Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disorders of lipid metabolism are an increasingly common consequence of modern industrial lifestyles. With increasing prosperity, the spread of Western-style diets and sedentary habits is resulting in dramatic rises in obesity in countries around the world. Poor lifestyle habits coupled with genetic predisposition commonly result in dyslipidemia, which is characterized by higher levels of the atherogenic cholesterol- and triglyceride-rich apolipoprotein (apo) B-100 lipoproteins, higher levels of triglyceride-rich apo B-48 lipoproteins, and lower levels of apo A lipoproteins. In the office setting, these apolipoproteins are reflected in levels of low-density lipoprotein (LDL) cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and non-HDL-cholesterol.
The most common clinical manifestation of lipid disorders is atherosclerotic cardiovascular disease (ASCVD) that results from elevated levels of apo B-100 lipoproteins. In the United States, 44% of adults have elevated non-HDL cholesterol levels above 130 mg/dL. Apo A lipoproteins have a smaller role in the development of (or protection from) ASCVD. Severe hypertriglyceridemia is primarily associated with an increased risk of pancreatitis.
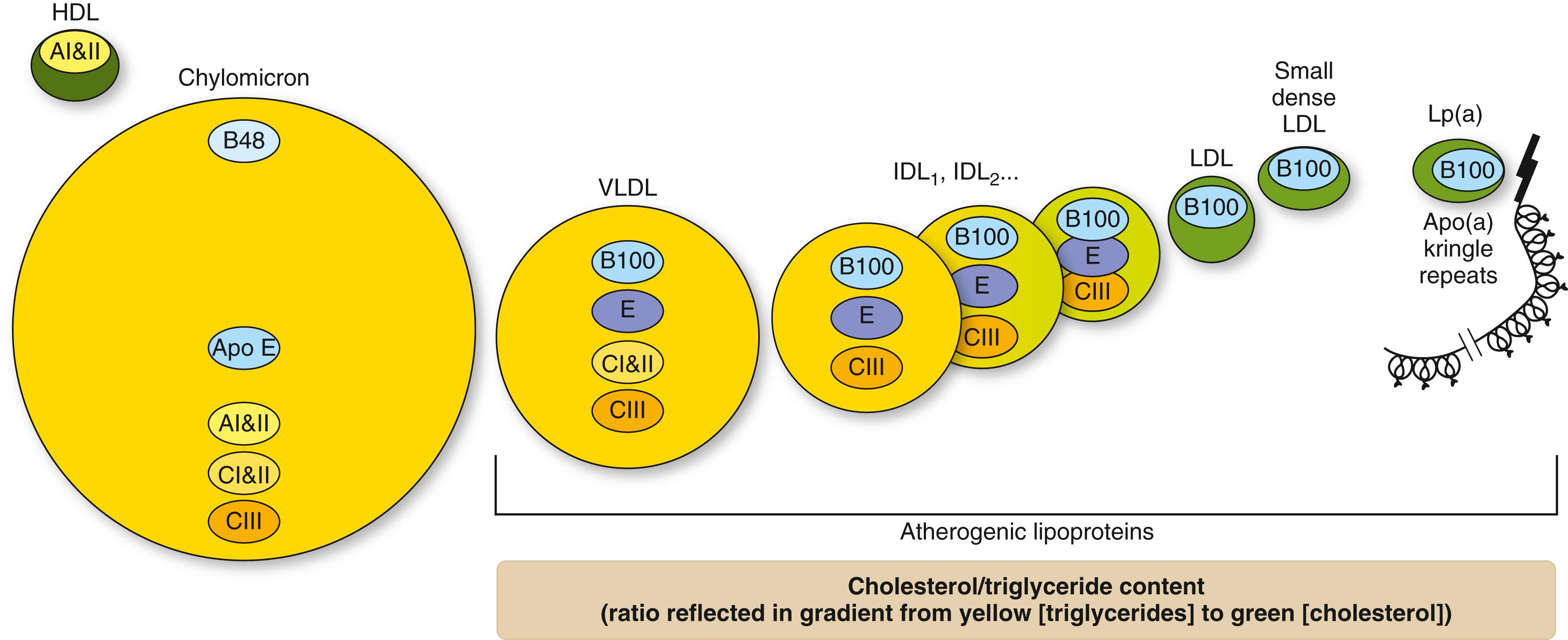
Cholesterol, which is an essential component of all animal cell membranes, functions as a precursor to fat-soluble vitamins and steroid hormones such as cortisol, estradiol, progestins, and testosterone. Plants utilize sterols rather than cholesterol as structural components of the cell membrane. Triglycerides are composed of three fatty acid chains attached to a glycerol molecule. Triglycerides are a source of energy, particularly in the fasting state. Both cholesterol and triglycerides are insoluble in water, so they require lipoprotein particles to transport them in plasma ( Fig. 190-1 ). Apolipoproteins, which are amphipathic molecules that are located on the surface of the lipoprotein particle and serve as biochemical keys to specific receptors or binding sites, allow delivery, entry, or modification of cholesterol ( E-Fig. 190-1 ).
