it gives three sensory branches: the posterior cutaneous nerve of the arm (which innervates a strip of skin overlying the triceps muscle), the lower lateral cutaneous nerve of the arm (which innervates the lateral half of the arm), and the posterior cutaneous nerve of the forearm (which innervates the skin of the extensor surface of the forearm). In the anterior compartment of the arm, the radial nerve, lying lateral to the humerus, innervates the brachioradialis and the extensor carpi radialis longus. The nerve then passes anterior to the lateral epicondyle and innervates the extensor carpi radialis brevis and supinator. The “radial tunnel” is a space (not an anatomical tunnel) where the radial nerve travels in the upper forearm from the humeroradial joint past the supinator muscle. Within that space, the radial nerve divides into its terminal branches, the superficial radial and posterior interosseous nerves (PINs). The PIN, a terminal pure motor branch, passes under the proximal edge of the supinator muscle (arcade of Frohse) and travels in the forearm and innervates all the remaining wrist and finger extensors. The superficial radial nerve is a terminal pure sensory nerve and innervates the skin of the proximal two-thirds of the extensor surfaces of the thumb, index, and middle fingers, and half of the ring finger, along with the corresponding dorsum of the hand.
Radial nerve compression in the arm often occurs at the spiral groove of the humerus during drunken sleep wherein the arm is draped over a chair (Saturday-night palsy) ( ). The radial nerve may also be injured following fractures of the humerus. Radial nerve lesions at the axilla are much less common; they may result from misuse of crutches or from the weight of a sleeping partner’s head (honeymoon palsy) or follow shoulder joint replacement. The radial nerve is also often involved in isolation or in combination with other single nerves in MMN with conduction block.
In radial nerve lesions in the spiral groove or midarm, there is weakness of the brachioradialis, wrist, and finger extensors, while the triceps is spared. Sensory abnormalities may occur over the dorsum of the hand, thumb, index finger, and middle finger. In lesions at the axilla, there is additional weakness of the triceps, and the sensory loss may extend into the extensor surface of the forearm and lateral half of the arm and over to the triceps owing to involvement of the posterior cutaneous nerve of the forearm and the lower lateral cutaneous and posterior cutaneous nerve of the arm.
The EDX studies are essential in confirming the site and extent of the lesion, excluding other causes of wrist drop, and estimating severity and prognosis. Low-amplitude or absent radial SNAP is common except when the pathology at the spiral groove is purely demyelinating. Conduction block across the spiral groove is seen in segmental demyelinating lesions, or the radial motor responses are low in amplitude in axon-loss lesions. Mixed lesions are also common. Needle EMG reveals denervation of all finger and wrist extensors, as well as the extensor carpi radialis and the brachioradialis. The triceps and anconeus are spared in midarm lesions and denervated in axillary lesions. Ultrasonography is a useful additional tool to electrodiagnosis for diagnosing radial neuropathy across the spiral groove. Enlarged radial nerve as determined by CSA above at 5.75 mm 2 is a highly specific finding ( ).
As with other peripheral nerve lesions, the prognosis depends on the primary pathology. Radial nerve lesions due to demyelinative conduction block, such as in most cases of Saturday-night palsy, usually improve in 6–8 weeks. Axon-loss lesions such as those often associated with humeral fracture, however, have a less favorable prognosis, with a protracted course and often incomplete recovery.
Lesions of the PIN are uncommon and usually occur in association with trauma, fracture, soft-tissue masses (e.g., lipomas, gangliomas), or exuberant synovium motor neuropathy (i.e., rheumatoid arthritis). Occasionally, a PIN lesion, in isolation or in combination with other single nerves is a manifestation of neuralgic amyotrophy, with acute arm pain followed within a few days by weakness ( ). The clinical manifestations of a PIN lesion are dropped fingers and inability to extend them at the metacarpophalangeal joints. Radial deviation of the wrist on wrist extension is often pathognomonic and is due to weakness of the extensor carpi ulnaris muscle with sparing of the extensor carpi radialis muscle, the latter innervated by the main trunk of the radial nerve. EMG study confirms the diagnosis by demonstrating normal radial SNAP and denervation of the muscles supplied by the PIN, with sparing of more proximal radial-innervated muscles including the brachioradialis, extensor carpi radialis, and triceps muscles.
In rheumatoid arthritis, local injection of corticosteroids may be helpful. If the syndrome is progressive, surgical exploration, including synovectomy or decompression of the PIN, may become necessary ( ).
Patients with persistent tennis elbow (lateral epicondylitis) are sometimes given a diagnosis of radial tunnel syndrome, an extremely rare and highly controversial entrapment of the radial nerve or its posterior interosseous branch within the radial tunnel ( ). The nerve appears most vulnerable to entrapment at the level of the supinator muscle. These patients present with forearm pain and tenderness at the lateral epicondyle and slightly distally into the forearm, with no associated muscle weakness or sensory loss in the radial or PIN distribution. Pain is induced by extension of the middle finger or supination with the elbow extended. The EMG study, including radial nerve conduction studies recording the extensor digitorum communis and extensor indicis proprius, is almost always normal. Local steroid injection may temporarily relieve symptoms. In patients with persistent pain, surgical division of the supinator muscle has been advocated, with variable results.
Cheiralgia paresthetica is a mononeuropathy of the superficial dorsal sensory branch of the radial nerve. It occurs as a result of trauma from tight wristbands or handcuffs, or may result from intravenous cannulation, fracture of the wrist, or wrist surgery (e.g., plating of forearm bones after fracture). The use of one-way (only tighten) ratcheting mechanism of handcuffs increases the risk of cheiralgia paresthetica ( ). In up to 50% of nontraumatic cases, it is also associated with de Quervain tenosynovitis , an inflammatory condition of thumb extensor muscles, predominantly extensor pollicis brevis ( ). In de Quervain tenosynovitis, there is tenderness of the anatomical snuffbox and thumb extensor tendons with forced ulnar deviation while holding the thumb wrapped in the palm (Finkelstien sign). Paresthesias and pain in the distribution of the superficial sensory branch of the radial nerve characterize this benign self-limiting condition. A small area of hypoesthesia in the dorsoradial aspect of the hand is frequently identified. Nerve conduction study often shows a low-amplitude or absent dorsal radial SNAP with normal needle EMG including all radial innervated muscles.
The musculocutaneous nerve arises from the lateral cord of the brachial plexus, with fibers originating from the C5 and C6 roots via the upper trunk. The nerve innervates and penetrates the coracobrachialis muscle and courses down the anterior aspect of the upper arm between the two muscles it innervates—the biceps brachii and brachialis. It then terminates as a sensory nerve, the lateral antebrachial cutaneous nerve, which innervates the lateral forearm to the base of thumb. This nerve may be damaged with shoulder dislocations, following general anesthesia, or with vigorous exercise such as weight lifting or repetitive movements such as occur in carpet carriers, where the nerve is repeatedly compressed by carrying carpets on the shoulder while held in place by the arm ( ). The musculocutaneous nerve may also be involved in neuralgic amyotrophy (Parsonage-Turner syndrome; ). The differential diagnosis includes C5 or C6 radiculopathy, upper trunk or lateral cord brachial plexopathy, and rupture of the biceps tendon.
Clinically, patients with musculocutaneous mononeuropathy present with weakness and atrophy of the biceps brachii and brachialis muscles, diminished biceps brachii reflex, and sensory loss over the lateral aspect of the forearm anteriorly. Nerve conduction studies show reduced musculocutaneous CMAP amplitude recording the biceps muscle and a low-amplitude or absent lateral antebrachial cutaneous sensory response. Needle EMG demonstrates denervation limited to the biceps brachii and brachialis muscles, often sparing the coracobrachialis muscle.
Spontaneous recovery is the rule. Local corticosteroid injection may provide some relief of pain. Surgical decompression is contemplated if no improvement occurs.
The suprascapular nerve is a pure motor branch of the upper trunk of the brachial plexus with innervation from the C5 and C6 roots. It then passes through the suprascapular notch, covered by the transverse scapular ligament, to innervate the supraspinatus muscle. It wraps around the spinoglenoid notch of the scapular spine and innervates the infraspinatus muscle. Entrapment at the suprascapular notch occurs after repetitive forward traction of the shoulders—a condition seen in certain athletes, especially volleyball players. This nerve also may be involved in a restricted form of neuralgic amyotrophy (Parsonage-Turner syndrome, ). Diffuse aching pain in the posterior aspect of the shoulder exacerbated by overhead activities is a cardinal symptom. The pain has an articular characteristic because the acromioclavicular joint and surrounding structures are innervated by the suprascapular nerve. Atrophy and weakness are confined to the infraspinatus and supraspinatus muscles. Slow and steady abduction of the arm starting from a vertical position alongside the chest is not possible with a severe lesion of the suprascapular nerve. Tendon ruptures of the rotator cuff have to be considered in the differential diagnosis. EMG shows denervation restricted to the supraspinatus and infraspinatus muscles. Local corticosteroid injection may give temporary relief of pain, although surgery is sometimes required ( ). In entrapment at the spinoglenoid notch, pain is usually absent, and there is atrophy, weakness, and denervation of the infraspinatus muscle only.
The intercostobrachial nerve is a cutaneous sensory nerve derived from the second and third thoracic nerve roots. It supplies the skin on the medial surface of the upper arm and axilla, as well as the adjacent chest wall. It may be injured in a modified radical mastectomy and other surgical procedures involving the axilla and lateral pectoral region ( ).
When a sizable cohort of patients with EDX evidence of distal upper-limb entrapment neuropathies was found to have either electrophysiological or radiological and clinical evidence of cervical radiculopathy, Upton and McComas proposed that focal compression of single nerve fibers proximally might so alter axoplasmic transport as to render the distal nerve more susceptible to symptomatic entrapment neuropathy ( ). They termed this the double crush syndrome . Although the concept of double crush syndrome has since been invoked in a wide variety of entrapment neuropathies, often as an explanation for failure or dissatisfaction with decompressive surgeries of the neck or limb or as a rationale to decompress a nerve in multiple proximal-to-distal sites along its course, this phenomenon is of uncertain validity ( ; ).
Entrapment neuropathies of lower limbs are shown in Table 106.5 .
The sciatic nerve is formed from nerve roots L4 through S3 and is composed of two distinct nerves, the common peroneal (renamed the fibular nerve by the Federative Committee on Anatomical Terminology [FCAT], owing to confusion between the terms peroneal and perineal ) and tibial nerves, which share a common sheath from the pelvis to the popliteal fossa. Usually the sciatic nerve emerges from the pelvis by passing beneath the piriformis muscle, but sometimes the fibular division only passes through or above the piriformis muscle. The sciatic nerve innervates all the hamstring muscles via the tibial nerve except the short head of the biceps femoris, which is innervated by the common fibular nerve. The common fibular and tibial nerves separate completely in the upper popliteal fossa or slightly above.
The sciatic nerve is occasionally vulnerable to entrapment as it crosses over the sciatic notch leaving the pelvis. Most sciatic nerve lesions are complications of hip replacement surgery ( ; ). Traumatic lesions include bullet and stab wounds, fractures, dislocations, hematomas in the posterior thigh compartment, and misplaced intramuscular injections. Recurrent sciatic mononeuropathy may be caused by endometriosis involving the nerve at the sciatic notch. Direct compression of the sciatic nerve is rare but occasionally occurs during coma, anesthesia, or prolonged sitting on a hard surface (“toilet seat palsy”) . Either or both divisions of the nerve may be compressed by a Baker cyst in the popliteal fossa.
A complete sciatic nerve lesion results in weakness of knee flexors and all muscles below the knee, as well as sensory loss of the entire foot and leg below the knee except for a region supplied by the saphenous nerve over the medial leg. The fibular division is more commonly involved than the tibial in proximal lesions of the sciatic nerve. Partial sciatic nerve lesions often affect the fibular nerve more than the tibial nerve and may mimic a more distal common fibular neuropathy. This is explained by the fewer fascicles with limited supportive tissue within the fibular nerve, which is also taut and secured at the sciatic notch and fibular neck. In such patients, evidence of denervation in the short head of the biceps femoris and tibialis posterior muscles and abnormal sural or medial plantar SNAPs help localize partial proximal sciatic nerve lesions ( ). Occasionally, the common fibular nerve gets injured selectively ( ). Young age, lack of severe initial weakness, and recordable distal motor or sensory responses are predictors of favorable outcome ( ; ).
On rare occasions, the piriformis muscle may entrap the sciatic nerve trunk as it passes through or over the piriformis muscle. Since the term was coined in 1947 by Robinson, the piriformis syndrome has been subject to controversy ( ; ). The syndrome fell out of fashion with the advancement of radiological techniques (myelography, CT, MRI) that often demonstrate that most patients with sciatica have nerve root compression and occasionally lesions of the sacral plexus or sciatic nerve at other locations.
The typical patient with piriformis syndrome has a history of buttock trauma and experiences maximal buttock pain during prolonged sitting (e.g., driving, biking), bending at the waist, or activity that requires hip adduction and internal rotation (e.g., cross-country skiing) ( ). The neurological and EDX examinations are usually normal. A bedside test maneuver in which the hip is placed passively in adduction, internal rotation, and flexion of the hip (AIF maneuver) may reproduce the pain and is considered diagnostic. Imaging is usually normal but occasionally shows hypertrophy of the piriformis muscle or abnormal vessels or bands in the region of the piriformis muscle. MR neurography may show sciatic nerve hyperintensity at the sciatic notch, a more specific sign of nerve entrapment ( ). Treatment consists of exercises that include prolonged stretching of the piriformis muscle by flexion, adduction, and internal rotation of the hip. CT- or MRI-guided corticosteroid injection into the piriformis muscle may alleviate the symptoms; a positive response is used as a confirmatory test. Surgical sectioning of the piriformis muscle is indicated in cases resistant to conservative therapy. Although good outcome is expected in carefully selected patients ( ), severe postoperative sciatic nerve injury was recently reported as a serious complication ( ).
