Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Iron metabolism and homeostasis 295
Hereditary iron overload 299
Miscellaneous iron overload 308
Acquired/secondary iron overload 310
The role of the liver biopsy in iron overload 318
Iron is by mass the most abundant trace element on Earth and is an essential micronutrient for most living organisms. Under an oxygen-free atmosphere, the chemistry of early life on our planet was founded on the capacity of water-soluble ferrous iron (Fe 2+ ) to exchange electrons with water-insoluble ferric iron (Fe 3+ ). In an oxygen-rich environment, however, the same chemistry limits its use and sets the basis for its toxicity. Iron enables fundamental reactions that are essential for life, including oxygen transport and delivery in haemoglobin and myoglobin, DNA synthesis, oxidative phosphorylation and host defences, but in the presence of oxygen, unchelated ‘free iron’ catalyses the generation of noxious reactive oxygen intermediates that damage macromolecules like proteins, lipids and nucleic acids. Therefore since humans have not developed mechanisms to actively rid the body of excess iron, blood and tissue iron levels must be kept within a very narrow range through fine-tuned regulatory systemic and local mechanisms. Moreover, a number of plasma and tissue proteins have evolved to absorb, transport and store highly reactive iron (see next section).
Based on these premises, it is no surprise that both iron deficiency and iron overload may represent serious threats to human health. A variety of abnormal states may arise from the disruption of iron homeostasis, ranging from iron deficiency anaemia to genetic iron overload, as typified by hereditary haemochromatosis (HC). Together, these reflect two of the more common disease states of humans. In recent years, there have been dramatic advances in understanding the handling of iron in the body and its homeostatic regulation. The liver, the main body iron store, is also the main site of iron toxicity when there is iron excess. However, the recent discovery that the liver is the main source of the iron hormone, hepcidin, has shed new light on its central role both in the regulation of body iron homeostasis and in the pathogenesis of numerous human diseases related to iron excess.
Total body iron content ranges from 3 to 5 g, mostly in haemoglobin or as storage iron in the liver and spleen, with smaller amounts in myoglobin and various enzymes. The daily requirement for iron in healthy adults is 20–25 mg, mainly for erythropoiesis, but only 1–2 mg of dietary iron is required daily in a healthy individual to compensate for minimal loss (1–2 mg daily), because most of the iron needed is recycled through the reticuloendothelial system by phagocytosis of effete (weak) red blood cells (RBCs).
Under physiological conditions, the only regulated step of iron metabolism is iron absorption by the small intestine. Dietary iron is released from bound proteins in the acidic environment of the stomach and taken up by the apical brush border of the small intestine, principally the duodenum. The ingested ferric iron must be reduced to its ferrous state through the action of a brush border ferrireductase, DcytB. It is then actively transported through the apical membrane of the duodenal enterocyte by the divalent metal transporter DMT1, which is also capable of transporting other divalent metals. Dietary haem, after its dissociation from globin, is transported across the brush border and released into the enterocyte cytoplasm by haem oxygenase or transported to the plasma as an intact porphyrin; the transporter responsible for haem uptake at the apical membrane has not yet been conclusively identified.
The absorbed iron, from either ionic or haem pathways, may be retained within the enterocyte as ferritin and eventually lost through desquamation, or transported actively through the basolateral membrane. The only iron-exporter protein currently identified in mammals is ferroportin , a multipass transmembrane iron channel also known as Ireg1 or MTP1.
This export of iron is facilitated by an accessory protein, hephaestin , a ferroxidase and caeruloplasmin homologue, thus returning the iron to the ferric state and promoting its binding to transferrin (Tf) and caeruloplasmin, also required for the export of iron from nonintestinal cells. The crucial relationship between ferroportin and the regulatory peptide hormone hepcidin is discussed later.
Tf is the major iron-binding glycoprotein in plasma. The Tf protein allows for high-affinity binding of two ferric iron atoms (Fe 3+ ) with Tf iron-binding sites and is normally about 30% saturated in the physiologic state. Given the potentially toxic nature of iron, Tf allows the iron to remain soluble and nonreactive in the aqueous environment and facilitates the cellular importation of iron from blood into cells. The latter occurs through the Tf cycle, whereby the two diferric Tf molecules bind with a high-affinity Tf receptor (TfR1) that is ubiquitously expressed on most cell surfaces. All nucleated cells have TfR1 on their cell surfaces, most importantly the developing RBCs in bone marrow, syncytiotrophoblasts and both Kupffer cells and hepatocytes, which receive first-pass blood from the portal circulation. The Tf–TfR1 complex is internalized, and after endocytosis the endosome is acidified by proton pumps, and the iron is released from Tf into the cytoplasm by a process that also requires DMT1. The unbound Tf, apotransferrin, and TfR1 recycle to the cell membrane to again participate in the transport pathway.
During iron overload states, non-Tf-bound forms of iron appear in the blood that are not tightly associated with Tf and whose labile fraction (‘labile plasma iron’) has a high propensity for oxygen-reduction (redox) activities. Labile plasma iron is rapidly taken up by hepatocytes through unregulated mechanisms, likely involving voltage-dependent calcium ion (Ca 2+ ) channels, the zinc ion (Zn 2+ ) transporter ZIP14 and others, and enters the cytosolic transit iron pool (‘labile cell iron’) where it can lead to uncontrolled production of reactive oxygen intermediates, particularly the highly reactive hydroxyl (OH − ) radicals through Fenton chemistry.