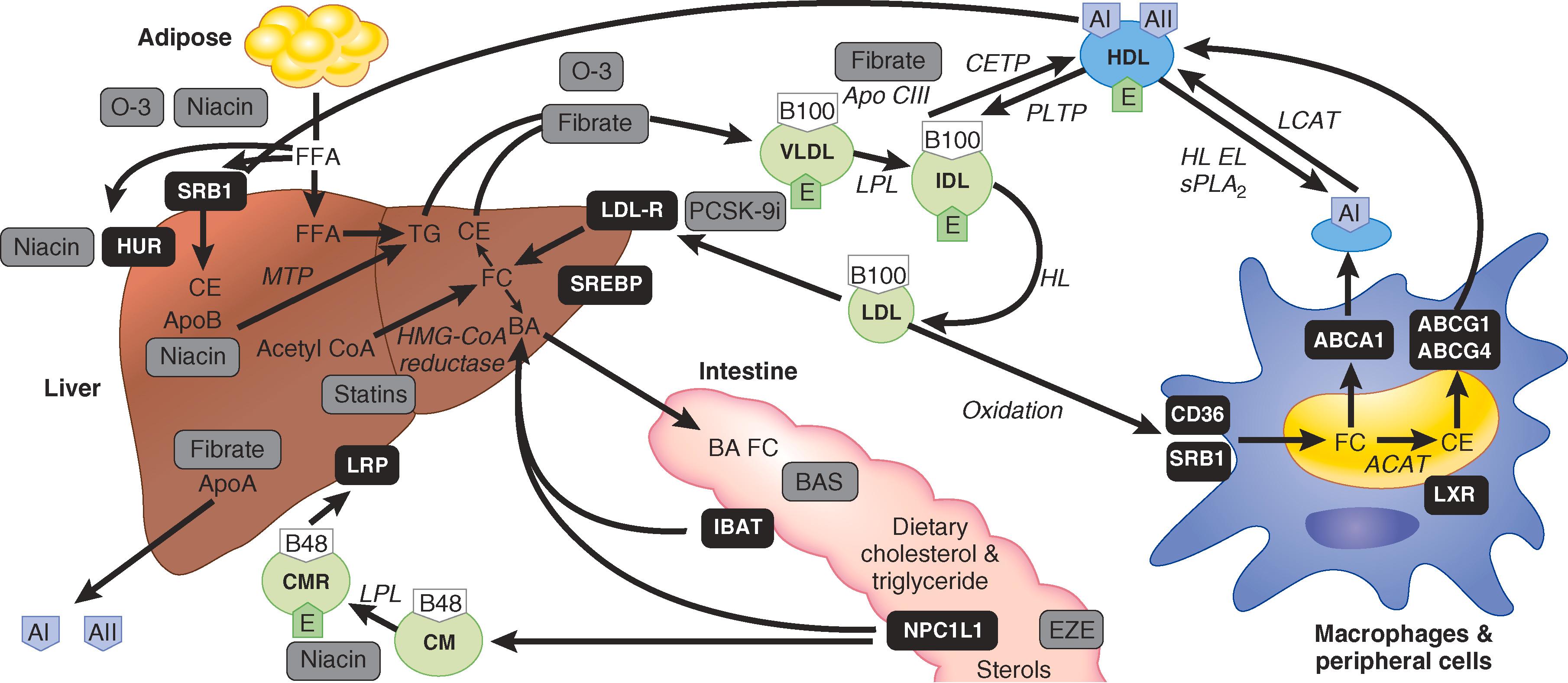
Cholesterol and triglycerides are either synthesized or absorbed from the intestine. Adults synthesize about 100 mg of cholesterol per day. The liver synthesizes about 25% of cholesterol, but the brain, reproductive organs, adrenal glands, and intestines synthesize it. Cholesterol is synthesized via multiple steps from acetyl CoA and acetoacetyl-CoA. The rate-limiting step in cholesterol synthesis is the reduction to mevalonate by 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, which is the target of statins. Downstream metabolites of mevalonate include several bioactive molecules, which have been implicated in some adverse effects of statins.
Diet provides 300 to 500 mg cholesterol per day. Bile acids provide about two thirds of daily cholesterol (800 to 1200 mg) and sloughed intestinal cells about 300 mg. Nonesterified cholesterol is secreted from the liver in bile acids, which are stored in the gallbladder and secreted into the small intestine, where they solubilize dietary fats and enhance fatty acid, lipid, and fat-soluble vitamin absorption. Bile acids are largely reabsorbed from the distal ileum and transported back to the liver via the enterohepatic circulation.
Dietary fats and animal products are broken down in the intestine, and the constituents are transported into enterocytes, where they are re-esterified into cholesteryl ester and triglycerides. Cholesterol is absorbed from the intestine via the Niemann-Pick C1-Like 1 (NPC1L1) receptor, which can be inhibited with ezetimibe.
In the enterocyte, triglycerides and cholesterol are packaged with apo B48 as well as apo CII and CIII and other apolipoproteins for secretion into the blood as triglyceride-rich chylomicrons (see Fig. 190-1 ). Chylomicrons undergo progressive hydrolysis of triglycerides into fatty acids by lipoprotein lipase in the capillary endothelium, which binds apo CII as well as other apolipoproteins. The fatty acids can be stored in adipose tissue or used as fuel by muscle tissue. Chylomicrons are converted into chylomicron remnants, which are relatively enriched in cholesteryl esters. Chylomicrons, which are the largest lipoproteins, are unlikely to contribute to atherosclerosis; they are not soluble in plasma, cause a “tomato soup” appearance to freshly drawn plasma, and rise to the top of the serum after overnight refrigeration to cause a “cream” layer. The smaller chylomicron remnants, which can enter the subendothelial space to be taken up by macrophages, may be the mechanism through which postprandial hypertriglyceridemia contributes to atherogenesis.