As noted earlier, the confusing similarity between peroneal and perineal led FCAT to rename the peroneal nerve the fibular nerve . Soon after the sciatic nerve divides close to the popliteal fossa, the common fibular nerve gives off the lateral cutaneous nerve of the calf, which innervates the skin over the upper third of the lateral aspect of the leg, and the fibular communicating nerve which joins the sural nerve. The common fibular nerve then winds around the fibular neck and passes through the origin of the peroneus longus muscle (“fibular tunnel”). Near that point, the common fibular nerve divides into its terminal branches, the deep and superficial fibular nerves. The deep fibular nerve traverses the lateral and then anterior leg compartments and innervates the tibialis anterior, extensor hallucis longus, peroneus tertius, and extensor digitorum longus. It then divides close to the ankle joint to innervate the extensor digitorum brevis and the skin of the web space between the first and second toes. The superficial fibular nerve innervates the peroneus longus and brevis and the skin of the lower two-thirds of the lateral aspect of the leg and the dorsum of the foot (except for the first web space). An accessory deep fibular nerve, seen in up to 28% of individuals, arises from the superficial fibular nerve, passes behind the lateral malleolus, and innervates the lateral part of the extensor digitorum brevis.
Compression of the common fibular nerve is the most frequent compressive neuropathy in the lower extremity. This nerve is particularly vulnerable to direct pressure in the region of the fibular neck as it passes through the origin of the peroneus longus muscle. Intraoperative compression due to improper positioning or padding during anesthesia is the leading cause of acute common fibular neuropathy at the fibular neck ( ). Weight loss, habitual leg crossing, or unrecognized pressure on the nerve in hospitalized critically ill, debilitated, or unconscious patients may also be responsible for this nerve injury ( ; ). Devices that may compress the fibular nerve include casts, orthoses, pneumatic compression, antithrombotic stockings, bandages, and straps. Fibular nerve stretch injury may result from an acute forceful foot inversion or prolonged squatting (strawberry pickers palsy). Blunt trauma (e.g., post fibular fracture, knee dislocation) and open injury (e.g., lacerations) account for a significant number of cases. Postpartum peroneal neuropathy may be due to stirrups compression, prolonged squatting, or direct hand compression. Fibular nerve injury is also a known complication of knee surgery, including arthroscopic surgery and lateral meniscus repair. Up to half of patients without a clear cause of fibular mononeuropathy across the fibular head have intraneural ganglia ( ). These are formed when disruption of the capsule of the superior tibiofibular joint results in dissection of synovial fluid along the articular branch of the fibular nerve into the tibialis anterior motor branch ( ). Other mass lesions such as osteochondromas or schwannomas are much less common.
A common fibular nerve lesion leads to weakness of foot and toe extension and foot eversion, with a foot drop and steppage gait. Sensory impairment is found over the lateral aspect of the lower two-thirds of the leg and the dorsum of the foot. Foot eversion and sensory loss may be spared (except for the first web space of the foot) when the lesion is selective, involving the deep fibular nerve. Pain is rare except with intraneural ganglia.
Fibular mononeuropathy is most often confused with other causes of unilateral foot drop, including L5 radiculopathy, sciatic nerve lesions (especially when predominantly affecting the common fibular nerve), and lumbosacral plexopathy (particularly when involving the lumbosacral trunk). A fibular nerve lesion must be differentiated from anterior tibial compartment syndrome , in which the deep fibular nerve is compressed by muscle swelling within the anterior compartment secondary to injury, heavy exercise, trauma, or ischemia. This results in an acute syndrome of severe lower leg pain, swelling, and weakness of foot and toe extensors. The anterior tibial compartment must be decompressed rapidly by fasciotomy to prevent irreversible nerve and muscle damage.
EDX studies are useful for localizing lesions and may provide clues to the underlying cause and a guide to prognosis. Although it is often possible by nerve conduction studies to demonstrate focal conduction block across the fibular head, contrary to common belief, the most frequent pathophysiological process is axonal loss, regardless of the cause ( ). Axon-loss lesions reveal diffusely low or absent fibular motor and sensory amplitudes. In contrast to ulnar lesions across the elbow and CTS, localized slowing in the region of the fibular head is not common. Needle EMG demonstrates denervation in common fibular-innervated muscles but not in the short head of the biceps femoris (innervated by the common fibular division of the sciatic nerve in the thigh), in the L5 nerve root-innervated muscles, such as the tibialis posterior, flexor digitorum longus, tensor fascia lata and gluteus medius, or the low lumbar paraspinal muscles.
Ultrasound and MRI are effective in visualizing intraneural ganglia and other soft-tissue masses or tumors. Ultrasound is slightly more accurate than MRI in compressive fibular neuropathies ( ). It shows thickened fibular nerve (cross-sectional area >8 mm 2 ) in about 70% of patients ( ), and establishes involvement of the anterior fascicles (corresponding to fibers for the deep fibular nerve) in the majority of compressive fibular neuropathies ( ).
The prognosis is uniformly good in cases of acute demyelinating lesions, whereas recovery is delayed in those with axonal lesions and stretch injuries. The distal fibular motor amplitude recording tibialis anterior serves as an accurate estimate of the extent of axonal loss and a good prognostic indicator of foot drop. Hence, fibular nerve studies should be performed bilaterally and compared. Bracing with a custom-made plastic ankle-foot orthosis is necessary to improve the gait in the presence of severe foot drop. The few patients who do not improve spontaneously after 3 months, or those who have pain or a slowly progressive fibular nerve lesion, may require MRI studies and surgical exploration ( ).
The tibial nerve innervates all the hamstring muscles except the short head of the biceps femoris. It then separates from the common fibular nerve, usually in the upper popliteal fossa, and gives off the sural sensory nerve, which is often joined by a branch from the common fibular nerve, the sural communicating nerve, to innervate the skin over the lateral aspect of the lower leg and foot, including the little toe. In the upper calf, the tibial nerve passes underneath the soleus muscle and innervates the gastrocnemius, soleus, tibialis posterior, flexor digitorum profundus, and flexor hallucis longus. At the ankle, the tibial nerve passes under the laciniate ligament, which covers the tarsal tunnel through which the nerve passes together with the tendons of the tibialis posterior, flexor digitorum longus, and flexor hallucis longus muscles and the tibial artery and veins.
Entrapment of the tibial nerve occurs behind and immediately below the medial malleolus. Burning pain is experienced in the toes and the sole of the foot. If the calcaneal sensory branches are involved, pain and numbness also involve the heel. Examination usually reveals plantar sensory impairment and wasting of the intrinsic foot muscles. Percussion at the site of nerve compression or eversion of the foot often elicit pain and paresthesias. EDX study results should confirm entrapment of the tibial nerve at the tarsal tunnel by demonstrating slowing of motor fibers to the abductor hallucis and/or abductor digiti minimi muscles, as well as involvement of the medial and/or lateral plantar mixed potentials fibers, with sparing of the sural nerve sensory action potential. Unfortunately, medial and/or lateral plantar mixed (or sensory) potentials are technically very difficult and may be unelicitable in normal subjects with plantar calluses, foot edema, previous surgical procedures in the foot, or in adults over the age of 45. Needle EMG shows denervation of the abductor hallucis and/or abductor digiti minimi muscles and normal S1-innervated and proximal muscles such as the gastrocnemius, soleus, biceps femoris, and gluteus maximus muscles. The majority of suspected cases of tarsal tunnel syndrome, particularly when symptoms are bilateral, turn out to have generalized peripheral neuropathy, S1 radiculopathy, or non-neurological foot pain such as plantar fasciitis, stress fracture, arthritis, or bursitis. Ultrasound plays an important role in identifying the cause ( ). This is particularly useful in elderly or patients with foot edema, calluses, or previous surgery as EDX studies may be difficult to perform or interpret.
Local injection with corticosteroids underneath the laciniate ligament may temporarily relieve symptoms. Surgical decompression is needed for permanent results in those rare cases in which objective evidence of this syndrome exists.
Although the vast majority of sural nerve lesions are iatrogenic as the result of diagnostic sural nerve biopsy or sural nerve harvesting for nerve grafts, mononeuropathy of the sural nerve has been reported with a number of other conditions including lower-limb vein-stripping surgery, ankle liposuction, Baker cyst or ankle joint surgery, local trauma such as with tightly laced high-topped footwear such as ski boots or ice skates, and rarely as the initial presentation of vasculitic mononeuritis multiplex ( ; ; ).
The femoral nerve is formed in the pelvis from the posterior divisions of the ventral rami of L2, L3, and L4 spinal roots, where it innervates the psoas muscle. It then passes within the iliacus compartment and innervates the iliacus muscle via a motor branch that originates 4–5 cm before the nerve crosses underneath the inguinal ligament. In the anterior thigh, the femoral nerve innervates the quadriceps and sartorius muscles and the skin of the anterior thigh and gives off the saphenous sensory nerve, which innervates the skin of the medial surface of the knee and medial leg.
The majority of femoral nerve lesions are iatrogenic ( ). Pelvic lesions follow a variety of gastrointestinal, vascular, urological, or gynecological operations such as abdominal hysterectomy, radical prostatectomy, renal transplantation, and abdominal aortic repair. During these procedures, the femoral nerve becomes compressed between the lateral blade of the retractor and the pelvic wall. Risk factors include the use of self-retaining retractors, a thin body habitus, and transverse abdominal incision ( ). Acute retroperitoneal hematoma is often iatrogenic following anticoagulant therapy, pelvic operations, or femoral vessel catheterization such as for cardiac catheterization. At the inguinal ligament, the femoral nerve may become kinked during lithotomy positioning, particularly when the leg is held in extreme hip flexion and external rotation, used during vaginal delivery, vaginal hysterectomy, prostatectomy, and laparoscopy. Total hip replacement, particularly surgical revisions and complicated reconstructions, may result in femoral nerve injury.
Femoral nerve injury due to spontaneous retroperitoneal hematoma may occur in hemophiliacs, patients with blood dyscrasias, or following a ruptured abdominal aortic aneurysm. Pelvic lymphadenopathy, primary malignancy of the colon or rectum, and neurofibromas or schwannomas are rare causes of femoral neuropathies. Hip hyperextension, such as in dancers or during Yoga exercise, may also cause a femoral stretch injury.
Femoral nerve lesions manifest with acute thigh weakness and anterior thigh and medial leg numbness. Thigh weakness often leads to falls. Pain is usually absent except in cases due to retroperitoneal hematomas. On examination, there is weakness of knee extension, with absent or depressed knee jerk. Thigh adduction is normal. Hip flexion is usually weak when the lesion is within the pelvis, although it may be difficult to assess hip flexion in the setting of severe quadriceps weakness.
Needle EMG reveals denervation of the quadriceps muscle. The iliacus muscle is often normal in inguinal lesions but shows denervation in femoral nerve lesions in the pelvis. Needle EMG of the thigh adductor muscles, innervated by the L2, L3, L4 roots via the obturator nerve, helps distinguish femoral nerve lesions from upper lumbar radiculopathy or plexopathy. Nerve conduction studies have prognostic value, since the amplitude and area of the femoral CMAP is a very good quantitative measure of motor axonal loss ( ). CT or MRI of the pelvis are urgently indicated in patients with suspected retroperitoneal hematoma or pelvic mass lesion.
Apart from patients with confirmed retroperitoneal hematoma who may require emergent drainage, most other femoral nerve lesions are treated conservatively. A knee or knee-ankle-foot orthosis is helpful for patients with unilateral severe weakness of the quadriceps and will assist in walking and prevent falls. Prevention of femoral nerve injury is of paramount importance. The surgeon should limit hip flexion, abduction, and external rotation during lithotomy positioning, particularly when “candy cane” stirrups are used. The incidence of femoral nerve lesions after pelvic and gynecological operations is significantly reduced when self-retractors are avoided; the retracting blades should also cradle the rectus muscle without compressing the psoas muscle ( ).
Saphenous nerve lesions may follow stripping of a long saphenous varicose vein, harvesting the vein for a coronary artery bypass, or surgical and arthroscopic operations on the knee. Entrapment of the saphenous nerve is rare and may occur as it exits the subsartorial (adductor or Hunter) canal or by pes anserine bursitis. Patients with saphenous mononeuropathy have sensory loss or hyperesthesia of the medial leg that may extend into the medial arch of the foot.
Saphenous nerve lesions should be differentiated from L4 radiculopathy, lumbar plexopathy, and femoral mononeuropathy. In addition to the clinical examination, EDX studies can confirm that the quadriceps, iliacus, and thigh adductors are normal in patients with saphenous nerve lesions. Saphenous SNAP is often unilaterally absent or low in amplitude, but this response is difficult to elicit, particularly in elderly or obese patients. Saphenous nerve lesions improve spontaneously with time; decompression underneath the subsartorial canal is occasionally performed.
The lateral femoral cutaneous nerve, which is a pure sensory nerve, passes medial to the anterior superior iliac spine under the inguinal ligament to enter the thigh under the fascia lata that it penetrates to supply the skin of the anterolateral part of the thigh. The site of entrapment is usually at the level of the inguinal ligament. Rarely, the nerve may be affected in its proximal segment by retroperitoneal tumors or be injured during appendectomy. The disorder occurs in about 4 per 10,000 individuals. It is most often seen in association with obesity, diabetes, and advancing age ( ). It is a common entrapment neuropathy during pregnancy, particularly the third trimester, and usually recovers after delivery. It may occur with ascites, or in other conditions that increase intraabdominal pressure. Direct compression by a belt, corset, beeper, or cellular phone; fracture of the anterior portion of the ilium; or pelvic tilt causing undue stresses on the abdominal musculature are other causes.
Patients develop numbness, painful burning, and itching over the anterolateral thigh. Pressure at the inguinal ligament medial to the anterior superior iliac spine may elicit referred pain and dysesthesias. Some patients report relief of pain when assuming a supine position.