Once in the cytosol, iron is rapidly directed to the functional utilization sites, mainly in cell organelles, where it catalyses enzymatic and nonenzymatic reactions essential for life. Unneeded iron is safely stored within the core of a multimeric protein, ferritin or, much less efficiently, in haemosiderin. Ferritin is the most important mechanism by which cells store iron. This large and complex protein comprises 24 similar subunits that sequester up to 4500 iron atoms in a central core, thus isolating the metal from the cellular environment. Ferritin is highly conserved across all organisms, thus indicating a critical function in iron homeostasis. Human ferritin comprises H (active) and L (inactive) subunits, whose relative proportions vary according to cell-specific iron and oxygen homeostasis, tissue type, development and, in animal models, iron overload. The H subunit carries a ferroxidase site that allows rapid oxidation of Fe 2+ with the formation of diferroxo-mineral precursors at the expense of generating hydrogen. Although the ferritin system is very efficient in iron cycling and storage, degradation of ferritin leads to the accumulation of haemosiderin that is, by contrast, a potentially pathological nonhomogeneous conglomerate of iron, protein and membrane breakdown products, one that is mobilized poorly if at all.
Under normal physiological conditions, hepatocytes and macrophages store iron to an amount of 0.5–1 g. The hepatocyte is the major storage facility for iron, whereas tissue macrophages are primarily responsible for scavenging and phagocytosing effete erythrocytes.
After the RBC is degraded, iron is released from haem by haem oxygenase and may be stored as ferritin or efficiently mobilized and exported as required to the bone marrow for purposes of erythropoiesis. The export process is incompletely understood, but apparently, it again occurs through the action of ferroportin and hephaestin. This in turn is controlled by the peptide hormone hepcidin, as reviewed later.
As previously noted, iron is a critical nutrient but is also a potentially toxic agent, and no physiological pathway for iron excretion exists in humans. Thus there must be meticulous coordination of the uptake, storage and utilization of the metal, both at the cellular level and systemically.
To orchestrate intracellular iron traffic, cells contain proteins that have the ability to register the presence or absence of iron, identified in mammalian cells as two homologous iron regulatory proteins IRP1 and IRP2. These discern cytosolic iron levels and alter the expression of proteins involved in iron movements, such as TfR1 (but not TfR2), DMT1 and ferroportin, or iron storage, such as ferritin. IRP1 and IRP2 share extensive sequence homology, but significant differences exist between the two. In addition, many proteins involved in iron metabolism have motifs called iron-responsive elements (IREs), which are stem-loop structures in their 3′- or 5′-untranslated regions.
Sensing of iron levels is coupled to the availability of oxygen and other oxidants. When, for example, intracellular iron is scarce, IRP binds to the 5′-untranslated region of the ferritin mRNA transcript and ferritin synthesis is halted, to prevent iron storage, while IRP binding at the 3′-untranslated region of TfR1 stabilizes its mRNA, leading to higher TfR1 protein expression and increased iron uptake. It has been suggested that the molecular basis for the iron-sensing process in mammalian cells further relies on stabilization or degradation of an iron- and oxygen-binding protein that targets IRPs to the ubiquitin (Ub)-proteasome. ,
Systemic iron homeostasis is also tightly regulated to avoid iron starvation or excess, both responsible for disease states. The nature of the iron regulatory signals, mediators and targets is now being uncovered. Storage iron in hepatocytes and tissue macrophages must be mobilized in response to need, and the concept of store and erythroid ‘regulators’ was originally conceived by Finch. The store regulator would control duodenal iron uptake by means of a tightly regulated feedback mechanism to prevent iron overload. The erythroid regulator would enhance intestinal absorption in response to erythroid demand when there is an increased requirement for iron but the capacity of storage cells is insufficient. In addition, iron could be regulated at the level of the duodenum by the amount of iron recently consumed, thus invoking a dietary regulator, probably resulting from the buildup of intracellular iron. Even in systemic iron deficiency, a bolus of iron may cause a so-called mucosal block. Furthermore, iron homeostasis is modified in conditions of hypoxia by a humoral hypoxia regulator, and cellular iron may be retained and its absorption interrupted by infection, through the inflammatory regulator, presumably to withhold iron from the pathogen.
Such ‘regulators’ were largely theoretical until the discovery of hepcidin (hepatic bactericidal protein) or LEAP-1 (liver-expressed antimicrobial peptide 1), the iron-regulatory hormone. Hepcidin functions at the point of convergence of all humoral iron regulators, including the erythroid, the store and the inflammatory regulators. Originally identified as a type II acute-phase protein produced by the liver, hepcidin is a 25-amino acid peptide containing four disulphide bonds that forms a hairpin stabilized by these bonds. Hepcidin is synthesized as an 84-amino acid prepropeptide containing a typical N-terminal 24-amino acid, endoplasmic reticulum (ER)-targeting signal sequence and a consensus furin cleavage site immediately preceding the C-terminal 25-amino acid bioactive peptide. In addition to prohepcidin and hepcidin-25, carboxyl terminal 22- and 20-amino acid forms of hepcidin are found in the circulation and urine, but hepcidin-25 is the bioactive form. Hepcidin is a member of the cysteine-rich, cationic, antimicrobial peptide family and, in fact, has retained some antibacterial and antifungal effects in vitro . It seems that among all known antimicrobial peptides involved in innate immunity, however, hepcidin has evolved the very unique capability of fighting pathogens by restraining serum iron that is necessary for the growth and proliferation of pathogens during infection. This task is accomplished by binding to ferroportin. As a result of its interaction to circulating hepcidin, ferroportin is internalized and degraded, thereby diminishing the cells’ ability to transfer iron to the plasma compartment. , Therefore low serum levels of hepcidin leads to increased iron flux from enterocytes and macrophages into the circulatory iron pool, whereas high serum hepcidin leads to low circulatory iron due to inhibition of its intestinal absorption or release from macrophages.