Chylomicrons and chylomicron remnants are also taken up by the liver by the LDL receptor–related protein, which interacts with apo E, and to a lesser extent by the LDL receptor and cell surface glycosaminoglycans. The liver breaks down the chylomicron particles, which can be stored as cholesteryl ester and triglycerides that are assembled into very-low-density lipoprotein (VLDL) particles for secretion into the blood, or the cholesteryl esters can be incorporated into bile acids for secretion into the intestine.
The liver assembles a VLDL particle from one apolipoprotein B100 molecule, cholesteryl esters, triglycerides, and a number of other apolipoproteins and lipids. Triglycerides are the rate-limiting step in the synthesis of VLDL. Microsomal transfer protein (MTP) transfers triglycerides to the growing apo B peptide. Drugs such as lomitapide that inhibit MTP can cause intrahepatic accumulation of triglyceride. Mipomersen, which is a drug that is no longer available in the United States, inhibits apo B synthesis and can also increase the intrahepatic accumulation of triglyceride, increase hepatic fat content, and cause hepatic steatosis.
Nascent VLDL is secreted into the blood, where it acquires apo E, apo CII, and apo CIII. Apo CIII and angiopoietin-like protein 3 activate lipoprotein lipase to hydrolyze the VLDL triglycerides into fatty acids for transport into tissues. As VLDL continues to deliver cholesterol and triglycerides to tissues, VLDL is transformed into increasingly cholesteryl-rich intermediate density lipoproteins (IDL) and ultimately into LDL (see Fig. 190-1 ). LDLs, which comprise the largest fraction of circulating lipoproteins, contain about 60 to 70% of total cholesterol in the circulation.
LDL is removed from the blood predominantly by the LDL receptor. The expression of the LDL receptor is regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9). In response to decreased intracellular cholesterol levels, sterol regulator element-binding proteins (SREBP) 1 and 2 upregulate the hepatic synthesis of both LDL receptors and PCSK9. LDL receptors travel to the cell surface, where they bind LDL particles from the blood. The LDL particle–LDL receptor complex is then taken up into vesicles, where the LDL particle undergoes degradation while the LDL receptor remains intact. The LDL receptor is then recirculated intact back to the cell surface to continue to remove LDL particles from the blood.
At the same time that the LDL receptor is traveling to the cell surface, the PCSK9 molecule is secreted into the blood, where it can bind the LDL-LDL receptor complex, tagging both the LDL receptor and the LDL particle for degradation. Inhibition or decreased levels of PCSK9 thus permit more LDL receptor to circulate and enhance removal of the LDL particles from the circulation.
The only hypertriglyceridemic polymorphisms that influence cardiovascular risk are those influencing VLDL levels. The atherogenicity of large VLDL particles is unclear, but smaller VLDL particles, IDL, and LDL are clearly atherogenic.
The liver assembles lipoprotein(a) (Lp[a]) from an apo(a) moiety connected to the apo B of an LDL-like particle (see Fig. 190-1 ). Apo(a) isoforms are quite variable owing to the number of kringle repeats as determined by the apo(a) gene. The size of apo(a), which is inversely related to its Lp(a) concentration, is thought to result from the longer assembly time of an apo(a) isoform with a large number of kringle repeats. The liver removes Lp(a) from the circulation. This process and the function of Lp(a) are not well understood. Polymorphisms causing very elevated Lp(a) levels are associated with increased cardiovascular risk, and Lp(a) levels correlate with the risk of developing ASCVD independent of LDL-cholesterol levels. Although Lp(a) can be reduced pharmacologically (e.g., with a hepatocyte-derived antisense oligonucleotide), it is unknown whether such therapy will reduce cardiovascular events.
HDL is the smallest and densest of the circulating lipoproteins (see Fig. 190-1 ). HDL carries apos AI and AII rather than apo B-100 or apo B-48. HDL is synthesized in the liver from apos AI, AII, and phospholipids to form nascent HDL or pre-β HDL ( E-Fig. 190-2 ). As this particle circulates through the body and interacts with peripheral cells, it acquires cholesterol and additional phospholipid by binding to adenosine triphosphate-binding cassette protein (ABC1) through a process called “reverse cholesterol transport.”