Lateral femoral cutaneous nerve SNAP is technically difficult to measure and may be absent in healthy subjects, particularly women and obese individuals. Asymmetrical low-amplitude or absent potential on the symptomatic side is a confirmatory finding. Electrophysiological studies of the femoral nerve and quadriceps femoris and iliacus muscles are normal, which helps exclude lumbar radiculopathy and plexopathy. A local anesthetic nerve block may have diagnostic value ( ). Treatment consists of symptomatic measures such as rest, analgesics, and weight loss. Postural abnormalities should be corrected. Neurolysis is rarely beneficial.
The ilioinguinal nerve is analogous to an intercostal nerve. Muscle branches innervate the lower portion of the transverse abdominal and internal oblique muscles. The cutaneous sensory nerve supplies the skin over the inguinal ligament and the base of the scrotum or labia. As the nerve takes a zigzag course, passing through the transverse abdominal and internal oblique muscles, it is subject to mechanical compression such as with a direct inguinal hernia. Trauma, surgical procedures, scar tissue, and increased abdominal muscle tone caused by abnormal posture are frequently responsible. Pain is referred to the groin, and weakness of the lower abdominal wall may result in the formation of an asymmetrical bulging of the lower abdominal wall.
Conservative treatment includes rest and NSAIDs. Neurolysis may be required in refractory cases when a mechanical lesion is suspected.
The obturator nerve is vulnerable to entrapment as it passes through the obturator canal (e.g., by an obturator hernia or osteitis pubis). An obturator neuropathy is most often associated with pelvic malignancies (prostate, cervical, or uterine cancers). It can also be seen with trauma and synovial cyst of the hip or as a surgical complication, especially with extensive retroperitoneal surgeries or laparoscopic pelvic lymphadenectomies and during total hip replacement.
Entrapment produces radiating pain from the groin down the inner aspect of the thigh, often difficult to distinguish from the pain of a recent procedure or trauma. There is weakness of hip adduction and sensory impairment in the upper medial thigh. Many patients appear to have hip-flexor weakness as a false localizing sign. Although this phenomenon may be explained by pain, it is more likely due to mechanical disadvantage of the hip flexors in the presence of weak thigh adductors. CT or MRI scanning of the pelvis is helpful in finding primary or metastatic pelvic tumors. EMG testing is essential for diagnosis by detecting selective denervation of the thigh adductor muscles, with normal quadriceps and iliacus muscles, thus excluding other causes of hip weakness including femoral nerve lesions, upper lumbar (L2, L3, or L4) radiculopathy or plexopathy, and diabetic amyotrophy (diabetic proximal neuropathy or radiculoplexopathy) ( ).
This entrapment neuropathy is treated conservatively, which often provides good results, especially in those with acute onset of symptoms. If such treatment fails or if symptoms progress to involve other nerves in the region, a careful search for occult pelvic or retroperitoneal malignancy must be pursued.
In this rarely reported but not uncommon condition, a pure and relapsing-remitting sensory mononeuritis multiplex is associated with loss of sensation and pain in the distribution of the affected nerves. The onset is usually sudden, and pain is precipitated by movements and (especially) stretching of the affected limbs. Many different cutaneous nerves may be involved. Commonly involved nerves include the superficial and deep peroneal sensory nerves, the median and ulnar digital nerves, the femoral and saphenous nerves, and the radial sensory nerve ( ). Motor nerve fibers are not affected. Laboratory tests fail to detect any underlying cause, but on occasion a sural nerve biopsy demonstrates inflammatory changes or a vasculitis, with patchy loss of nerve fibers and evidence of axonal degeneration suggestive of an ischemic process. Rarely, immunoglobulin (Ig) G deposits are also observed around blood vessels. The pain and areas of sensory loss often recover over weeks to months, but the improvement may be partial. Symptoms may recur at the same or other sites. The discrete areas of sensory deficit and nerve irritation in several cutaneous nerves should indicate the proper diagnosis. The differential diagnosis should always include conditions like DM, leprosy, vasculitis, sarcoidosis, sensory perineuritis, and rarely HNPP ( ; ).
A slowly progressive painless mononeuropathy that cannot be localized to entrapment sites and is caused by a focal fusiform enlargement of the affected nerve, termed localized hypertrophic neuropathy or perineurioma , is an uncommon condition affecting young adults ( ). Although any nerve may be involved, it often occurs in the radial, posterior interosseous, tibial, and sciatic nerves. The fusiform enlargement is mainly composed of “onion bulblike whorls” formed by layers of perineurial cells. The lamellae of the whorls stain for epithelial membrane antigen. The cause of the perineurial cell proliferation is unknown. It typically results in painless, slowly progressive weakness and atrophy in the distribution of the affected nerve. Sensory symptoms are minor, although sensory nerve fibers are obviously involved. EDX study shows an axonal mononeuropathy and help in the precise localization of the focal nerve lesion. MRI shows a focal enlargement of the affected nerve, increased signal on T2-weighted images, and enhancement with gadolinium.
Surgical exploration and a fascicular biopsy by a surgeon experienced in peripheral nerve microsurgery may confirm the diagnosis and exclude malignant peripheral nerve sheath tumors, which are difficult to exclude without biopsy. Surgical resection of the involved nerve segment with graft repair has been proposed, but because of the benign nature of the “tumor” and its very slow progression, the involved nerve should be preserved if it has even partial function.
The hereditary neuropathies constitute a complex heterogeneous group of diseases that usually share the clinical features of insidious onset and indolent course over years to decades. The number of hereditary disorders for which a metabolic or molecular defect is known is rapidly increasing, allowing a more accurate classification. For those inherited neuropathies for which the underlying genetic abnormality has yet to be identified, the classification still depends on the clinical phenotype, mode of inheritance, and class of neurons predominantly affected. Major advances in understanding the molecular basis of inherited neuropathies have come from identifying chromosomal loci or causative genes for a given disease phenotype, leading to identification of an ever-increasing number of genes coding for a specific gene product essential to myelin or axonal function ( ; ; ; ; ).
Hereditary neuropathies are common disorders, accounting for nearly 40% of chronic polyneuropathies, and as many as 50% of previously unidentified peripheral polyneuropathies. Their inherited nature may go unrecognized in a surprisingly large percentage of patients ( ). Eliciting historical evidence of long-standing neuromuscular symptoms; obtaining detailed family histories; looking for skeletal abnormalities such as hammer toes, high arches, or scoliosis; performing neurological and electrophysiological evaluations in relatives of patients; and, more importantly, testing for confirmed genes are essential in identifying a previously unsuspected inherited neuropathy. Because of the paucity of positive symptoms, patients may not volunteer information about their own or relatives’ conditions. For example, paresthesias are spontaneously reported three times more commonly in acquired than in inherited neuropathies. Even in the face of a truly negative family history, the possibility of an inherited neuropathy cannot be dismissed. Such a situation may arise in cases of early death of one or both parents, few blood relatives, or autosomal recessive (AR) disease. Also, available diagnostic deoxyribonucleic acid (DNA) testing has shown that about a third of isolated cases of inherited neuropathies may arise from de novo gene mutations ( ). It is advisable to consider the possibility of an inherited neuropathy in any patient with a chronic polyneuropathy that remains cryptogenic or refractory to treatment.
The syndrome of peroneal muscular atrophy, or CMT disease, was first described in 1886 by Charcot and Marie in Paris and Tooth in London ( ; ). CMT disease is the most common inherited neuropathy, with an estimated prevalence of 1 per 2500 individuals ( ).
Major advances have been made in recent years in the molecular genetics of CMT disease ( ; ; ). Mutations in more than 80 genes cause CMT (Inherited Neuropathy Variant Browser: http://hihg.med.miami.edu/neuropathybrowser ). These mutations in CMT affect proteins involved in Schwann cell membrane structure (PMP22, MPZ, Cx32) mitochondrial movement (MFN2), signal transduction (GDAP1), cell cycle (MTMR2), cytoskeleton (NEFL, INF2, gigaxonin), transcription factors (EGR2), and protein degradation (LITAF/SIMPLE).
CMT may be classified by mode of inheritance (autosomal dominant [AD], X-linked [XL], and AR), electrophysiological studies, chromosomal locus, or causative genes ( Table 106.6 ). CMT1 and the vast majority of subtypes of CMT2 display AD inheritance. A minority of cases occur sporadically or in siblings only and have therefore been attributed to AR inheritance or to de novo gene mutations. Because a great variability in clinical expression exists among affected kin in the dominant disorders, a recessive inheritance can only be accepted if the clinical and electrophysiological examinations of both parents have proved to be normal. Even when the cause is nonparental, most of these patients phenotypically resemble CMT1.
| Disorder | Locus | Gene | Mechanism | Testing Available |
|---|---|---|---|---|
| CMT1 | ||||
| CMT1A | 17p11.2 | PMP22 | Duplication > pm | Yes |
| CMT1B | 1q22-q23 | MPZ | Pm | Yes |
| CMT1C | 16p13.1 | LITAF | Pm | Yes |
| CMT1D | 10q21 | EGR2 | Pm | Yes |
| CMT1E | 17p11.2 | PMP22 | Pm | Yes |
| CMT1F | 8p21 | NEFL | Pm | Yes |
| CMTX | ||||
| CMTX1 | Xq13.1 | GJB1 (Cx32) | Pm | Yes |
| CMTX4 | Xq24 | AIFM1 | Pm | Yes |
| CMTX5 | Xq22.3 | PRPS1 | Pm | Yes |
| CMTX6 | Xq22.11 | PDK3 | Pm | Yes |
| CMT2 | ||||
| CMT2A2 | 1p36.22 | MFN2 | Pm | Yes |
| CMT2A1 | 1p36.22 | KIFBβ | Pm | — |
| CMT2B | 3q21.3 | RAB7 | Pm | Yes |
| CMT2B1 | 1q22 | LMNA | Pm | Yes |
| CMT2B2 | 19q13.33 | MED25 | Pm | Yes |
| CMT2C | 12q24 | TRPV4 | Pm | Yes |
| CMT2D | 7p15 | GARS | Pm | Yes |
| CMT2E | 8p21 | NEFL | Pm | Yes |
| CMT2F | 7q11-21 | HSPB1 | Pm | Yes |
| CMT2I | 1q23.3 | MPZ | Pm | Yes |
| CMT2J | 1q23.3 | MPZ | Pm | Yes |
| CMT2K | 8q21.11 | GDAP1 | Pm | Yes |
| HNPP | ||||
| HNPP | 17p11.2 | PMP22 | Deletion > pm | Yes |
| DSD Phenotype | ||||
| DSD-A | 17p11.2 | PMP22 | Pm | Yes |
| DSD-B | 1q22-q23 | MPZ | Pm | Yes |
| DSD-C | 10q21-q22 | EGR2 | Pm | Yes |
| AR CMT (CMT4) | ||||
| CMT4A | 8q21 | GDAP1 | Pm | Yes |
| CMT4B1 | 11q22 | MTMR2 | Pm | — |
| CMT4B2 | 11p15.4 | SBF2 | Pm | Yes |
| CMT4C | 5q23-q33 | SH3TC2 | Pm | Yes |
| CMT4D | 8q24 | NDRG1 | Pm | Yes |
| CMT4E | 10q21-q22 | EGR2 | Pm | Yes |
| CMT4F | 19q13 | Periaxin | Pm | Yes |
| CMT4G | 10q23 | HK1 | Pm | — |
| CMT4H | 12q11.1-q13.11 | FGD4 | Pm | — |
| CMT4J | 6q21 | FIGURE4 | Pm | Yes |
The majority of CMT neuropathies are demyelinating, although up to one-third are primary axonal disorders. Clinical studies combined with electrophysiological studies of a large number of families allowed a simple separation of CMT into two main groups: (1) the demyelinating form, or CMT1 (sometimes known as hereditary motor and sensory neuropathy [HMSN-I]), in which there are marked reductions in motor NCVs and nerve biopsy findings of demyelination and onion bulb formation; and (2) the axonal form, or CMT2 (HMSN-II), in which motor NCVs are normal or near normal, and nerve biopsy reveals axonal loss without prominent demyelination ( ). A more severe phenotype of severe demyelinating polyneuropathy with onset occurring in early childhood and very slow conduction velocities (<10 m/sec in forearm) is referred to as Dejerine-Sottas disease (DSD). DSD, formerly CMT3, may no longer be a useful designation because it is genetically heterogeneous, caused by different structural myelin protein and transcription factor gene mutations. A CMT phenotype without sensory involvement on either clinical or electrophysiological examination has been classified as hereditary motor neuropathy or hereditary distal spinal muscular atrophy .
More recent discoveries and phenotype-genotype correlations have identified patients with CMT and intermediate conduction velocities with X-linked inheritance (CMTX), thus revising the electrophysiological classification of patients with suspected CMT. These are now divided into at least four groups based on forearm (ulnar or median) motor conduction velocities : Group 1 are patients with velocities ranging from 15 to 35 m/sec , with the majority diagnosed as CMT1 (mostly CMT1A); Group 2 are patients with normal or near-normal velocities (>45 m/sec) , with most patients diagnosed as CMT2; Group 3 are patients with intermediate velocities (35–45 m/sec), diagnosed as CMTX, but also sometimes CMT1; and Group 4 are patients with extremely slow velocities (<15 m/sec), many presenting in early childhood as DSD phenotype and others presenting in adolescence or early adulthood as CMT1 phenotype.