Hepcidin has evolved as part of the innate immune defence. As such, hepcidin is induced by infection and inflammation and plays a central role in the anaemia and hypoferraemia of chronic disease, alternatively called ‘anaemia of inflammation’. Hepcidin responds to a variety of inflammatory signals and mediators, particularly interleukin-6 (IL-6), IL-1, IL-22 , and activin B. In this regard, hepcidin is in essence an acute-phase protein and senses, beyond inflammation, a number of intracellular and extracellular stress signals ( Fig. 4.1A ). ER stress is primarily associated with disruption of ER homeostasis and accumulation of unfolded or misfolded proteins in the ER. ER stress is also involved in a number of pathophysiological states, including inflammatory responses, nutrient disorders and viral infections. Exogenous and endogenous ER stressors can trigger hepcidin transcription through the cyclic adenosine monophosphate (cAMP) response element-binding protein 3–like 3 (CREB3L3, also known as CREBH) and leads to perturbation of iron homeostasis in vivo . Interestingly, CREBH appears to be at the centre of an intricate hub of liver metabolic pathways, including involvement with regulation of lipogenesis, lipolysis and fatty acid oxidation and gluconeogenic genes. To this end, PPARGC1A, a transcriptional co-activator, cooperates with CREBH to activate hepcidin and regulate iron traffic in vivo during food deprivation. The latter finding has important implications during human disorders associated with insulin resistance and induced gluconeogenesis, such as obesity, diabetes and nonalcoholic fatty liver disease (NAFLD). Hepcidin induction in the presence of stress factors that perturb the internal homeostasis—our legacy to the innate immune response to pathogens—aims at preserving and retaining in the body (or within the cells) the precious iron needed for vital energy production.
![Figure 4.1, (A) Stimulatory and inhibitory signals and pathways controlling hepcidin transcription. The main hepcidin stimulatory signals identified include iron, inflammation/infection and endoplasmic reticulum (ER) /nutrient stress. The iron signal converge on a membrane-associated heterotetrameric signalling complex, composed of transferrin-iron, bone morphogenetic protein (BMP) ligands, two type I and two type II serine-threonine-kinase receptors, a co-receptor (haemojuvelin [HJV] ) and ancillary proteins (including HFE and TfR2) that trigger a common signal transduction cascade involving r-SMADs and co-SMADs and activate transcription of the hepcidin gene. Both HFE and transferrin receptor 2 ( TfR2 ) act as sensors for the iron signal and are necessary for optimal induction of the BMP/SMAD pathway. A key mediator of hepcidin response to inflammation is interleukin-6 (IL-6) , which stimulates hepcidin transcription through STAT3, possibly by interacting with the BMP/SMAD pathway. Activin B likely uses the BMP/SMAD pathways to induce hepcidin during inflammation. Hormonal and nutrient signals during activated gluconeogenesis induce hepcidin through cyclic adenosine monophosphate (cAMP) and involve the transcriptional co-activator PPARGC1A and CREBH, a transcription factor also responsible for hepcidin regulation by a variety of ER stressors. The main signals for hepcidin inhibition arise in the bone marrow during active erythropoiesis and include growth differentiation factor 15 (GDF15) , a member of the TGF-β superfamily, twisted gastrulation protein ( TWSG1) , a BMP-binding protein, and erythroferrone (ERFE) . Three ‘negative modulators’ of the BMP/SMAD signaling pathway have also been identified: the soluble form of HJV, a membrane serine protease matriptase-2 (TMPRSS6) that possibly cleaves HJV and SMAD7. Neogenin, a membrane receptor for RGM, has been proposed to stabilize HJV and participate in HJV shedding, but its role is still controversial. (B) The iron-sensing machinery in the liver. Iron transferrin from the portal vein enters the sinusoids and induces the local production of BMPs, such as BMP6, by sinusoidal cells and perisinusoidal cells. Both iron transferrin and BMPs engage the membrane-associated heterotetrameric signaling complex, in a manner similar to that as described in A. From Pietrangelo A, Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin. Gastroenterology. 2015;149(5):1240–1251 . Figure 4.1, (A) Stimulatory and inhibitory signals and pathways controlling hepcidin transcription. The main hepcidin stimulatory signals identified include iron, inflammation/infection and endoplasmic reticulum (ER) /nutrient stress. The iron signal converge on a membrane-associated heterotetrameric signalling complex, composed of transferrin-iron, bone morphogenetic protein (BMP) ligands, two type I and two type II serine-threonine-kinase receptors, a co-receptor (haemojuvelin [HJV] ) and ancillary proteins (including HFE and TfR2) that trigger a common signal transduction cascade involving r-SMADs and co-SMADs and activate transcription of the hepcidin gene. Both HFE and transferrin receptor 2 ( TfR2 ) act as sensors for the iron signal and are necessary for optimal induction of the BMP/SMAD pathway. A key mediator of hepcidin response to inflammation is interleukin-6 (IL-6) , which stimulates hepcidin transcription through STAT3, possibly by interacting with the BMP/SMAD pathway. Activin B likely uses the BMP/SMAD pathways to induce hepcidin