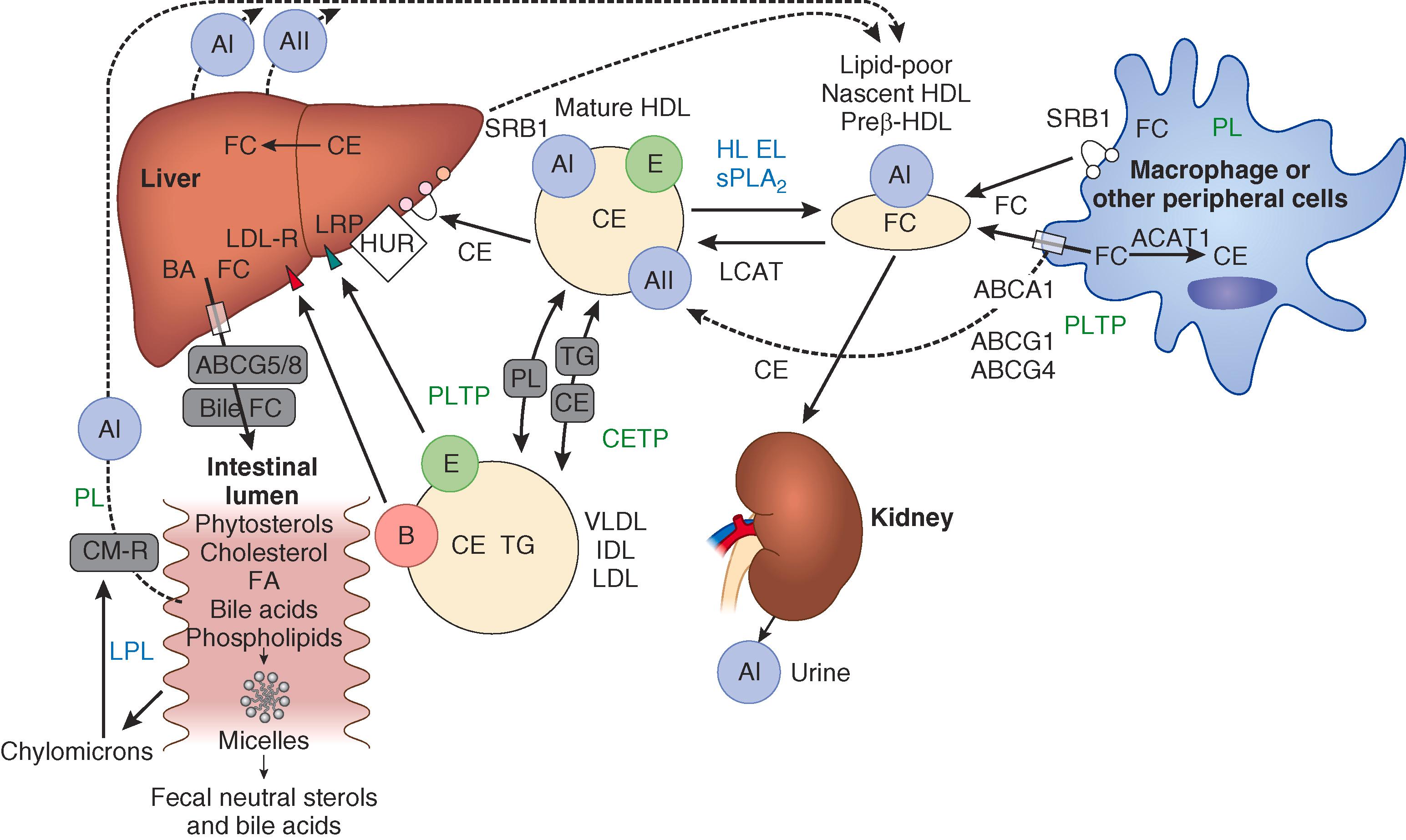
HDL carries about 30% of blood cholesterol. Inside the HDL particle, unesterified cholesterol is esterified by lecithin-cholesterol acyl transferase (LCAT). Esterified cholesterol can be removed from HDL via the scavenger receptor B1 (SR-B1) in the liver. In the blood, esterified cholesterol can be exchanged for triglycerides from apo B lipoproteins via the cholesteryl ester transfer protein (CETP) in the plasma. Phospholipid transfer protein transfers phospholipids from apo B lipoproteins to HDL. Lipoprotein lipase hydrolyzes triglycerides from VLDL and chylomicrons to HDL. Hepatic lipase removes triglycerides from HDL.
The functionality of HDL as measured by in vitro reverse cholesterol assays has been associated with reduced cardiovascular risk, independent of HDL-cholesterol levels. An uncommon apo AI mutation, apo AI-Milano, causes low HDL-cholesterol levels but is associated with reduced cardiovascular risk that is thought to be mediated by more efficient reverse cholesterol transport.
In epidemiologic studies, low levels of HDL, HDL-cholesterol, and apo AI are associated with increased cardiovascular risk. However, low HDL-cholesterol, which is often accompanied by elevated triglyceride levels, does not clearly appear to be a direct cause of cardiovascular disease but rather a marker of impaired glucose metabolism and insulin resistance. For example, mendelian randomization studies have failed to find an association between HDL-cholesterol–raising genes and cardiovascular risk. Several genetic polymorphisms that markedly increase HDL-cholesterol levels are associated with increased cardiovascular risk, HDL-cholesterol levels above 80 mg/dL are associated with an increased risk of coronary heart disease events, and apo AII is associated with increased cardiovascular risk through unknown mechanisms. In randomized trials, HDL-cholesterol–raising drugs have not reduced cardiovascular events.
The accumulation of cholesterol and triglyceride-rich apo B lipoproteins in the arterial subendothelium is the pathologic initiating factor in the development of an atherosclerotic plaque ( Chapter 41 and Fig. 190-2 ). Apolipoproteins with up to approximately 70-nm diameter (LDL, IDL, smaller VLDL, remnant chylomicron particles, and Lp[a]) more efficiently cross the endothelium. High plasma concentrations increase the probability that these particles will enter the subendothelial space of the artery. This size limitation is likely why very high plasma levels of the large apo B-48 chylomicrons in lipoprotein lipase deficiency do not cause atherosclerosis.