The classification of CMT remains fluid and continues to change as experts alter and revise these designations based on new molecular findings. The classification of CMT subtypes based on alphabet has become unwieldy as the number of genes and mutations have increased steadily; most neurologists, including specialists in neuromuscular medicine and neurogenetics, do not memorize this nomenclature. In addition, recent studies have confirmed that the same gene defect may manifest as different phenotypes. For example, mutations in MPZ causes AD demyelinating CMT1B as well AD axonal varieties (CMT2I/J). A recent proposal is to abandon the cumbersome numerical designation of CMT subtypes and precisely identify the disorder by using the mode of inheritance (AD, AR, X), electrophysiological hallmark (De, Ax, In), and name of the gene ( ; ). For example CMT1A would be named as AD-CMTDe-PMP22dup (i.e., AD CMT, demyelinating due to PMP22 duplication). Similarly, CMT2A would be AD-CMTAx-MFN2 (AD CMT, axonal due to MFN2 mutation) and CMTX would be XL-CMTIn-GJB1 (X-linked CMT, intermediate due to GJB1 mutation).
In CMT1, symptoms often begin during the first or second decade of life. It is characterized by slowly progressive weakness, muscular wasting, and sensory impairment predominantly involving the distal legs. Foot deformities and difficulties in running or walking resulting from symmetrical weakness and wasting in the intrinsic foot, peroneal, and anterior tibial muscles are often present. In two-thirds of patients, the upper limbs are involved later in life. Inspection reveals pes cavus and hammer toes in nearly 75% of adult patients, mild kyphosis in approximately 10%, and palpably enlarged hypertrophic peripheral nerves in 25% of patients ( Fig. 106.8 ). The foot deformities occur because of long-term muscular weakness and imbalance between the intrinsic extensor and long extensor muscles of the feet and toes (a similar process causes clawing of the fingers in more advanced cases). Absent ankle reflexes are universal and frequently associated with absent or reduced knee and upper limb reflexes. Some degree of distal sensory impairment (diminished vibration sense and light touch in the feet and hands) is usually discovered by examination but rarely gives rise to positive sensory symptoms. Occasionally, patients have an essential or postural upper-limb tremor. Such cases have been referred to as Roussy-Lévy syndrome , but current evidence suggests that this is not a separate clinical or genetic entity.
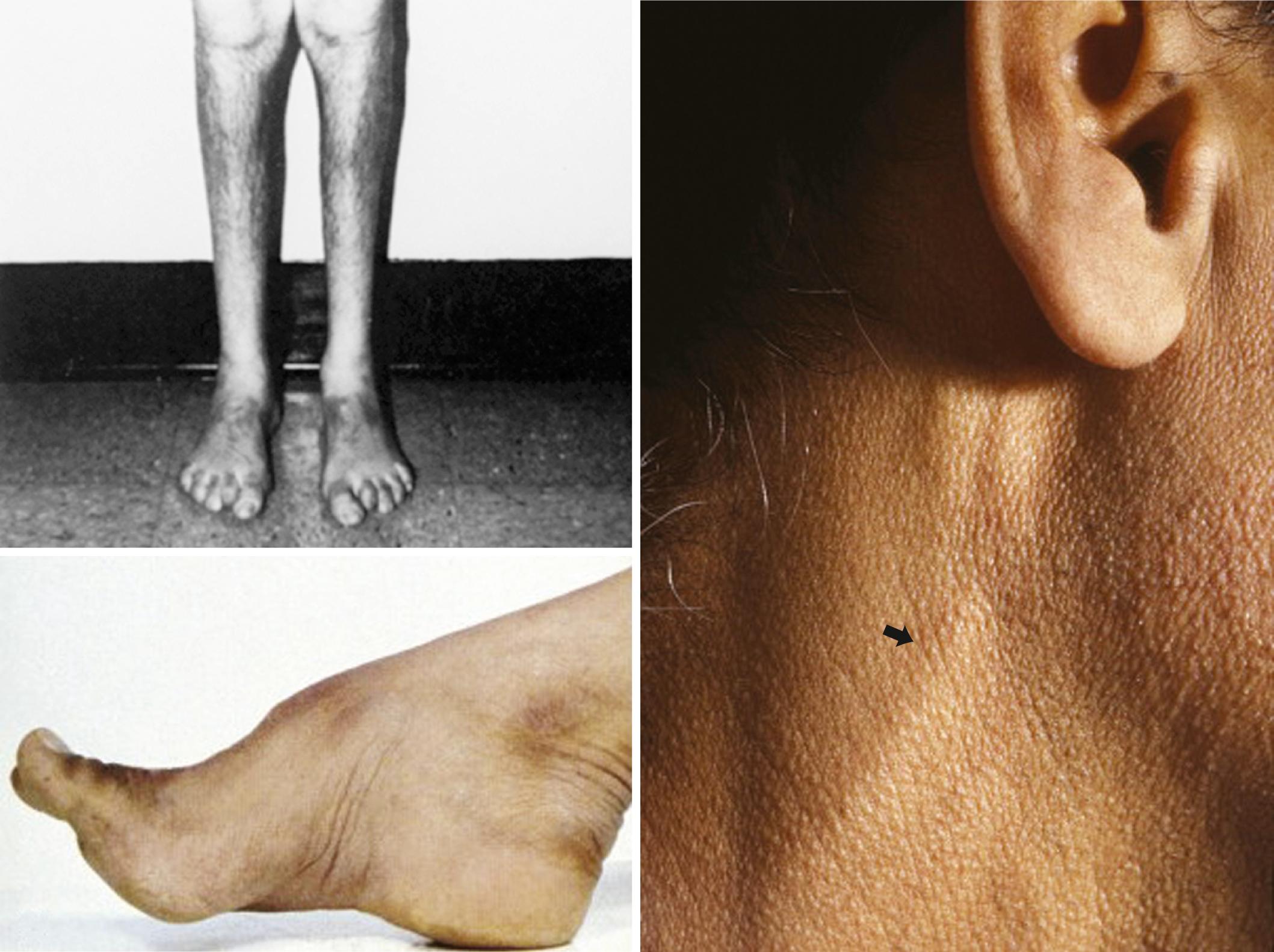
Severity of neuropathy in affected family members varies considerably. Approximately 10% of patients with slowed NCVs may remain asymptomatic. In women with CMT1, the disease may exacerbate during pregnancy. Such worsening is temporary in about a third of patients but becomes progressive in the remainder. Slow deterioration in strength and decline in axonal function continues throughout adulthood, although much of this deterioration likely represents the effects of aging superimposed on decreased reserves ( ).
SNAPs are usually absent with surface recordings. Motor nerve conduction studies show uniform slowing to less than 75% of the lower limits of normal in all nerves. Motor conduction velocities of upper-limb nerves prove more useful than studies of lower-extremity nerves because distal denervation in the feet is often severe and sometimes complete. A motor conduction velocity below 35 m/sec in the forearm segment of the median or ulnar nerves is a proposed cutoff value to distinguish CMT1 from CMT2 and CMTX. Although this cutoff is useful, it can be misleading if applied too rigidly. The conduction slowing evolves over the first 5 years of age and does not change appreciably afterward. Neurological deficits correlate with reductions in CMAP and SNAP amplitudes rather than conduction velocity, indicating that clinical weakness results from loss of axons.
Uniform conduction slowing is often used to differentiate CMT1 from acquired demyelinating neuropathies. Uniform slowing along the entire length of nerves and among neighboring nerves suggests an inherited myelinopathy affecting conduction in all nerves and nerve segments in the upper extremities or lower extremities to the same degree. In contrast, acquired demyelinating neuropathies result in multifocal or nonuniform conduction slowing together with excessive temporal dispersions and conduction blocks. Uniform conduction slowing is found in CMT1A with PMP22 duplication or point mutations; CMT1B with MPZ point mutations; DSD phenotype, including PMP22, MPZ, and EGR2 gene mutations; as well as metachromatic leukodystrophy (MLD); Cockayne disease; and globoid cell (Krabbe) leukodystrophy ( ).
Neuromuscular ultrasound in adults and children with CMT1 displays significantly larger nerve CSA compared with control ( ). Nerve enlargement is commonly diffuse and more pronounced than in acquired demyelinating polyneuropathies (such as CIDP and MMN), where the enlargement is often regional. In children with CMT1A, the CSA correlates with neurological disability and the expected increase in nerve CSA with age is disproportionately greater in CMT1A, suggesting ongoing nerve hypertrophy throughout childhood ( ).
Routine hematological and biochemical studies are normal. CSF is also normal, which helps differentiate the condition from chronic inflammatory demyelinating polyneuropathy (CIDP), in which the CSF protein is usually elevated. Sural nerve biopsy typically shows the changes of a hypertrophic neuropathy, characterized by onion bulb formation, increased frequency of fibers with demyelinated and remyelinated segments, an increase in endoneurial area, and loss of large myelinated fibers ( Fig. 106.9 ). Gene mutations, predominantly affecting genes for myelin and Schwann cell proteins, have been recognized that account for more than three-quarters of families with CMT1 ( Fig. 106.10 ). CMT1A is the most common CMT subtype, accounting for 70%–80% of CMT1 cases and more than 50% of all CMT cases. The disease is caused by duplication of a 01.5-Mb fragment in the short arm of chromosome 17p11.2-12 harboring peripheral myelin protein 22 (PMP22). Rarely, the disease is caused by PMP22 point mutation. PMP22 is a membrane glycoprotein found in the compact portion of the peripheral myelin sheath. The precise function of PMP22 in normal nerve remains unknown. Deletion of the same 1.5-megabase region on chromosome 17p11.2 results in a single copy of the normal PMP22 gene, a finding observed in 85% of patients with HNPP. The CMT1A duplication or HNPP deletion is caused by reciprocal recombination events that occur in male germ cell meiosis. The PMP22 duplication or deletion can be detected in blood samples using pulse-field electrophoresis followed by hybridization with specific CMT1A duplication junction fragments or cytogenetic testing with a PMP22 probe by fluorescence in situ hybridization.
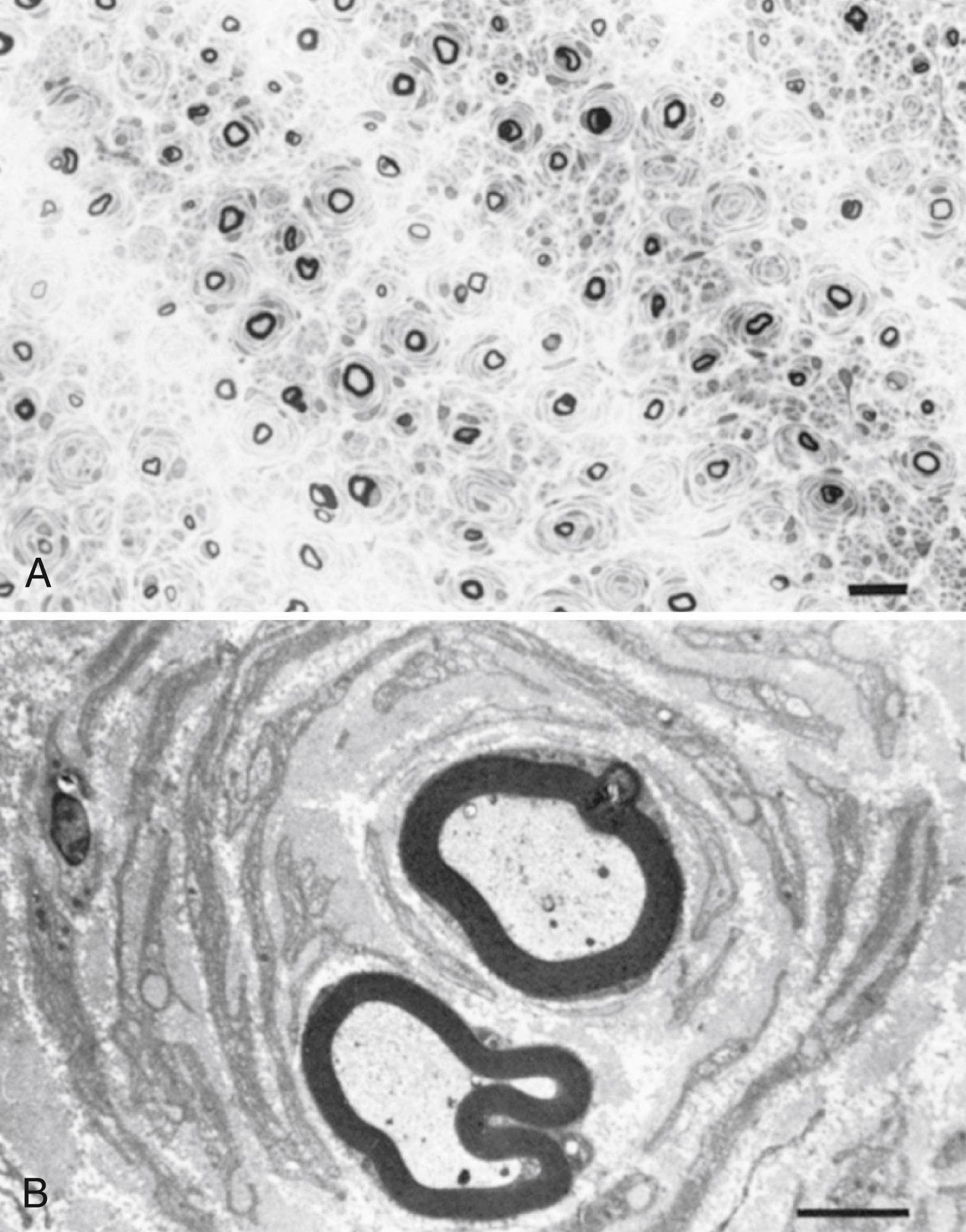
![Fig. 106.10, A , Charcot-Marie-Tooth disease (CMT) and related disorders: CMT1, CMT with X-linked inheritance (CMTX) , hereditary neuropathy with liability to pressure palsies (HNPP) , Dejerine-Sottas disease (DSD) , and most of CMT4 are inherited disorders of myelin. CMT2 is a primary axonal disorder. Alterations in dosage of peripheral myelin protein 22 (PMP22) gene account for the majority of patients with CMT1A and HNPP. B , Point mutations of these genes (connexin-32 [Cx32], myelin protein zero [MPZ, P0], PMP22, EGR2, periaxin) result in CMTX, CMT1B, CMT1A, DSD, and CMT4. Mutations of the LITAF gene result in CMT1C. C , Point mutations of the KIF1B and NF-L genes and specific MPZ missense mutations result in CMT2. NCV , Nerve conduction velocity. Fig. 106.10, A , Charcot-Marie-Tooth disease (CMT) and related disorders: CMT1, CMT with X-linked inheritance (CMTX) , hereditary neuropathy with liability to pressure palsies (HNPP) , Dejerine-Sottas disease (DSD) , and most of CMT4 are inherited disorders of myelin. CMT2 is a primary axonal disorder. Alterations in dosage of peripheral myelin protein 22 (PMP22) gene account for the majority of patients with CMT1A and HNPP. B , Point mutations of these genes (connexin-32 [Cx32], myelin protein zero [MPZ, P0], PMP22, EGR2, periaxin) result in CMTX, CMT1B, CMT1A, DSD, and CMT4. Mutations of the LITAF gene result in CMT1C. C , Point mutations of the KIF1B and NF-L genes and specific MPZ missense mutations result in CMT2. NCV , Nerve conduction velocity.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersofPeripheralNerves/9_3s20B9780323642613001066.jpg)
CMT1B is clinically indistinguishable from CMT1A but it only accounts for 4% to 5% of CMT1 cases. It is caused by mutations in the myelin protein zero (P0; gene symbol, MPZ) gene, mapped to chromosome 1q22-23. MPZ is the major peripheral myelin glycoprotein and is thought to function as an adhesion molecule in the formation and compaction of peripheral myelin. It is a member of the immunoglobulin superfamily, with distinct extracellular transmembrane and intracellular domains. Mutations in the gene encoding for MPZ have also been associated with DSD, and congenital hypomyelination neuropathy. Different MPZ mutations result in divergent morphological effects on myelin sheaths, consisting of uncompacting of myelin or focal myelin foldings ( ). Motor conduction block was reported rarely in CMT1B patients with specific MPZ mutations ( ). Specific MPZ missense mutations have also been reported with a CMT2 phenotype, showing only mild slowing of NCVs ( ). The Thr124 Met mutations in the MPZ gene have been detected in several families with a distinct CMT2 phenotype (CMT2J) characterized by late onset, marked sensory loss, and sometimes deafness, chronic cough, and pupillary abnormalities ( ).