during inflammation. Hormonal and nutrient signals during activated gluconeogenesis induce hepcidin through cyclic adenosine monophosphate (cAMP) and involve the transcriptional co-activator PPARGC1A and CREBH, a transcription factor also responsible for hepcidin regulation by a variety of ER stressors. The main signals for hepcidin inhibition arise in the bone marrow during active erythropoiesis and include growth differentiation factor 15 (GDF15) , a member of the TGF-β superfamily, twisted gastrulation protein ( TWSG1) , a BMP-binding protein, and erythroferrone (ERFE) . Three ‘negative modulators’ of the BMP/SMAD signaling pathway have also been identified: the soluble form of HJV, a membrane serine protease matriptase-2 (TMPRSS6) that possibly cleaves HJV and SMAD7. Neogenin, a membrane receptor for RGM, has been proposed to stabilize HJV and participate in HJV shedding, but its role is still controversial. (B) The iron-sensing machinery in the liver. Iron transferrin from the portal vein enters the sinusoids and induces the local production of BMPs, such as BMP6, by sinusoidal cells and perisinusoidal cells. Both iron transferrin and BMPs engage the membrane-associated heterotetrameric signaling complex, in a manner similar to that as described in A. From Pietrangelo A, Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin. Gastroenterology. 2015;149(5):1240–1251 .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersofIronOverload/0_3s20B9780702082283000041.jpg)
During iron deficiency and anaemia, hepatic hepcidin transcription must be turned down so that more iron can be transferred from the intestine and storage sites to serum Tf and to the bone marrow. Hypoxia and erythropoietin inhibit hepcidin synthesis and increase iron absorption. Circulating factors derived from maturing erythroblasts in the bone marrow have been reported to downregulate hepcidin transcription in the liver, including growth differentiation factor 15 (GDF15), , a member of the transforming growth factor-β (TGF-ß) superfamily, twisted gastrulation protein, a BMP-binding protein, , and more recently erythroferrone (ERFE), which mediates hepcidin suppression during stress erythropoiesis by sequestering BMP ligands in the context of erythropoietic drive (see also later).
The main humoral signal for hepcidin synthesis is iron itself ( Fig. 4.1A, B ). The iron-sensing system resides within the liver and disruption of this system is responsible for human HC. Iron-sensing involves Tf-iron (i.e. the extent of Tf saturation) and a class of ligands of the TGF-ß superfamily, the bone morphogenetic proteins (BMPs), which normally play a crucial role in embryonic development and in other fundamental processes during postnatal life. Tf-iron interacts with a multiprotein complex at the hepatocyte plasma membrane composed of BMPs, BMP receptors, a BMP co-receptor (hemojuvelin, HJV) and a number of ancillary proteins (including HFE and the second Tf receptor [TfR2]) ( Fig. 4.1A, B ). , This interaction triggers the phosphorylation of SMAD1/5/8 complex (receptor-associated SMADs [R-SMADs]), its subsequent binding to SMAD4 (common-partner SMAD, co-SMAD), the translocation of the SMAD complex to the nucleus and activation of hepcidin transcription. The BMP co-receptor HJV, which is present in either a soluble or a cell-associated form, provides specificity to the iron signal in the liver and functions as an enhancer for iron signalling to hepcidin. At least two other proteins are required for normal signalling of iron status to hepcidin via the BMP6/SMAD1,5,8 pathway: HFE and TfR2. , In fact, functional loss of HFE in mice and humans , leads to low hepcidin and HC. Functional loss of TfR2 in mice and humans is also associated with blunted hepcidin expression and iron overload. The details of HFE and TfR2 function in the context of the BMP/SMAD signalling pathway are still not completely understood. HFE is a major histocompatibility complex (MHC) class-I-like protein that interacts with TfR1, the receptor for serum Tf that mediates uptake of Tf-bound iron. The C282Y HFE mutation, which is associated with human HC, disrupts a disulphide bond required for HFE binding to ß2-microglobulin and transport to the cell surface and endosomal membranes, where it interacts with TfR1. The H63D mutation, a common HFE polymorphism, does not impair this important HFE-TfR1 interaction. HFE gene function is not required for transcriptional regulation of BMP6 in response to dietary iron, but loss of HFE protein reduces BMP6 signalling, in vitro and in vivo . , HFE interacts with the BMP type I receptor ALK3 to stabilize it and increase its expression, thereby inducing hepcidin expression. TfR2 mediates the uptake of Tf-bound iron by hepatocytes in vitro , but its in vitro affinity for Tf is much lower than that of TfR1. It has been postulated that also TfR2 interacts with HFE, forming a unique iron-sensing complex with TfR1-HFE that modulates hepcidin expression in response to Tf-iron. However, human studies in patients with combined TfR2 and HFE mutations and studies in HFE/TfR2 double knockout mice , , have shown that the contemporary loss of HFE and TfR2 has additive phenotypic effects. This suggests that HFE and TfR2 regulate hepcidin and iron metabolism in an independent manner. Both proteins seem to be important for BMP signalling and necessary for an optimal response to BMPs.