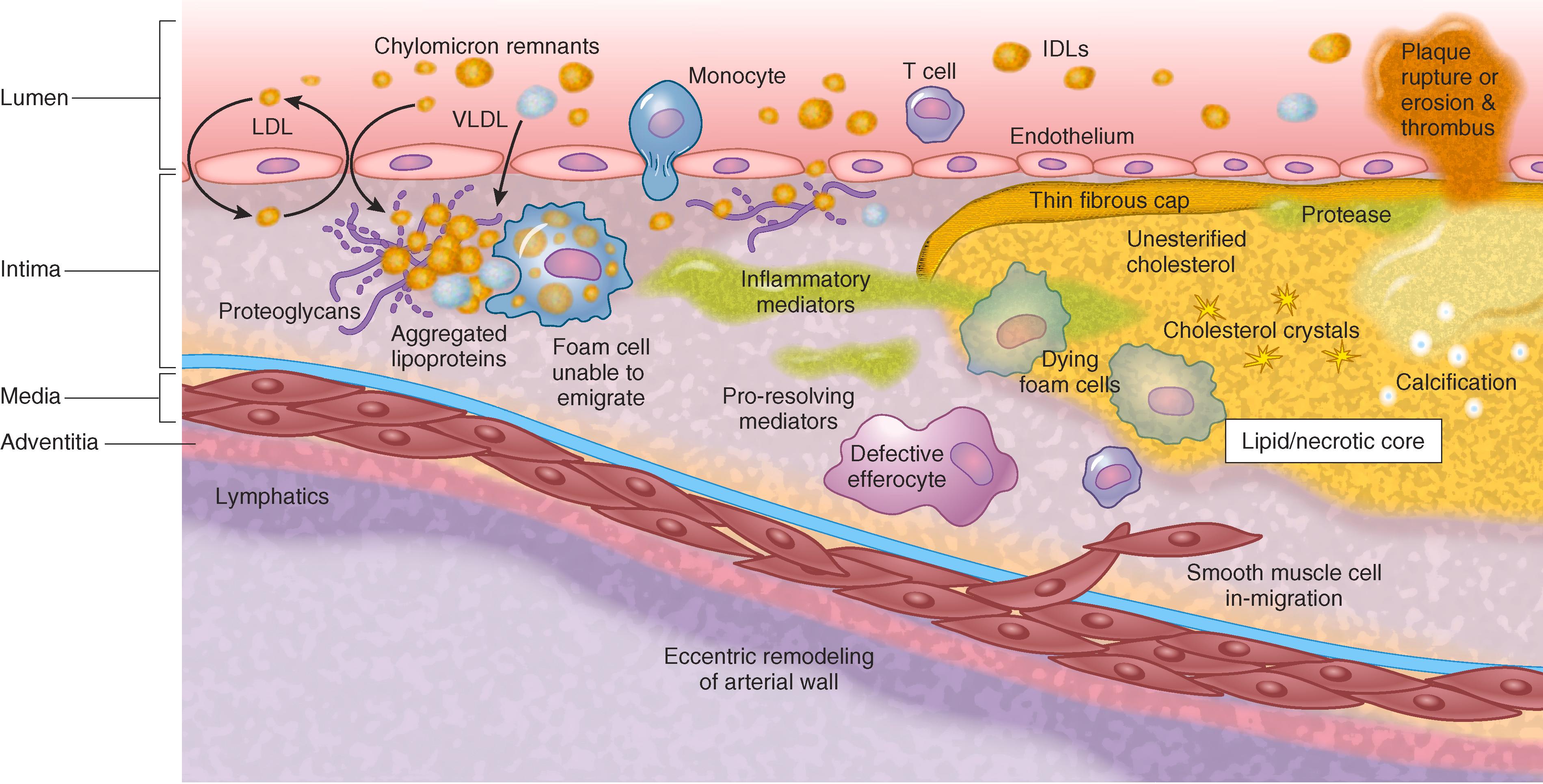
Normally, few apo B lipoproteins are retained in the subendothelium, and most return to the circulation. The key initiating factor of atherogenesis is the subendothelial retention of apo B lipoproteins, a process that is influenced by cardiovascular risk factors and genetic susceptibility. The presence of atherosclerotic plaque increases endothelial permeability to apo B lipoproteins, thereby accelerating their accumulation and the growth of plaque. Treatment of hyperlipidemia results in rapid decreases in endothelial permeability and increased fractional degradation of the LDL entering the plaque; as a result, plaque can stabilize and even regress.
In the earliest stages of atherogenesis, negatively charged proteoglycans in the extracellular matrix of the arterial intima trap the positively charged apo B-100 and apo B-48 moieties. Other characteristics of the LDL molecule that may affect atherogenicity by influencing apo B binding or proteoglycan interactions include the composition of surface core lipids. Apo E mediates binding to arterial glycosaminoglycans but also facilitates hepatic disposal of apo B lipoproteins. Conversely, apo CIII increases both the affinity of LDL for arterial wall proteoglycans and decreases hepatic uptake of apo B lipoproteins, thereby promoting retention of the cholesterol and triglyceride-rich apo B lipoproteins.