CMT1C is caused by a mutation in lipopolysaccharide-induced tumor necrosis factor-alpha (LITAF/SIMPLE) gene, mapped to chromosome 16p13-12 expressed on Schwann cells. This gene encodes a lysosomal protein that may play a role in protein degradation pathways ( ). Affected individuals in these families manifest characteristic CMT1 symptoms.
CMT1D is mapped to chromosome 10q21-q22 and is due to mutation of the early growth response 2 gene (EGR2) which encodes a zinc-finger transcription factor expressed in myelinating Schwann cells that regulates the expression of myelin proteins including PMP22, P0, Cx32, and periaxin ( ). EGR2 gene missense mutations have also been reported in patients with DSD, or congenital hypomyelination neuropathy ( ; ). Respiratory compromise and cranial nerve dysfunction are commonly associated with EGR2 mutations ( ). Other rare CMT1 subtypes include CMT1E and CMT1F.
CMT2 constitutes about one-third of all AD CMT disease. It is associated with mutations in genes affecting intracellular processes such as axonal transport, membrane trafficking, and translation (see Chapter 48 ). Clinical symptoms begin later than in CMT1, most commonly in the second decade, but may be delayed until middle age or beyond. Foot and spinal deformities tend to be less prominent than in CMT1. The clinical features closely resemble those of CMT1 but differ in that peripheral nerves are not enlarged, and upper limb involvement, tremor, and diffuse areflexia occur less frequently. However, in individual cases, it is often impossible to determine the type of CMT disease on the basis of clinical manifestation alone. Approximately 20% of affected individuals are asymptomatic.
CMT2A is the most common CMT2 subtype and accounts for 30% of CMT2 cases (see Table 106.6 ). CMT2A2, which is responsible for most CMT2 families, shares clinical features of weakness and atrophy with other CMT variants, but has an earlier onset and is more severe, often resulting in earlier disability and wheelchair dependence. It may also be associated with optic atrophy. It is caused by mutations in the mitofusin 2 (MFN2) gene, with a locus on chromosome 1p36.22. MFN2 protein is a mitochondrial fusion protein ubiquitously expressed in many tissues including peripheral nerves. CMT2A1, linked to chromosome 1p36.22, is caused by a mutation in kinesin protein involved in axonal transport of synaptic vesicles ( ; ). In CMT2B, which is linked to chromosome 3q13-22, there is prominent sensory loss with foot ulcerations ( ). A mutation in the RAB7 gene, which encodes a small guanosine triphosphatase (GTPase) late endosomal protein, has been found to be causative ( ). This form of CMT is clinically very similar to hereditary sensory neuropathy type 1 (HSN1) but lacks spontaneous lancinating pain. CMT2B1 and CMT2B2 are AR disorders, caused by mutation in the lamin A/C gene on chromosome 1q22, and mediator of RNA polymerase II transcription, subunit 25 gene (MED25) on chromosome 19q13.33, respectively. Another distinct subgroup of severely affected patients, designated CMT2C (mapped to chromosome 12q24), develop vocal cord, intercostal, and diaphragmatic muscle weakness ( ). Because of respiratory failure, the life expectancy of these patients is shortened. CMT2D, mapped to chromosome 7p14, is characterized by weakness and atrophy that is more severe in the hands than in the feet ( ). In CMT2E, some patients within the same kindred and with an otherwise typical CMT2 phenotype may exhibit slowed motor nerve conduction that is much below the forearm cutoff value of 38 m/sec and a more severe clinical phenotype. This form of CMT is caused by mutations in genes that encode neurofilament light (NEFL) subunit, and patients may have axonal swelling (giant axons) and significant secondary demyelination on sural nerve biopsies ( ; ). CMT2F, caused by mutations in small heat shock protein 27 (Hsp27), is characterized by later onset (35–60 years), mild sensory impairment, and moderate to severely slowed NCVs of lower limbs but normal or mildly reduced velocities in the upper limbs. Mutation in Hsp27 may impair formation of the stable neurofilament network that is essential for the maintenance of peripheral nerves. CMT2G has the same gene locus as CMT4H (see later discussion on type 4 disease) on chromosome 12q12-q13.3, with the age onset from 9 to 76 years. CMT2I and CMT2J are designated as CMT2 with MPZ (myelin protein zero) gene mutations. CMT2J is associated with pupillary abnormalities (Adie pupil) and hearing loss.
Motor NCV may be normal or mildly reduced. SNAPs are either absent or reduced in amplitude. Sural nerve biopsy specimens show preferential loss of large myelinated fibers, without significant demyelination; there may be clusters of regenerating myelinated fibers, a hallmark of axonal regeneration.
X-linked Charcot-Marie-Tooth disease (CMTX) is phenotypically similar to CMT1. CMTX1 is caused by many mutations in gap junction protein B1 (GJB1), the gene that encodes connexin 32 (Cx32), on chromosome Xq13.1. Affected male subjects tend to be more severely affected, and females with the gene mutation are asymptomatic or may have a mild neuropathy. CMTX1 should be considered in any patient whose family history does not exhibit a male-to-male transmission. CMTX1 accounts for 7%–16% of all forms of CMT, making it the second most common form of CMT (following CMT1A).
The connexins are a family of highly related genes encoding a group of channel-forming proteins. Cx32 is a gap junction protein found in noncompacted paranodal loops and Schmidt-Lanterman incisures of Schwann cell cytoplasm, which is encoded by a four-exon gene located on chromosome Xq. As a gap junction protein, Cx32 forms small channels that facilitate transfer of ions and small molecules between Schwann cells and axons. More than 200 different mutations in Cx32 have been identified in CMTX1 families. Genotype-phenotype correlations among patients with Cx32 mutations suggest that most missense mutations result in a mild clinical phenotype, whereas nonsense and frameshift mutations produce more severe phenotypes ( ).
Cx32 is expressed in Schwann cells and oligodendrocytes, regions of noncompact myelin (incisures and paranodes), as well as other non-neural cells. Some mutations of Cx32 have been reported to be associated with central nervous system (CNS) involvement with white-matter MRI and MR spectroscopy abnormalities, abnormal brainstem auditory evoked potentials, and deafness ( ). An interesting phenomenon of transient and acute ataxia, dysarthria, and weakness occurring after visiting high altitudes and associated with CNS white-matter MRI abnormalities has been described in patients with two mutations: R142W and C168Y ( ). This suggests that CMTX1 patients should be cautioned about travel to high-altitude locations. It has been proposed that Cx32 mutations may cause these abnormalities by reducing the number of functional gap junctions between oligodendrocytes and astrocytes, making them more susceptible to changes in intercellular ions and small-molecule exchange that occur in situations of metabolic stress (e.g., high altitude or physical activity).
Men with CMTX1 show significant slowing in NCV, and brainstem auditory evoked responses are often abnormal. A picture of both axonal loss and demyelination is revealed on nerve biopsy. There is debate as to whether CMTX1 should be classified as a primary axonal or demyelinating disorder ( ). However, careful studies of individual patients suggest nonuniform conduction slowing consistent with demyelination ( ; ). NCVs in males with CMTX1 with Cx32 mutations are often slow, usually in the intermediate, slowing between 35 and 45 m/sec. Conduction slowing in heterozygous women may be subtle and frequently is in the range found in patients with axonal polyneuropathies leading to a suspected diagnosis of CMT2. The absence of male-to-male transmission on family history, the presence of mild to intermediate conduction velocities (>42 m/sec) in female carriers, and delayed brainstem auditory evoked response latencies in affected men is highly suggestive of CMTX1 and Cx32 mutations ( ). Much less common X-linked CMT subtypes have been described (see Table 106.6 )
DSD, previously designated as CMT3, is an uncommon progressive hypertrophic neuropathy with onset in childhood. Although originally the disorder was thought to be AR, most cases are sporadic and in some instances have been shown to result from a de novo dominant mutation. The majority of patients have mutations that are common in other types of CMT, including PMP22 duplication or point mutation, MPZ mutation, or EGR2 mutation.
Motor development is delayed; proximal weakness, global areflexia, enlarged peripheral nerves, and severe disability are the rule. Motor conduction velocities are markedly slowed, often to less than 10–15 m/sec in the forearms. Temporal dispersion and amplitude reduction on proximal stimulation may be found in such cases, owing to high electrical stimulation thresholds in hypertrophic nerves. CSF protein is frequently increased. Pathologically, pronounced onion bulb changes are associated with hypomyelination and loss of myelinated fibers. Defective myelination is confirmed by an increased axon-to-fiber diameter ratio. Cases of congenital hypomyelination neuropathy probably represent a variant of DSD at the far end of a spectrum of defective myelination. DSD is genetically heterogeneous and is caused by different structural myelin protein and transcription factor gene mutations (see Chapter 48 ).
The majority of Charcot-Marie-Tooth disease type 4 (CMT4) patients have AR inheritance. They are less common, accounting for less than 10% of all CMT cases. They are characterized by onset in early childhood and progressive weakness leading to inability to walk in adolescence. Both demyelinating and axonal types have been identified ( ). Common to all the demyelinating subgroups is a disturbance in normal myelination of the axons; clinical and electrophysiological features are similar in several of these subtypes with severe forms of CMT1 or DSD. Conduction velocities are slowed (20–30 m/sec). CSF protein is normal. Nerve biopsy shows loss of myelinated fibers, hypomyelination, and onion bulbs.
CMT4 consists of several subgroups (see Table 106.6 ). Each subgroup is rare and tends to be more common in certain inbred populations. CMT4A is the most common and accounts for 25%–30% of all AR cases. The disease has been mapped to chromosome 8q13 because of ganglioside-induced differentiation-associated protein 1 (GDAP1) mutations, the most common cause of CMT4, and may result in demyelinating as well as axonal phenotypes ( ). CMT4B1 is mapped to chromosome 11q21 caused by myotubularin-related protein 2 (MTMR2) mutations, with findings of redundant loops of focally folded myelin ( ), while CMT4B2 is mapped to chromosome 11p15.4 caused by set-binding factor-2 gene (SBF2) mutations. In both, irregular folding and redundancy of loops of myelin are evident on nerve biopsies. Children affected with CMT4B2 also exhibit congenital glaucoma, leading to loss of vision. CMT4C, characterized by frequent and severe scoliosis, is linked to chromosome 5q31-q33 and is caused by SH3TC2 gene mutation ( ). CMT4D has onset in childhood but may progress into the fifth decade of life. It is associated with dysmorphic features and hearing loss. CMT4E is a form of congenital hypomyelinating neuropathy, often diagnosed as DSD, associated with mutations in PMP22 and ERG2 (early growth response) genes. The phenotypic presentation of CMT4F is also severe, similar to that for DSD phenotype, but the mutations occur in the periaxin gene, which produces a membrane-associated protein solely expressed in myelinating Schwann cells. Periaxin is a cytoskeleton-associated protein that links the cytoskeleton of the Schwann cell with the basal lamina, a necessary function to stabilize the mature myelin sheath ( ). CMT4H is similar to CMT2G in terms of genetic locus but is more severe clinically, with an onset in early childhood and prominent nerve hypomyelination.