The BMP-SMAD pathway is likely counter-regulated by a number of feedback regulatory signals that prevent overshooting of hepcidin expression in response to positive stimuli. A key role appears to be played by a membrane serine protease 2 (also called TMPRSS6) that functions by inhibiting the BMP pathway, possibly by cleaving HJV, although this aspect is still debated. , In fact, a recent study suggests that TMPRSS6 could act independently of HJV in vivo and shows that it cleaves multiple components of the hepcidin induction pathway including HFE. Loss of TMPRSS6 in humans causes a syndrome known as ‘iron-deficiency, iron-refractory anaemia’ (IRIDA), characterized by hyperhepcidinaemia and a microcytic iron deficiency anaemia resistant to oral iron supplementation. , , A second BMP pathway inhibitor is SMAD7, induced by chronic dietary iron loading and by BMP-SMAD signalling pathway activity in the liver. Another player in the BMP-SMAD pathway is neogenin , a member of the DCC (deleted in colorectal cancer) family of tumour suppressor molecules. Neogenin appears to interact with HJV, , but its role in HJV biology and hepcidin synthesis is controversial. , , Interestingly, the livers of neogenin-mutant mice exhibit reduced BMP signalling, low levels of hepcidin and iron overload. BMPs and the BMP-SMAD pathway are therefore central in hepcidin transcription in the liver. BMP6 in particular seems to play a key role in this process. BMP6 is largely produced by hepatic sinusoidal cells and nonparenchymal cells ( Fig. 4.1B ) and is directly induced by excess iron. In fact, blocking BMP6 in vivo inhibits hepcidin expression and increases serum iron, whereas genetic BMP6 ablation in mice leads to low hepcidin expression and HC, , indicating that BMP6 is an endogenous regulator of hepcidin expression and iron metabolism in vivo . BMP2 has also been shown to have a physiologic role on hepcidin regulation, appearing to control the iron hormone cooperatively and independently from BMP6.
In summary, iron homeostasis requires specific transportation of iron across membranes and meticulous intracellular iron storage. Diminution of iron stores due to dietary deficiency, iron loss, or infection may give rise to iron restriction and progression to anaemia. The converse may arise, however, in which the large ionic iron molecule, in excess and in solution with oxygen, may generate free-radical formation via Fenton and Haber–Weiss chemistry with hydrogen peroxide (H 2 O 2 ) being changed into its radical (HO − ). This leads to consequent damage to DNA, proteins and membranes.
The classic example of iron overload in human pathology is HFE -hereditary HC, but numerous other entities are now identified that cause pathological iron overload. Some are well described and have a verified hereditary basis, whereas in others, the genetic and hereditary basis is still speculative. Another subgroup results from iron overload on an inflammatory or infectious basis or as a reactive response to systemic or as-yet unknown disease processes.
Controversy surrounds nomenclature and classification for these iron overload states. HC, as identified in the liver, has previously been applied to iron loading primarily in hepatocytes, whereas the term ‘haemosiderosis’ was the terminology of choice when the iron overload was predominantly within Kupffer cells. Many overload states, however, may show elements of both HC and haemosiderosis. A further caveat is that current classifications, including the Online Mendelian Inheritance in Man (OMIM) database, are founded on the basis of single-gene defects and have significant shortcomings in that numbers of atypical cases have been identified that relate to multiple gene mutations. It appears now that failure to produce adequate amounts of the iron hormone hepcidin or its impaired activity, mostly due to genetic changes but also to acquired factors, results in a syndromic entity that resembles the historical definition of HC. Thus hereditary HC can be defined and classified phenotypically, regardless of the underlying gene defect, as an inherited disorder resulting from an inborn error of iron metabolism causing hepcidin deficiency that leads to progressive loading of parenchymal cells of the liver, pancreas, heart and endocrine organs and leads to damage and disease states.
Trousseau first described a diabetic patient with an ‘almost bronzed’ appearance in 1865, while Emile Troisier shortly afterwards detailed the first autopsied case of a diabetic patient with cirrhosis and a red-brown liver containing clumps of pigment. Von Recklinghausen later termed the condition ‘haemochromatosis’. Two main theories grew out of subsequent reports: the primary disease was diabetes that then gave rise to cirrhosis and pigmentation or, alternatively, that the pigment was primarily derived from the blood. After years of dispute as to its pathogenesis, Sheldon consolidated the existing knowledge of HC with its classic triad of diabetes, cirrhosis and melanin-based pigmentation. He described the entity as ‘an inborn error of metabolism, which has an overwhelming incidence in males and which at times has a familial incidence’.
The autosomal recessive inheritance pattern of classic hereditary HC was thereafter established, but it was only in 1976 that Simon et al. , confirmed an association between the disorder and the human leukocyte antigens (HLAs) A3 and B978-0-7020-8228-3. The subsequent discovery that ß 2 -microglobulin knockout mice developed iron overload analogous to human HC raised the postulate that the defective gene would be within an MHC molecule. , Positional cloning experiments using linkage disequilibrium and haplotype analysis allowed the identification of the affected gene, an atypical class 1 HLA molecule originally designated HLA-H and subsequently redefined as HFE . Subsequent knockout of the mouse Hfe gene demonstrated iron overload, thus confirming HFE to be the defective gene in classic hereditary HC. ,
As genetic testing for HFE mutations became more widespread, it rapidly became clear that the situation was more complicated than initially thought. Other iron genes were discovered whose mutations were associated with hereditary iron overload syndromes with some, or many, or apparently even all, of the phenotypic features of classic HC ( Table 4.1 ): ferroportin-associated iron overload ( FPN ), , TfR2-associated HC ( TfR2 ), hepcidin-associated ( HAMP ) and hemojuvelin-associated ( HJV ) ‘juvenile’ HC.