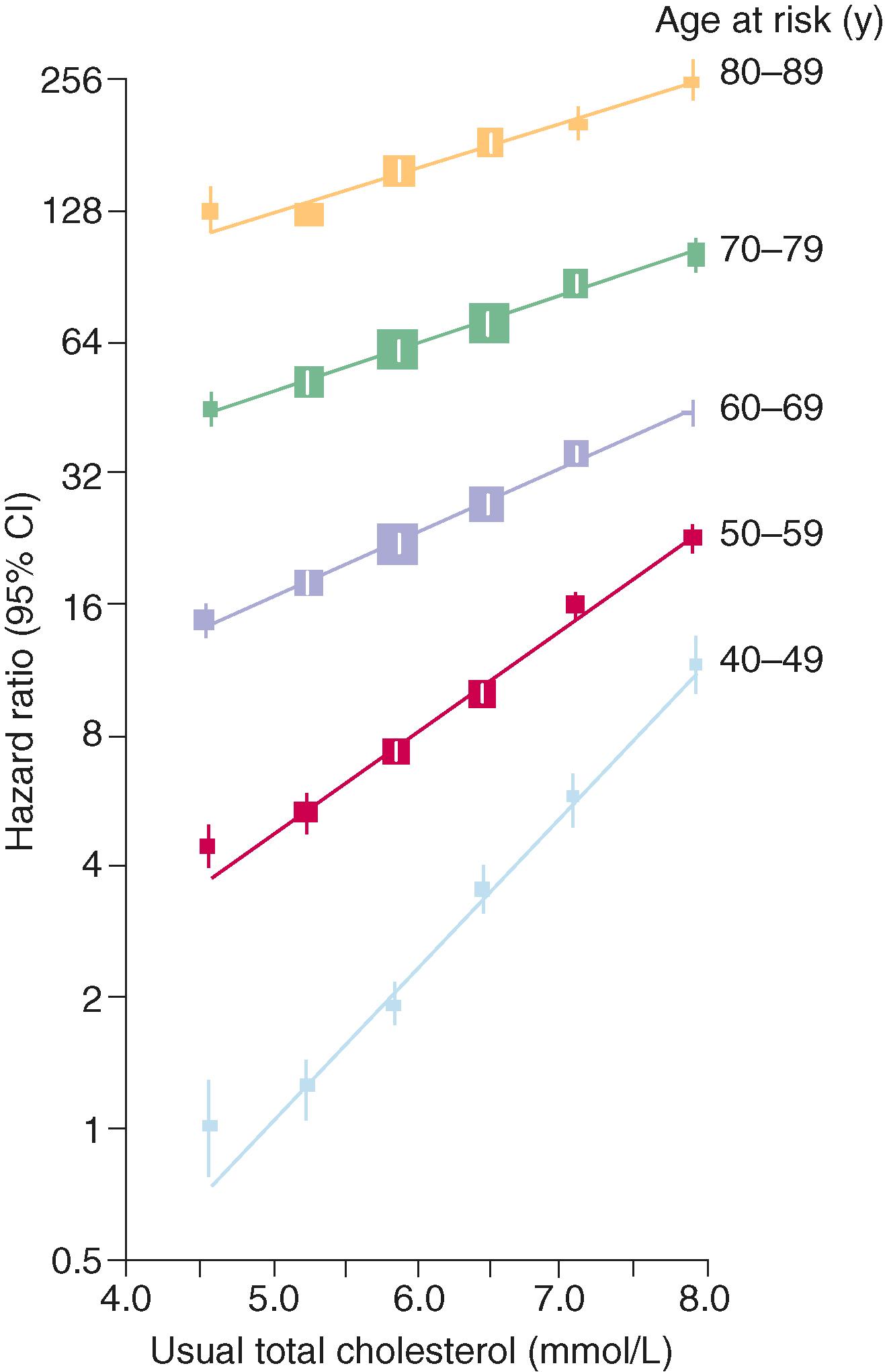
Atherogenesis begins in childhood and may progress to advanced plaque by late adolescence or early adulthood, a process that is accelerated in the presence of other cardiovascular risk factors and genetic predisposition. Apo B lipoproteins, as measured by total, LDL-cholesterol, and non-HDL-cholesterol levels, predict ASCVD risk in all ages, genders, races/ethnicities, and regions. Mendelian randomization studies have consistently found that genetic polymorphisms that increase LDL-cholesterol or non-HDL-cholesterol increase cardiovascular risk and that the converse is true for those that cause lower LDL-cholesterol or HDL-cholesterol levels. The increase in the relative risk of ASCVD per increment increase in total or LDL-cholesterol depends on age and the level and duration of exposure ( Fig. 190-3 ). Cumulative exposure to LDL-cholesterol levels greater than 100 mg/dL or non-HDL-cholesterol levels greater than 130 mg/dL since adolescence or early adulthood markedly increases the lifetime risk of ASCVD. Elevated cholesterol levels remain a risk factor into old age, although the relative contribution to risk is attenuated.