Some dominant forms of CMT have displayed features intermediate between CMT1 and CMT2, with conduction velocities between 35 and 45 m/sec. These forms have been classified separately as dominant intermediate CMT (DI-CMT) and include types A, B, C, and D. DI-CMTA maps to chromosome 10q24-25, but its gene defect has not been discovered. DI-CMTB is caused by mutations in the dynamin 2 (DNM2) gene and maps to chromosome 19p12-13. This typically presents as a classic mild to moderately severe CMT phenotype. Some families with this variety have developed neutropenia and early cataracts ( ). A mutation in tyrosyl-tRNA (transfer ribonucleic acid) synthetase has been found to be the cause of DI-CMTC, which maps to chromosome 1p34-35 and typically displays a mild, very slowly progressive course ( ). DI-CMTD maps to chromosome 1q22 MPZ gene mutations.
A number of families with CMT exhibit additional features such as optic atrophy, pigmentary retinal degeneration, deafness, and spastic paraparesis. Cardiac involvement is encountered in occasional patients, but prospective family studies find no association between cardiomyopathy and CMT disease. A syndrome of CIDP responding to prednisone and immunosuppression has been reported in patients with inherited CMT disease due to MPZ mutation ( ), providing evidence that nongenetic factors may play a role in clinical expression of the mutant gene. It has been suggested that any patient with a hereditary neuropathy who suffers a recent rapid deterioration should be considered as having a secondary CIDP and be treated with immunosuppressants such as corticosteroids or high-dose intravenous immunoglobulin (IVIG).
Molecular diagnostic testing should be considered in CMT and related peripheral neuropathies. Commercial reference laboratories can detect point mutations or PMP22 duplication/deletion by DNA sequencing of PMP22, Cx32, MPZ, EGR2, periaxin, GDAP1, and NEFL, among others, in samples of peripheral blood ( Table 106.7 ). It is, however, advisable to use the clinical and EDX findings supplemented by a detailed family history and plan a logical approach to obtaining DNA studies. An all-inclusive “battery” of available genetic tests of CMT disease is tempting but interpretation of results may be more difficult because of frequent detection of genes with variants of unknown significance. Population studies confirmed that CMT1A (PMP22 duplication or PMP22 deletion), CMT1X (Cx32 mutation), CMT1B (MPZ mutation), and CMT2A (MFN2 mutation) account for about 65%–70% of all CMT cases ( ; ).
In families with at least two generations with the disease, known male-to-male transmission, and uniform conduction slowing (<35 m/sec if forearm), CMT1A should be considered first and the PMP22 duplication test should be obtained.
If normal, PMP22 sequencing should be done.
If normal, CMT1B should be excluded by obtaining MPZ DNA sequencing.
Patients who have neither the PMP22 duplication nor male-to-male transmission should be screened for CMTX1 by looking for Cx32 mutations.
For patients displaying an axonal pattern, the MFN2 mutation should be investigated first, as this is the most common type of CMT2 ( ).
Given the high spontaneous mutation rate, the diagnosis of CMT1A should be considered even in the absence of a positive family history.
The PMP22 duplication test followed by DNA sequencing of PMP22, MPZ, EGR2, and periaxin should be considered in childhood cases with severe demyelinating neuropathy suggestive of DSD or congenital hypomyelination neuropathy.
Because of the severe reactions to vincristine and other chemotherapeutic neurotoxic drugs in CMT patients, before initiating cancer chemotherapy it is best to rule out CMT1A in any patient with either unexplained chronic neuropathy or a family history of neuropathy ( ).
| Test | CMT1 | HNPP | CMTX | CMT2 | DSD/CHN |
|---|---|---|---|---|---|
| PMP22 dup/del FISH | X, duplication | X, deletion | — | — | X, duplication |
| DNA sequencing: Cx32 PMP22 MPZ EGR2 Periaxin NEFL |
|||||
| X | X | ||||
| X | X | ||||
| XX | XX | X |
The rates of progression of CMT1 and CMT2 are slow, disability occurs relatively late, and lifespan may be normal. Management is mainly symptomatic. Patients should be instructed in proper foot care and advised to wear broad, well-fitting shoes. Insoles may be used to distribute body weight more evenly in patients with a foot deformity. Ankle-foot braces or orthopedic procedures are indicated to correct severe foot drop. Patients should be warned to avoid neurotoxic drugs because of greater susceptibility to agents such as vincristine. Issues like genetic counseling, family planning, prenatal diagnosis, and psychological concerns must be carefully approached, preferably by a multidisciplinary team including a genetic counselor. Recent studies suggest that CMT is associated with higher risk for complication during delivery ( ). Also, during pregnancy the symptoms of CMT may worsen.
Despite astonishing advances in the molecular genetics of CMT, there is still no effective treatment available for any form of the disease. Rats overexpressing PMP22 worsen with progesterone administration. This has led to proposed use of progesterone antagonists for CMT1A, though potentially unacceptable side effects have prevented such trials. The same animal model showed a convincing clinical and pathological improvement following administration of ascorbic acid (vitamin C), a known promoter of myelination. Though pharmacological treatment trials in CMT are rare, this discovery prompted a multicenter double-blind placebo-controlled study on the use of ascorbic acid at 1 or 3 g/day in CMT1A. Ascorbic acid was found to be well tolerated, but no significant benefit was demonstrated in the CMT neuropathy score at 12 months, concluding vitamin C does not improve the course of CMT1A in adults or children ( ; ). The possibly beneficial role of neurotrophins, particularly neurotrophin 3 (NT3), in CMT1A and nerve regeneration has recently been demonstrated in a small pilot study ( ). Treating cells with curcumin, derived from the curry spice turmeric, releases the MPZ mutants from the endoplasmic reticulum into the cytoplasm, thus reducing the number of apoptotic cells and becoming a potential treatment for CMT1B ( ). Some CMT2 patients with MPZ mutations may respond to corticosteroids ( ).
HNPP is an AD disorder of peripheral nerves leading to increased susceptibility to mechanical traction or compression. It occurs with an estimated prevalence of 16 per 100,000 population. Patients have recurrent episodes of isolated mononeuropathies, typically affecting, in order of decreasing frequency, the fibular nerve, ulnar nerve, brachial plexus, radial nerve, and median nerves. Painless brachial plexopathy is seen in up to a third of patients. Most HNPP patients experience the initial episode in the second or third decade of life. Attacks usually are provoked by minor compression, slight traction, or other trivial trauma. Most episodes are of sudden onset, painless, and usually followed by complete recovery within days or weeks. Less-common presentations include transient positionally induced sensory symptoms, progressive mononeuropathy, chronic sensory polyneuropathy, mild CMT phenotype with pes cavus, and a diffuse chronic sensorimotor neuropathy resembling CIDP ( ). About 15% of mutation carriers remain asymptomatic.
Nerve conduction studies in patients with HNPP associated with PMP22 deletion typically demonstrate a characteristic pattern of prolonged distal motor latencies with only mild slowing in forearm segments of median and ulnar nerves, focal slowing and conduction blocks of median, ulnar, and fibular nerves at compression sites, and diffuse reduction of SNAP amplitudes ( ; ). The median forearm motor NCV is typically above 38 m/sec, but sensory studies demonstrate velocities in the demyelinating range and reduced or absent SNAPs. The sural SNAP is absent or abnormal in more than 90% of patients, while the tibial motor nerve is slowed in about 60% of the patients. The most common focal entrapment neuropathy at compressive sites are the ulnar nerve at the elbow and median nerve at the wrist, each occurring in more than three-fourths of patients, followed by the fibular nerve at the fibular head, seen in one-third of the patients ( ). Prolonged median distal motor latencies and abnormal sensory conduction studies are frequently found in asymptomatic carriers ( ).
Sural nerve biopsy specimens demonstrate focal sausage-like thickenings of myelin, termed tomacula ( Fig. 106.11 ), segmental demyelination, and axonal loss. Linkage studies show a 1.5-megabase deletion of chromosome 17p11.2-12 that includes the PMP22 gene and corresponds to the duplicated region in CMT1A in 85% of affected patients with HNPP. The remaining patients have a variety of mutations in PMP22 that lead to frameshift or nonsense mutations causing functional changes in the protein ( ; ). Exactly how the deletion of PMP22 causes HNPP remains unclear, though loss of function of the PMP22 protein is the likely explanation for HNPP, and a toxic gain of function in CMT1A ( ; ). Molecular diagnosis of the 17p11.2 deletion has replaced nerve biopsy for the diagnosis of HNPP. Testing should be considered regardless of family history in any patient presenting with painless multiple mononeuropathies, brachial plexopathy, or recurrent demyelinating neuropathy ( ). The primary treatment strategy in HNPP is to prevent nerve injury by avoiding pressure damage.
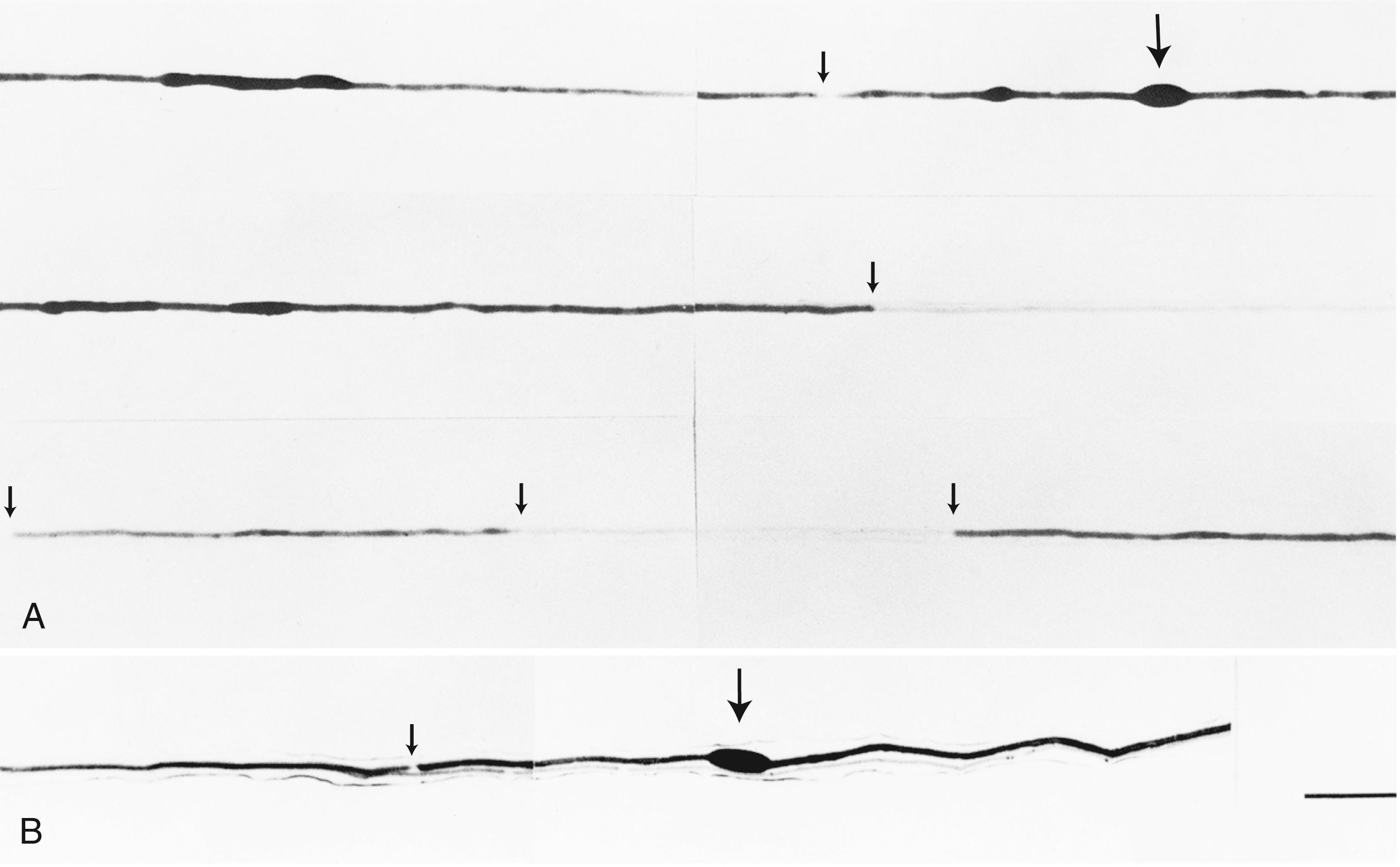
Recurrent brachial plexopathy, often preceded by severe ipsilateral limb pain, is the hallmark of hereditary neuralgic amyotrophy, an AD disorder. Most patients recover over weeks to a few months, with accumulating evidence of residual neurological deficit over time. Patients also have dysmorphic features, including hypotelorism, epicanthal folds, microstomia, and dysmorphic ears. The disorder maps to chromosome 17q25 and is associated with mutations in the SEPT9 gene ( ).
GAN is a rare AR multisystemic neurodegenerative disorder of intermediate filaments affecting the peripheral and CNS. GAN presents as a slowly progressive axonal sensorimotor neuropathy in early childhood and leads to death by late adolescence. Most affected children have tightly curled hair and distal leg weakness. Some develop a peculiar gait disturbance with a tendency to walk on the inner edges of the feet With disease progression, evidence of CNS involvement occurs, including optic atrophy, nystagmus, cerebellar ataxia, upper motor neuron signs and intellectual decline, and abnormal visual, auditory, and somatosensory evoked potentials. MRI of the brain demonstrates cerebellar and cerebral white-matter abnormalities. Nerve conduction studies show reduced CMAP and SNAP amplitudes with normal to only slightly reduced conduction velocities. Sural nerve biopsy demonstrates the pathognomonic changes of large focal axonal swellings that contain densely packed disorganized neurofilaments ( eFig. 106.12 ). Axonal function and axoplasmic transport are impaired. The disease locus was mapped to chromosome 16q24. GAN is caused by mutations of the GAN gene, which encodes a novel protein called gigaxonin ( ; ). Gigaxonin is a ubiquitously expressed cytoskeletal protein and a member of the BTB/kelsch superfamily, which is involved in diverse cellular functions and may be important in the cross-linking of intermediate filaments ( ).