| Hereditary (gene locus) | Acquired | Miscellaneous |
|---|---|---|
| Hereditary haemochromatosis | Transfusion-dependent iron overload (thalassaemia major, sideroblastic anaemia, etc.) | Neonatal haemochromatosis * |
| Adult onset | Enteral iron overload | African/American iron overload † |
| HFE (6p21.3) | Parenteral iron overload | |
| TFR2 -associated (7q22) | Porphyria cutanea tarda | |
| FPN -associated (2q32) | Anaemia of inflammation | |
| Juvenile-onset | Chronic liver diseases | |
| HJV -associated (1q21) | Hepatitis B and C viruses ‡ | |
| HAMP -associated (19q13) | Alcoholic siderosis ‡ | |
| TfR2/HFE -associated | Insulin resistance (NAFLD/NASH) | |
| HFE/HJV-HAMP -associated | End-stage liver disease ‡ Portocaval shunt | |
| Ferroportin disease (2q32) | ||
| Atransferrinaemia (3q21) | ||
| Acaeruloplasminaemia (3q23–24) | ||
| DMT1 deficiency (12q13) | ||
| Hereditary iron-loading anaemias with inefficient-erythropoiesis |
† Possible environmental and genetic (ferroportin polymorphism) influences.
‡ Direct inhibition of hepcidin transcription in the liver is also postulated as a cofactor for hepatic iron overload. DMTI, Divalent metal transporter-1; FPN, ferroportin; HAMP, hepcidin; HJV, hemojuvelin; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; TFR2, transferrin receptor-2.
Sheldon almost certainly described patients having the characteristics of ‘juvenile’ HC. However, the entity was only formally recognized after the case study and literature review of Lamon et al. They described a 36-year-old woman with heart failure, diabetes mellitus, hepatomegaly and secondary amenorrhoea and reviewed another 52 cases published over the previous 85 years. Essential differences between these cases and classic hereditary HC are notable, when compared with the large series of Finch and Finch, namely that juvenile HC is a rare form of iron overload with rapid and severe progression of disease, leading to significant complications before the age of 30 years. Lamon et al. also noted the inherited nature of this rare disease and favoured an autosomal recessive inheritance pattern. Subsequent genotyping identified the disorder to be distinct from HFE -linked iron overload. A high frequency of consanguinity is observed in patients with juvenile HC, with the disease occurring in siblings but not parents. A human-genome search demonstrated linkage between the disease and numerous markers on the long arm of chromosome 1q. Subsequent analysis of Greek, Canadian and French families carrying the disorder identified multiple deleterious mutations at LOC148738, whose protein product is identified as hemojuvelin (HJV), previously known as HFE2. In addition, the investigation of juvenile HC identified a second cohort that is even rarer, demonstrating a mutation, C70R on 19q13, the gene encoding hepcidin. , The concept and spectrum of juvenile HC has been further extended by the recent identification of two young patients presenting with typical features of the disease, namely severe endocrinopathy and cardiomyopathy, but testing positive for combined mutations for C282Y/H63D and TfR2 . In addition, there is evidence that selected HJV and HAMP mutations, when carried simultaneously with mutant HFE genes, may influence iron status in older patients being evaluated for a molecular diagnosis of HC because of increased Tf saturation and/or serum ferritin. This further challenges the current classification system for hereditary HC in that some cases that fit the clinical picture for juvenile HC do not represent a distinct monogenic disorder but may be variant of the adult-onset form of hereditary HC.