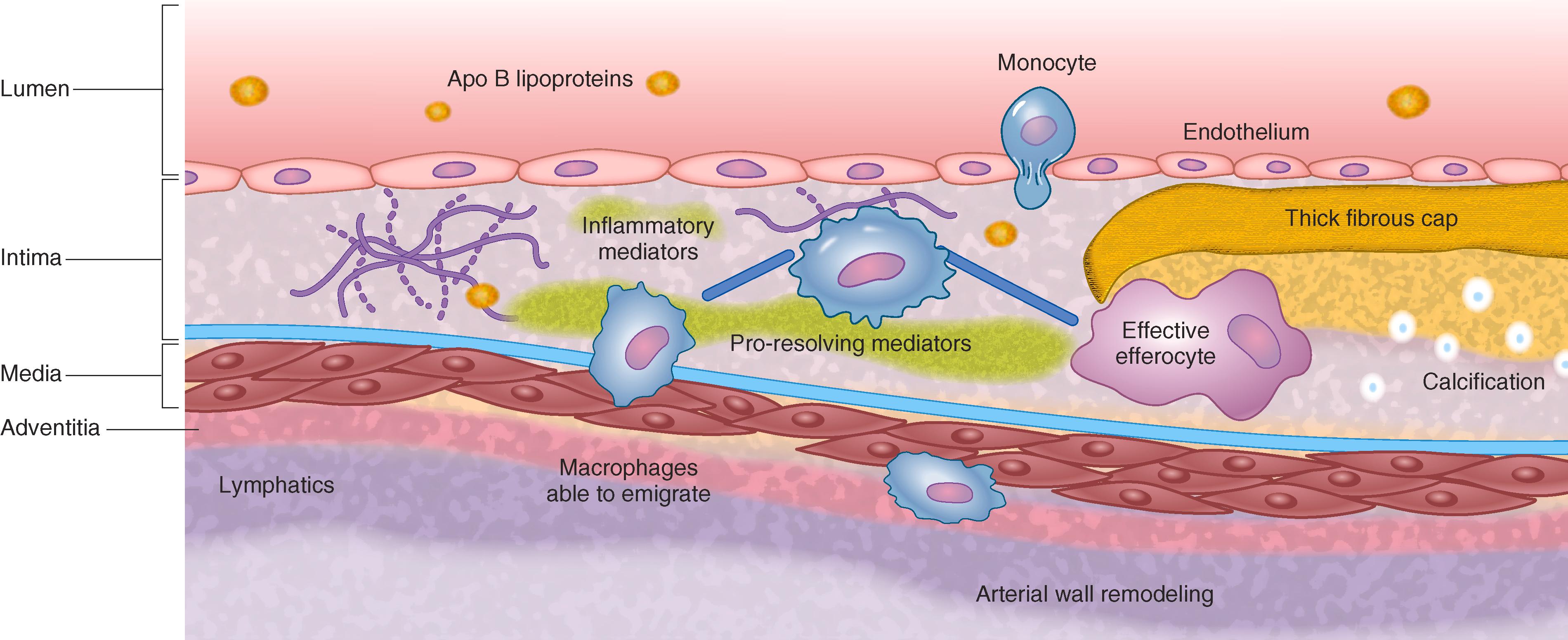
Reducing LDL and other apo B lipoprotein levels can regress atheroma. Markedly lowering apo B lipoprotein levels results in reduced subendothelial uptake and retention, as well as decreased recruitment of monocytes and macrophages ( E-Fig. 190-3 ). Reducing the toxic input of apo B lipoproteins allows the normal scavenger and phagocytic clearance mechanisms to clear the overload of apo B lipoproteins. Reverse cholesterol transport mechanisms mobilize cholesterol out of the foam cells, which decrease owing to emigration into adventitial lymphatics and transmigration into the lumen. More effective efferocytosis clears apoptotic cells and necrotic core. Inflammatory cells out-migrate, and smooth muscle cells migrate into the subendothelium; collagen synthesis falls, and fibrosis resolves. In animal models, very low LDL-cholesterol levels of less than 20 to 40 mg/dL completely regress early atheroma and normalize vascular function. In more advanced atheroma, substantial regression can occur, but residual stabilized plaque remains.
In the clinical laboratory, lipoprotein particles are usually not measured directly but rather by density ultracentrifugation, which separates them into bands based on their cholesterol and triglyceride content and reports levels of triglycerides, total cholesterol, LDL-cholesterol, and HDL-cholesterol. Patients who have high triglyceride levels have elevated numbers of chylomicrons, chylomicron remnants, or VLDL particles, or they have increased triglyceride content in VLDL. Triglycerides do not exist as independent entities in the blood, so blood triglyceride levels reflect the triglyceride content of the lipoprotein particles. High blood cholesterol levels reflect either increased numbers of LDL, VLDL, IDL, or Lp(a) particles circulating in the blood or an increased cholesteryl content in these particles.
On fasting or nonfasting samples (≥8 hours), LDL-cholesterol is usually calculated by the Friedewald equation when the triglyceride level is lower than 400 mg/dL.
Calculated LDL-cholesterol is not accurate in the presence of type III hyperlipidemia (see later) or when cholesteryl ester transfer protein inhibitors are used.
More recently developed methods facilitate more accurate indirect estimation of LDL-cholesterol when triglyceride levels are elevated as high as 800 mg/dL. LDL-cholesterol also can be measured directly. Direct LDL-cholesterol levels are 5 to 10 mg/dL lower than calculated LDL-cholesterol levels unless corrected for the Lp[a] level. HDL-cholesterol levels are measured directly using a number of methods, the results of which may vary to modest extent.
On nonfasting or fasting samples when triglycerides are greater than 500 mg/dL, non-HDL-cholesterol (non-HDL-C) can be calculated as:
Thus, non-HDL-cholesterol represents the atherogenic apo B lipid component (see Fig. 190-1 ). Non-HDL-cholesterol is generally about 30 mg/dL higher than LDL-cholesterol.
Measurement of Lp(a) has been problematic due to interindividual variation in the number of kringles in the apo(a) moiety. Several methods are available, but no standardized method has been used consistently. Large variations by race support the need to develop better reference ranges.
Advanced lipid testing can measure apo B and apo A directly, but the clinical utility of such testing is not clearly established. The vast majority of patients can be managed with calculated LDL-cholesterol or non-HDL-cholesterol levels, which is the least expensive and widely available method. Apo B measurement may be helpful in select patients with hypertriglyceridemia.