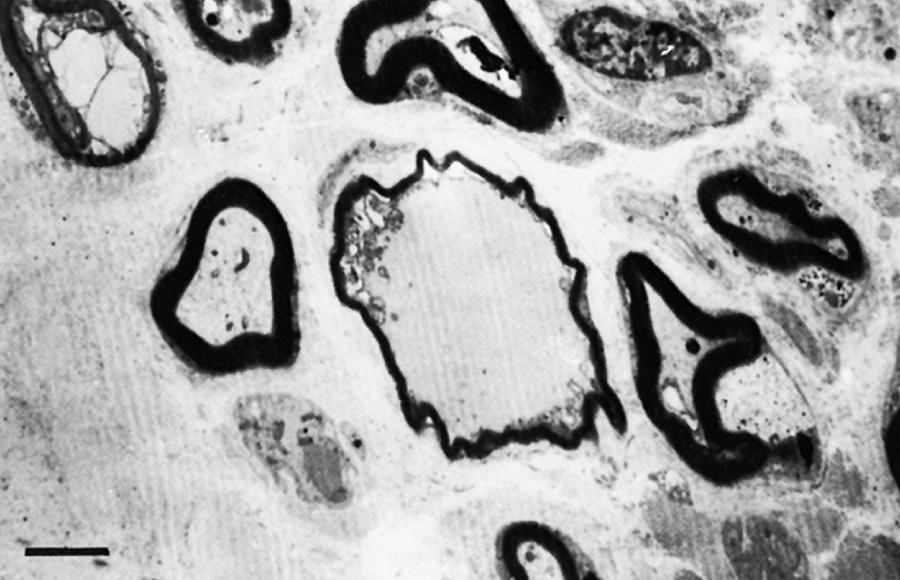
HSANs are a clinically and genetically heterogeneous group of neuropathies characterized by prominent sensory loss and variable autonomic features but without significant motor involvement ( ). Currently these neuropathies are divided into five main groups based on the inheritance, clinical features, and type of sensory neurons involved ( eTable 106.8 ). Compared with CMT, HSANs are distinctly rare. Molecular genetic studies have identified seven disease-causing gene mutations in these disorders. More genetic discoveries are likely forthcoming; a study of 100 patients with familial HSAN and isolated patients diagnosed with HSAN found that only 14% of the isolated cases and 31% of the familial cases were found to have mutations in the known causative genes ( ). The pronounced sensory loss in HSAN predisposes these patients to unnoticed recurrent trauma, leading to neuropathic (Charcot) joints, nonhealing ulcers, infections, and osteomyelitis resulting in acral mutilations (acrodystrophic neuropathy). These complications are preventable by careful avoidance of trauma to the insensitive extremities.
| Disease | Inheritance | Locus | Gene | Clinical Features |
|---|---|---|---|---|
| HSAN I (HSN I) | AD | 9q22 | SPTLC1∗ | Small > large MF sensory loss, distal weakness, onset in second to fourth decade |
| HSAN II | AR | WNK1/HSN2 | Pansensory loss in infancy | |
| HSAN III (FD) | AR | 9q31 | IKBKAP ∗ | Sensory loss, autonomic dysregulation, absent tears, fungiform tongue papillae |
| HSAN IV | AR | 1q21 | NTRK1/NGF receptor | Insensitivity to pain, anhidrosis at birth, mental retardation, nl SNAPs |
| HSAN V | AR | NGFB NTRK1/NGF | Insensitivity to pain at birth, nl SNAPs, no mental disability, absent small MF |
HSAN type I is an AD disorder and the most common hereditary sensory neuropathy ( ). Symptoms begin in the second to fourth decade with sensory loss and subsequent tissue injury mainly affecting the feet and legs. Sensory loss initially affects pain and temperature perception more than touch-pressure sensation, but it involves all modalities as the disease progresses. Autonomic involvements are limited to hypohidrosis. Patients present with calluses on the soles, painless stress fractures of the feet, neuropathic foot and ankle joints, and recurrent plantar ulcers. If ulcers are neglected and become infected, severe acromutilation may result (acrodystrophic neuropathy). Lancinating or shooting pains are often prominent and are considered the hallmark feature of HSAN-I. Distal muscle weakness and wasting are present in advanced cases. Such motor involvement, albeit without the lancinating pain, may create diagnostic confusion with CMT2B with dense sensory loss (linked to chromosome 3q13) or with certain axonal MPZ gene mutations. Variable neural hearing loss or rarely spastic paraparesis may be seen in HSAN-I. Some families present with burning feet or neurogenic arthropathy, suggesting clinical as well as genetic heterogeneity both within and between families with HSAN-I ( ; ).
SNAP amplitudes are reduced late in the disease. Motor conduction velocities remain normal, but CMAP amplitudes are reduced in advanced cases. Sural nerve biopsy confirms a severe loss of small myelinated axons and, to a lesser degree, of unmyelinated and large myelinated fibers ( eFig. 106.13 ). Pathological evidence of regenerative activity is usually minimal. HSAN-I has been mapped to chromosome 9q22.1-22.3. Mutations in the SPTLC1 gene encoding a subunit of serine palmitoyltransferase are identified in 90% of patients ( ). These mutations result in increased de novo ceramide synthesis. Since ceramide plays a role in the regulation of programmed cell death, the neuronal degeneration in HSAN-I may be caused by ceramide-induced apoptosis. Molecular genetic testing for HSAN-I is clinically available. Families without linkage to chromosome 9q22 have been described, suggesting genetic heterogeneity ( ). A subtype, HSAN-IB, is associated with paroxysmal cough, cough syncope, and gastroesophageal reflux ( ). Additional features include hoarseness of voice and hearing deficit; motor involvement, acral mutilations and ulceration are usually absent. The association of cough and gastroesophageal reflux is not unique to HSAN-IB, as it has also been associated with CMT2 with MPZ mutation.
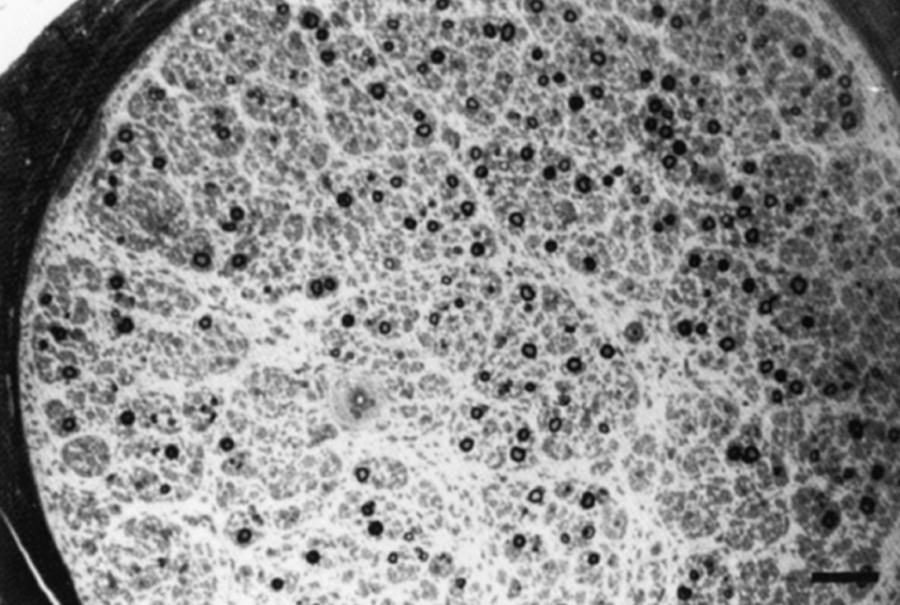
HSAN-II is recessively inherited and rarely begins later than infancy. All sensory modalities of distal upper and lower limbs and, to a lesser extent, of trunk and face are affected. The hands, feet, lips, and tongue are at risk for mutilation because of generalized sensory loss and insensitivity to pain. Autonomic symptoms are minimal, and mental development is normal. There is loss of tendon reflexes. Rarely, associations with spastic paraplegia, retinitis pigmentosa, mild motor weakness, or neurotrophic keratitis have been described. The clinical course is slowly progressive, with progressive axonal loss. SNAPs are absent. Sural nerve biopsy specimens show almost complete absence of myelinated fibers and reduced unmyelinated fiber populations ( eFig. 106.14 ). Mutations in the HSN2 nervous-system-specific exon of the with-no-lysine(K)-1 (WNK1) gene on chromosome 12q13.33 cause HSAN-II ( ). All mutations result in a truncation of the HSN2 protein, with the protein loss or inactivation (or both) causing the peripheral neuropathy. The exact function of HSN2 protein remains unknown, but it may play a role in the development or maintenance of sensory neurons or accompanying Schwann cells.
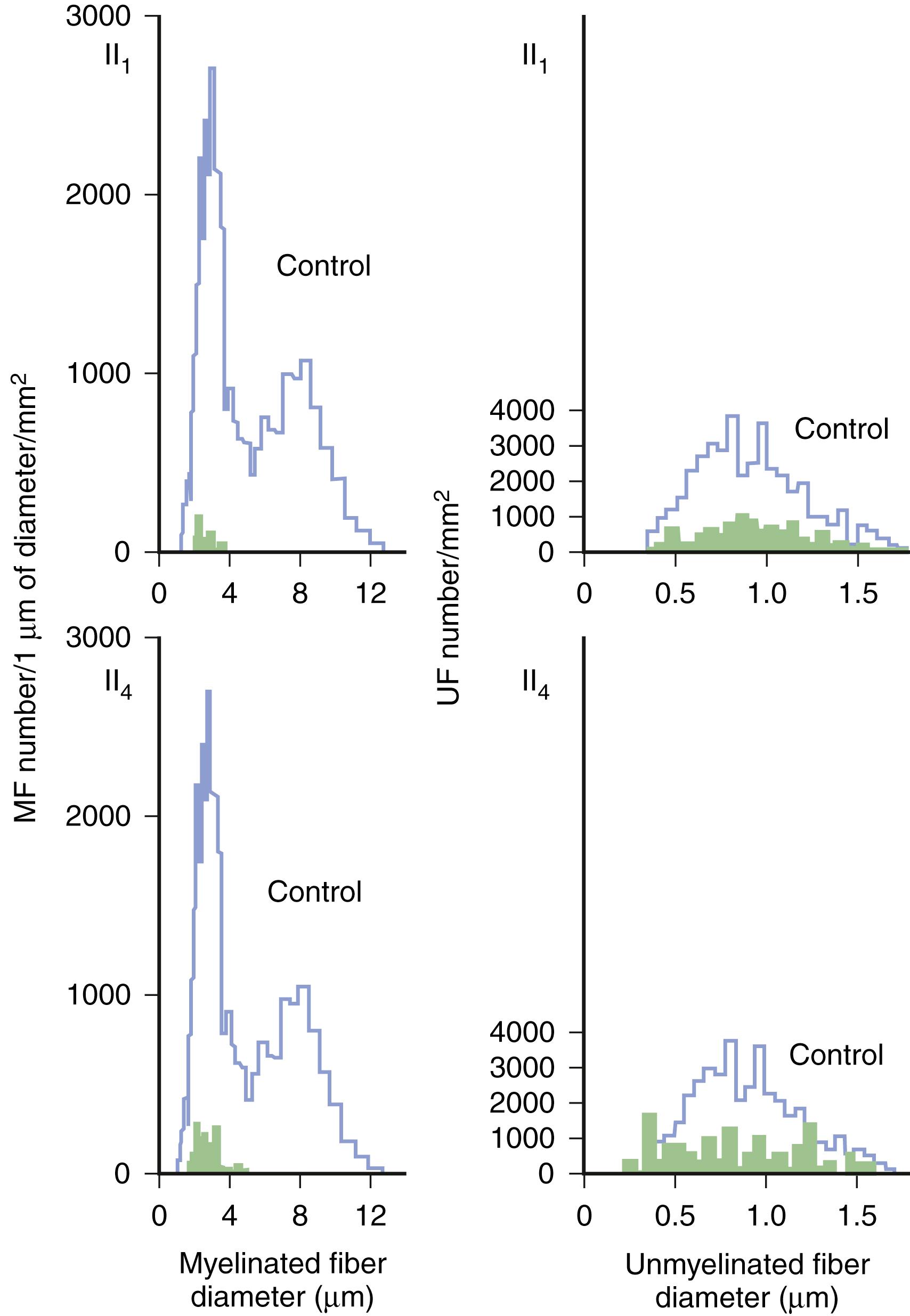
HSAN-III, or familial dysautonomia (FD) or Riley-Day syndrome , is an AR inherited sensory neuropathy with prominent autonomic manifestations particularly affecting children of Ashkenazi Jewish ethnicity. Symptoms begin at birth and include poor sucking, uncoordinated swallowing due to esophageal dysmotility, episodes of vomiting, recurrent pulmonary infections largely due to oropharyngeal incoordination, attacks of fever, and cardiovascular instability. Emotional stimuli provoke episodic hypertension, profuse sweating, and marked skin blotching caused by defective autonomic control. Hypotonia in infancy contributes to delayed motor milestones. Later in childhood, hyporeflexia, insensitivity to pain, gait ataxia, stunted growth, and scoliosis become apparent. Defective lacrimation (absence of overflow tears with crying), absence of fungiform papillae of the tongue giving it a smooth appearance, and pupillary hypersensitivity to parasympathomimetic agents are telltale signs. Patients with FD are susceptible to periodic episodes of paroxysmal hypertension, tachycardia, excessive sweating, and vomiting, often termed dysautonomic crises . These episodes are caused by uncontrolled catecholamine releases and occur in 40% of patients, usually in response to stress, either emotional or physical. They are also at risk to develop profound hypoxemia and tachypnea following anesthesia or with high-altitude travel as a result of diminished respiratory response to hypercapnia and hypoxia. In older patients, clinical manifestations of orthostatic hypotension may become apparent but because of adaptation of cerebrovascular autoregulation rarely lead to syncope. As affected children grow older, sexual maturation is delayed, but normal pregnancies have occurred and male patients have fathered children.