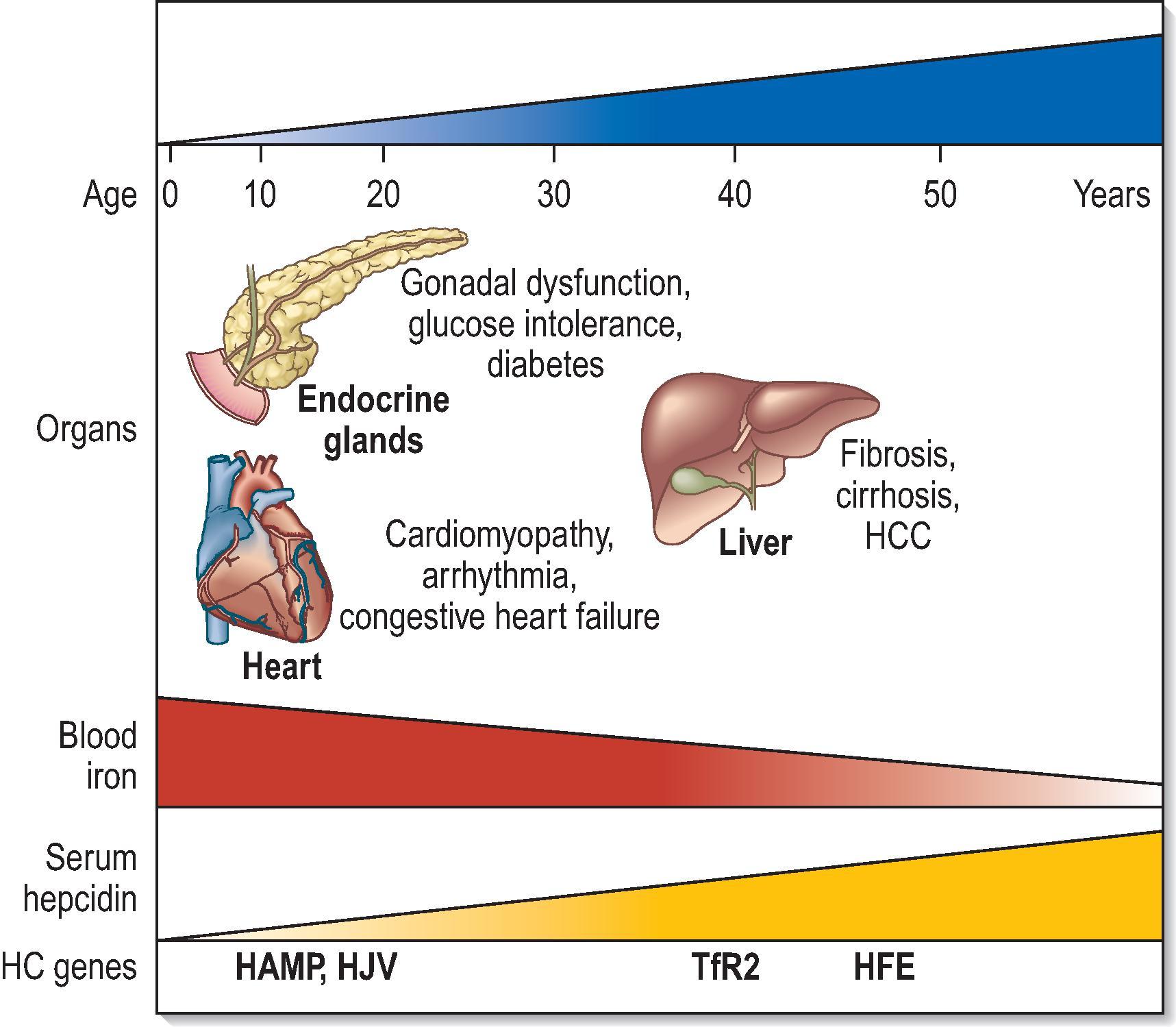
The present definition of hereditary HC embraces the classic disorder related to HFE C282Y homozygosity (the prototype for this syndrome and by far the most common form) and the rare disorders more recently attributed to loss of TfR2, HAMP and HJV or, in very rare cases, FPN ( Table 4.1 ). This concept of HC stems from the idea that, beyond their genetic diversities, all known haemochromatoses belong to the same clinicopathological entity: they all originate from the failure to prevent unneeded iron from entering the circulatory pool as a result of genetic changes compromising the synthesis or activity of hepcidin, the iron hormone ( Fig. 4.2 ). Depending on the gene involved and its role in hepcidin regulation, the phenotype of HC varies, ranging from the rare HJV and HAMP juvenile forms characterized by massive iron loading with severe health and endocrine disorders to the adult-onset phenotype dominated by liver disease and usually associated with HFE , TfR2 and, rarely, FPN mutations. In brief, the features that distinguish HC from all other iron-loading disorders listed in Table 4.1 are hereditary nature (usually autosomal recessive); early and progressive expansion of the plasma iron compartment (increasing Tf saturation); progressive parenchymal iron deposits with potential for severe damage and disease that may involve, liver, endocrine glands, heart and joints; nonimpaired erythropoiesis and optimal response to therapeutic phlebotomy; and inadequate hepcidin synthesis/activity. It is anticipated that disruption of any regulatory mechanisms that, beyond the central hepcidin homeostatic axis, prevents an unregulated flux of iron towards the blood in the presence of normal erythropoietic activity will lead to a syndrome similar to HC.
An autosomal recessive disease, classical HFE -associated hereditary HC is the most common monoallelic inherited disorder in Western society. The dominant missense polymorphism identified in 80% of cases, C282Y, is characterized by a single nucleotide change, G to A, resulting in a Cys → Tyr at position 282 of the unprocessed protein, occurring in a highly conserved region involved in the disulphide bridge in the MHC class 1 protein. A less common polymorphism, H63D, is a C to G change resulting in a Hys → Asp substitution at amino acid 63, showing limited clinical effects, although compound heterozygosity for C282Y and H63D may cause disease expression. Of several other uncommon polymorphisms, S65C, in which cysteine replaces serine at position 65, has been associated with compound heterozygosity for either C282Y or H63D. It has been related to the development of mild to moderate hepatic iron overload but without clinical manifestations. Its significance is still controversial.
The C282Y polymorphism is most prevalent amongst populations of northern European origin. Over 90% of patients with clinically penetrant HC in the United Kingdom are homozygous for C282Y, and worldwide, allele frequencies are calculated at 1.9% for C282Y and 8.1% for H63D, with highest frequencies of 10% for C282Y in Irish chromosomes and 30.4% for H63D in Basque chromosomes. The origin of the genetic mutation was initially thought to represent a unique event in chromosome HLA-A3 and -B7 originating from the Celts in central Europe between 65 and 70 generations ago and spreading north and west by population migration. However an alternative Viking origin has been postulated, and it been more recently proposed that the initial C282Y polymorphism occurred in mainland Europe before 4000 bc , earlier than both the Celtic and Viking periods. Its high prevalence in White populations suggests that it may have constituted or constitutes an environmental or genetic advantage for asymptomatic carriers.