All patients with a newly identified LDL-cholesterol level of 160 mg/dL or higher or a triglyceride level of 500 mg/dL or higher should be evaluated for secondary causes of hyperlipidemia ( Table 190-1 ). The most common causes of secondary hypercholesterolemia include obesity ( Chapter 201 ), high intake of saturated or trans fats, and glucocorticoids. The most common causes of hypertriglyceridemia are poorly controlled diabetes ( Chapter 210 ), obesity ( Chapter 201 ), and high dietary intake of refined carbohydrates, alcohol, or fat. After secondary causes are addressed or stabilized, a repeat fasting lipid panel can be used to guide therapy.
| SECONDARY CAUSE | ELEVATED LDL-CHOLESTEROL OR NON-HDL-CHOLESTEROL | ELEVATED TRIGLYCERIDES |
|---|---|---|
| Diet | Saturated or trans fats , large weight gain , anorexia | Large weight gain , high fat intake , high refined carbohydrate intake , excessive alcohol intake , very-low-fat diets if high in refined carbohydrates |
| Drugs | Glucocorticoids , cyclosporine, anticonvulsants, oral contraceptives, anabolic steroids, diuretics, sirolimus, amiodarone | Glucocorticoids , oral estrogens, anabolic steroids, bile acid sequestrants, highly active retroviral therapy, retinoic acid (isotretinoin), sirolimus, tacrolimus, raloxifene, tamoxifen, β-blockers (not carvedilol), thiazides, cyclophosphamide, L-asparaginase, second-generation antipsychotics (clozapine and olanzapine) |
| Diseases | Biliary obstruction, nephrotic syndrome, monoclonal gammopathy | Proteinuria , nephrotic syndrome, chronic renal failure, glomerulonephritis, Cushing syndrome, HIV, lipodystrophies, monoclonal gammopathy, systemic lupus erythematosus, autoimmune chylomicronemia, chronic idiopathic urticaria |
| Disorders and altered states of metabolism | Obesity , hypothyroidism, pregnancy ∗ | Diabetes (poorly controlled), obesity , lipodystrophy, hypothyroidism, pregnancy, ∗ polycystic ovarian syndrome |
Newly identified LDL-cholesterol ≥160 mg/dL or non-HDL-cholesterol ≥190 mg/dL
Newly identified triglycerides ≥500 mg/dL
Worsening LDL-cholesterol, non-HDL-cholesterol, or triglyceride levels despite adherences to lifestyle and drug therapy
Fasting glucose or hemoglobin A 1C (HbA 1C )
Thyroid-stimulating hormone (TSH)
Alkaline phosphatase, bilirubin, and alanine aminotransferase (ALT)
Creatinine/glomerular filtration rate (GFR)
Urinary albumin
Total protein
Women of childbearing age—beta human chorionic gonadotropin (βhCG)
∗ Cholesterol and triglycerides rise progressively throughout pregnancy.
Blood levels of LDL-cholesterol, non-HDL-cholesterol, triglycerides, and HDL-cholesterol are influenced by genetics, lifestyle habits, medications, and medical conditions. Secondary causes of hyperlipidemia should be identified and treated. Severe primary elevations in LDL-cholesterol (≥190 mg/dL), non-HDL-cholesterol (≥220 mg/dL), or triglycerides (≥1000 mg/dL) almost always occur in the setting of a significant genetic disorder. However, treatment approaches are guided by LDL-cholesterol or non-HDL-cholesterol levels unless triglyceride levels are higher than 1000 mg/dL.
In the United States, individuals with LDL-cholesterol higher than 190 mg/dL have a five-fold higher lifetime risk of ASCVD, and individuals with familial hypercholesterolemia have a 20-fold higher lifetime risk. In the Netherlands, where nationwide efforts to identify patients with familial hypercholesterolemia have resulted in earlier treatment, patients with familial hypercholesterolemia who receive statins have an ASCVD risk similar to that of the nonfamilial hypercholesterolemia population. Therefore, all patients older than 20 years with a primary elevation in LDL-cholesterol higher than 190 mg/dL should receive high-intensity statin therapy unless a contraindication is present.
Familial hypercholesterolemia is a common genetic disorder, affecting about 1 in 250 persons in populations around the world. Familial hypercholesterolemia may be more common in some populations such as Ashkenazi Jews, some Lebanese groups, French Canadians, and other populations with frequent consanguineous marriages. Homozygous familial hypercholesterolemia or compound heterozygous familial hypercholesterolemia is present in about 1 in 500,000 adults.
Heterozygous familial hypercholesterolemia is characterized by LDL-cholesterol levels higher than 190 mg/dL and a family history of severe LDL-cholesterol elevations, or premature cardiovascular disease, and/or a genetic familial hypercholesterolemia mutation. About 60 to 75% of individuals with heterozygous familial hypercholesterolemia have a monogenetic autosomal dominant mutation, and the remainder have a polygenic disorder. The most common familial hypercholesterolemia mutations result in loss of function in the LDL receptor. Uncommon mutations are PCSK9 gain-of-function mutations, which result in more rapid degradation of the LDL receptor, and mutations that alter the apo B LDL receptor binding site.
Homozygous familial hypercholesterolemia manifests with ASCVD events in childhood. Heterozygous familial hypercholesterolemia is a common cause of premature atherosclerotic coronary heart disease in the 30s and 40s in men and 40s and 50s in women. The first sign or symptom can be sudden death in younger adults, thereby leaving no opportunity for prevention.
Because heterozygous familial hypercholesterolemia is a common and completely reversible genetic disorder if treated early, universal screening with a fasting or nonfasting lipid panel is recommended between the ages of 8 and 11 years. Screening should occur at an even earlier age if there is family history of an LDL-cholesterol level higher than 190 mg/dL or onset of ASCVD before age 55 in a male first-degree relative or age 65 in a first-degree female relative. All adults should be screened with a lipid panel by no later than age 20 years.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here