The number of neurons in the sympathetic, parasympathetic, and spinal ganglia is reduced. Peripheral blood vessels also demonstrate lack of autonomic nerve terminals. A marked reduction in the density of unmyelinated axons and small myelinated fibers is seen in sural nerve biopsy specimens, even in the youngest patients. Recent studies have confirmed that FD is a disorder of afferent nerve function, with relative sparing of the efferent motor neurons. This is confirmed by selective failure of baroreceptor afferent reflex and impaired brainstem reflexes involving the trigeminal nerves ( ; ). On tilt-table study, there is a significant drop in blood pressure and heart rate. Plasma norepinephrine and vasopressin levels do not increase with head-up tilt ( ). Motor NCVs are generally normal, whereas SNAP amplitudes are frequently reduced. Linkage studies have mapped the gene locus to chromosome 9q31-q33. The disease is caused by a point mutation in the IKBKAP gene that affects the splicing of the elongator-1 protein (ELP-1, also known as IKAP), an essential protein of the human elongation complex. The major FD mutation is a splice mutation that results in aberrant tissue-specific messenger (m) RNA splicing ( ). The elongator complex is thought to be involved in the regulation of cell-surface transport of exocytosis, and its impairment results in the dysregulation of neural endocytosis.
FD is a potentially life-threatening disorder with a high mortality rate due to aspiration pneumonia or autonomic crises. Improved supportive treatment has extended the survival of patients into adulthood. There is a greater than 50% probability for infants with FD to reach 30 years of age ( ).
HSAN-IV is a rare AR disorder characterized by congenital insensitivity to pain and thermal sensation as well as anhidrosis. This leads to repeated episodes of fever, thick and calloused skin, dystrophic nails, self-mutilating behavior, and mild mental retardation associated with emotional lability and hyperactivity. Tendon reflexes, muscle strength, and SNAPs are preserved, but sympathetic skin responses are absent. Biopsy of sensory nerves shows selective total or near-total loss of unmyelinated axons and small myelinated fibers. Confirmation of a neuropathic abnormality in cases of congenital indifference to pain without apparent neurological signs therefore depends on the morphometric study of unmyelinated and myelinated fiber populations in nerve biopsy specimens, and is supported by quantitative sensory testing and lack of sweating by the QSART. Intradermal histamine injection produces a wheal but no flare response. Skin biopsy has demonstrated a lack of intradermal nerve fibers and sweat glands devoid of nerve fibers ( ). The gene locus for HSAN-IV maps to chromosome 1q21-22. Mutations in the NTRK1 (formerly trkA) gene encoding the tyrosine kinase receptor for nerve growth factor (NGF) have been described in patients with HSAN-IV ( ). These findings indicate that the NGF-trkA system plays a crucial role in the development of unmyelinated nociceptive and sudomotor fibers.
Clinically similar cases with selective loss of only small myelinated fibers have been designated HSAN type V. These patients do not have mental retardation. Mutations in the NTRK1 gene have been found in some cases, suggesting that the two disorders may be allelic ( ). A related disorder with loss of deep pain perception but normal sweating has been linked to chromosome 1p13.2-p11.2, encoding the NGFB gene ( ).
The prevention of stress fractures and plantar ulcers is of utmost importance in most patients with HSAN. This can be achieved by meticulous foot care, avoiding barefoot walking, daily inspection of feet and shoes, and proper skin care with moisturizing lotions. Whenever plantar ulcers develop, weight bearing should be discontinued until the ulcers heal. Infusion of pamidronate, a bisphosphonate, has been suggested to be helpful in the management of Charcot neurogenic arthropathy.
Treatments for patients with AF are also limited, and mainly preventive and symptomatic. They include managing dysphagia, protecting airway passages, prompt treatment of aspiration pneumonia, and using nighttime noninvasive ventilation for apneas and hypercapnia.
At present, no specific treatment effectively delays the progression of any of the HSANs. Clinical trials of compounds that slow the progression of FD by increasing levels of ELP-1 (IKAP) are in progress.
Friedreich ataxia is an AR neurodegenerative disease characterized by degeneration of large sensory neurons and spinocerebellar tracts, cardiomyopathy, and increased incidence of diabetes (see Chapter 96 ). Even in the early stages of the disease, examination reveals lower-limb areflexia and impaired joint position and vibration sense, with preserved pain and temperature sensation. Pes cavus and hammer toes occur in approximately 90% of cases.
SNAPs are invariably reduced in amplitudes or absent. However, motor nerve conduction studies are normal or slightly reduced. A selective loss of large myelinated fibers occurs in the sural nerve. Friedreich ataxia is the result of a large GAA triplet repeat expansion on chromosome 9q13-q21.1, leading to loss of frataxin expression (see Chapter 96 ).
Peripheral nerve involvement in spinocerebellar ataxias (SCAs) is inconsistent. This is a mainly axonal sensorimotor peripheral polyneuropathy or pure sensory polyneuropathy. The prevalence of peripheral neuropathy, based on nerve conduction studies, in SCA1, SCA2, and SCA3 ranges from 87% to 96% ( ). SCA4, SCA18, and SCA25 have also an associated peripheral polyneuropathy.
Inherited or primary erythromelalgia is a rare AD neuropathic condition with an incidence of 0.36–1.1 per 100,000 persons. The onset of symptoms is often in the first decade of life. It is characterized by recurrent attacks of erythema and intense burning pain of the hands and feet in response to mild warmth or moderate exercise. To get relief, patients often attempt to cool the affected areas. The most frequently affected areas are the feet, involved in 90%–100% of patients, followed by the hands in 25%–60%. The head and neck are rarely involved, reported in 2%–15% of patients. These symptoms may lead to significant disability. Patients may function normally between episodes.
Sensory and motor nerve conduction studies are normal in all patients, confirming the absence of large-fiber polyneuropathy. QSART and thermoregulatory sweat testing showed evidence of a small-fiber neuropathy in about half of children ( ). Mutations in the voltage-gated sodium channel Na(v)1.7, encoded by the gene SCN9A, are responsible for this syndrome. Na(v)1.7 is preferentially expressed in small dorsal-root ganglia neurons. Gain-of-function mutations in Nav1.7 that enhance activation and impair fast inactivation cause the sensation of pain ( ; ; ; ). Other mutations in SCN9A that cause a loss of Na(v)1.7 function result in the opposing condition of “congenital inability to experience pain,” emphasizing the importance of sodium channels in nociception ( ). A third disorder has also been linked to Na(v)1.7: paroxysmal extreme pain disorder, which was formerly known as familial rectal pain syndrome ( ). The linkage between pain and temperature in erythromelalgia is in keeping with other diseases of sodium channels.
No treatment is consistently effective and has to be individualized. Sodium channel blockers such as lidocaine (gel, patch, or infusion), carbamazepine, and mexiletine are frequently used ( ). Aspirin, tricyclic antidepressants (TCAs), and vasoactive drugs have been used with varying success.
FAP is a group of AD disorders characterized by the extracellular deposition of amyloid proteins in peripheral nerves and other organs. Amyloid is a fibrillar conformation of a protein characterized by (1) green birefringence of Congo red-stained sections viewed in polarized light, (2) the presence of nonbranched 10-nm amyloid fibrils on electron microscopy, and (3) a β-pleated sheet structure on x-ray diffraction. More than 20 different proteins are known to undergo conformational changes leading to the generation of amyloid deposits in tissues. These deposits may be widespread or restricted to certain organs (localized amyloidosis). The clinical presentation depends on the organs involved and the size of amyloid fibrils. In FAP, one of three aberrant proteins (transthyretin, apolipoprotein A1, or gelsolin [ ; ]) may be found in the peripheral nerves. The latter two are rare and restricted to a few families. In acquired primary systemic amyloidosis, polypeptides of immunoglobulin light-chain origin are deposited in tissues as amyloid, which leads to the term amyloid light (AL) amyloid (see Primary Systemic Amyloidosis, later).
The classification of FAP was traditionally based on clinical presentation. However, progress in understanding the protein composition and molecular genetics of these disorders justifies a different approach ( eTable 106.9 ).
| Type | Aberrant Protein | Decade of Onset | Neuropathy | Associated Organ Involvement |
|---|---|---|---|---|
| FAP I and II | TTR | Early onset: Third through fourth decade Late onset: Sixth through eighth decade |
Length-dependent sensorimotor neuropathy, life-threatening autonomic neuropathy, carpal tunnel syndrome | Heart, eye |
| FAP III | Apolipoprotein A1 | Third through fourth decade | Sensorimotor neuropathy (not prominent) | Kidney, liver, gastrointestinal tract |
| FAP IV | Gelsolin | Third to fourth decade | Cranial neuropathy | Cornea (lattice dystrophy) and skin (cutis laxa) |
The majority of patients with FAP have mutations of the plasma protein TTR. This is a bifunctional transport protein for thyroxin and retinol-binding protein. It is predominantly synthesized in the liver and choroid plexus and consists of a single polypeptide chain of 127 amino acid residues. The gene for TTR is located on chromosome 18q11.2-q12.1. Most patients are heterozygous for TTR gene mutations (more than 90 described) that result in transcriptions of aberrant proteins with predisposition toward amyloid formation and deposition in peripheral nerve, heart, kidney, eye, and (rarely) leptomeninges.
TTR-FAP is rare, with endemic populations predominantly in Portugal, Sweden, Japan, and Brazil. TTR amyloidosis demonstrates two disparate clinical phenotypes. The original cases described by Andrade in Portugal are referred to as FAP type I . Two other large foci of patients are found in Sweden and Japan. This is the most common form of FAP and has been observed in 30 different countries and in many ethnic groups ( ). The neuropathy begins insidiously in the third and fourth decades with dissociated sensory impairment (loss of pain and thermal sense) in the lower extremities, often associated with lancinating pain and paresthesias. Because of its similarity to other neuropathies, it may be difficult to diagnose this condition ( ; ). Autonomic dysfunction commonly includes impotence, postural hypotension, bladder dysfunction, distal anhidrosis, and abnormal pupils with scalloped margins. Gastrointestinal symptoms characterized by constipation alternating with diarrhea, delayed gastric emptying, and weight loss may be prominent. Eventually panmodality sensory loss, distal wasting, weakness, and areflexia develop. Systemically, amyloid is deposited in the ocular vitreous, heart, and kidneys. Cardiac manifestations include cardiac hypertrophy, arrhythmias, ventricular blocks, or cardiomyopathy. The pattern of myocardial involvement varies according to specific TTR mutations. The disorder is relentlessly progressive. Untreated patients usually die, often of cardiac disease and less often from renal failure or malnutrition 10–15 years after the onset ( ).
EDX studies reveal a distal axonal neuropathy that affects sensory fibers earlier and more prominently than motor fibers. Early changes include low-amplitude or absent SNAPs, mild reduction in CMAP amplitudes, and preserved motor conduction velocities. Evidence of denervation is found in distal leg muscles. Until specific biochemical and genetic studies became available, the diagnosis was confirmed by the demonstration of amyloid in tissue biopsy specimens. In early cases, sural nerve biopsy specimens show a predominant loss of unmyelinated and small myelinated fibers. Amyloid deposits of variable size are usually seen within the endoneurium or around vasa nervorum. Immunostaining with antibodies to TTR can identify the specific type of amyloid. Many but not all mutations have evidence of myocardial infiltration on echocardiography. The mechanisms of nerve fiber injury and their relationship to amyloid deposits are incompletely understood. It has been proposed that the preferential deposition of amyloid in sensory and autonomic ganglia interferes with or is toxic to neuronal function, leading to a length-dependent axonal degeneration. An alternative theory suggests that endoneurial edema associated with amyloid deposition in blood vessels and the endoneurium results in ischemic nerve fiber injury. However, this does not explain the selective involvement of smaller nerve fibers.
A more restricted form of the disease, referred to as FAP type II , has a later onset than FAP I in the sixth to eighth decade, may present with CTS, and slowly progresses to peripheral polyneuropathy. Autonomic manifestations are also less prominent. Vitreous opacities are common and cardiac involvement may develop. Surgical decompression of the carpal tunnel provides symptomatic relief despite the fact the CTS is often more severe than in patients with idiopathic CTS. Demonstration of amyloid infiltration of the flexor retinaculum obtained at surgery or in other tissues establishes the diagnosis.
More than 90 different amino acid substitutions of the TTR protein have been identified as causing the clinical TTR-FAP phenotypes. Among these, substitution of valine by methionine at position 30 (Val30Met) is by far the most frequent; it is found in clusters in distinct areas of Portugal, Sweden, and Japan and accounts for 50% of mutations worldwide. Other TTR variants, including isoleucine 33, alanine 60, and tyrosine 77 substitutions, have similar features of generalized polyneuropathy with varying degrees of autonomic involvement. A serine substitution at position 84 and histidine at position 58 are the two TTR mutations seen most commonly in FAP-II. The age of onset varies greatly with specific TTR mutations. Even in families with the Val-30Met mutation, variation in age of onset is observed in different geographic regions ( ). Specific TTR mutations may produce unique phenotypes with predominantly cardiac or leptomeningeal amyloidosis ( ).
This is a rare form of familial amyloidosis, described first by Van Allen and colleagues in Iowa. The clinical manifestations of the type III variant have much in common with those of type I, except for a less prominent length-dependent sensorimotor polyneuropathy and for early renal involvement and a high incidence of duodenal ulcers. Uremia is the most common cause of death, typically occurring 12–15 years after the onset of neuropathy. Sixteen mutations of apolipoprotein A1 are known to cause FAP. Those involving codons 1–75 more commonly cause hepatic and renal amyloidosis, while those involving codons 173–178 cause cardiac, laryngeal, and cutaneous amyloidosis ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here