The genetic defect, which caused no serious obstacle to reproduction and may even confer some advantages, was passed on and spread through population migration. The distribution of the C282Y mutation coincides with its northern origin, with frequencies ranging from 12.5% in Ireland to 0% in southern Europe. In addition to C282Y, H63D, the ‘minor’ HFE polymorphism, is found more frequently in HC patients than in the control population. The frequency of the H63D polymorphism shows less geographic variations with an average allelic frequency of 14%, but its clinical impact appears to be limited. An additional HFE polymorphism is S65C, which can be associated with HC when inherited in trans with C282Y on the other parental allele. The prevalence of C282Y homozygosity among patients with liver disease is around 5%, 10-fold higher than the reported prevalence in the general population. This figure increases if patients with liver disease are preselected for increased Tf saturation. Even higher C282Y frequencies can be found in patients with hepatocellular carcinoma (HCC), a known complication of HC.
HC is associated with homozygosity for the C282Y HFE mutation in approximately 80% of clinically characterized patients of European ancestry. Therefore almost 20% of such patients have the disease in the absence of C282Y. Although compound heterozygosity (H63D/C282Y) appears to be disease causing in some cases, cofactors are implicated in the clinical expressivity.
Other genes associated with clinical HC are TfR2 , HJV , HAMP and FPN ( Table 4.1 ). None of these non- HFE HC appears to be restricted to northern Europeans. The global prevalence of non- HFE HC has recently been estimated, and TfR2- , HJV- and HAMP -related forms are predicted to be rare, with allele frequencies ranging from 0.00007 to 0.0005; however, the most prevalent non- HFE associated form appears to be one associated with FPN (allele frequency 0.0004), accounting for the high incidence in Black Africans. Although the TfR2 mutation is rare, at least nine individual TfR2 mutations have been identified. With the exception of one patient of Portuguese descent with a c2069 A→C, Q690P mutation in the TfR2 gene mutation and two affected homozygous female siblings, most represent largely inbred families of Italian extraction with various mutations of the TfR2 gene; consanguinity is a common feature. The AVAQ 594–597 deletion originally described by Girelli et al. in their Italian cohort has also been identified in three members of a Japanese family. Subsequent investigation of a further nine, unrelated Japanese patients with HC of unknown origin described two more novel TfR2 mutations, L490R and V561X. The patient with the V561X mutation, age 58, was a member of a consanguineous family. Both of these patients had cirrhosis and diabetes, one with associated skin pigmentation. HC is rare in the Far East, particularly among the Japanese. It would thus appear that TfR2 -hereditary HC is the most common form of hereditary iron overload amongst Japanese patients, although iron loading secondary to acaeruloplasminaemia is also identified in this population. However, in 2001, a Y231del mutation in the HFE gene was found in the Huh-7 hepatoma cell line (obtained from a Japanese donor) and was shown to prevent the translocation of HFE to the cell surface, similar to the C282Y mutation. More recently, this same HFE mutation has been reported in a Japanese pedigree affected by HC, indicating the occurrence of an HFE -related form of HC also in Asian populations.
The juvenile form of HC is rare. Most cases are due to mutations of HJV . One common HJV mutation, G320V, has been identified in at least some patients in all studies and it is present in half of juvenile HC families. Analysis of patients from the central and northern parts of Europe, specifically Germany, Slovakia, Croatia and Ireland, has identified the G320V mutation as the most common. , Numerous other mutations have subsequently been identified. , A small proportion of patients with the juvenile form of HC carry mutations in the gene hepcidin.
Although most FPN mutations give rise to a distinct form of hereditary iron overload called ‘the ferroportin disease’ (FD), unusual FPN mutations cause rare forms of HC similar to HFE -HC.
The first biochemical manifestation of HC is an increase of Tf saturation, which reflects an uncontrolled influx of iron into the bloodstream from enterocytes and macrophages. Except for menstruation, the body has no effective means of significantly reducing plasma iron levels. Without therapeutic intervention, overload in the plasma compartment will lead to the progressive accumulation of iron in the parenchymal cells of key organs, creating a distinct risk for oxidative damage. This stage is reflected in increasing serum ferritin levels. The time of onset and pattern of organ involvement in HC vary depending on the rate and magnitude of plasma iron overloading, which in turn depend on the underlying genetic mutation. For this reason, milder adult-onset forms (e.g. HFE and TfR2 related) and more severe juvenile-onset forms (e.g. HJV and HAMP related) are recognized. The extent of iron release from enterocytes and macrophages into the bloodstream in humans is under the control of the hepcidin–ferroportin axis ( Fig. 4.1A ) and HFE and TfR2 play a role in conveying the iron signal to hepcidin in the hepatocyte.
In view of these findings, HC should be seen as a genetically heterogeneous disease that results from the complex interaction between genetic and acquired factors. If the altered gene plays a dominant role in hepcidin synthesis (e.g. HAMP itself or HJV ), circulatory iron overload occurs rapidly and reaches high levels ( Fig. 4.2 ). In these cases, the modifying effects of acquired environmental and lifestyle factors will be negligible and the clinical presentation will invariably be dramatic, with early onset (first to second decade) of a full-blown organ disease. In contrast, C282Y- HFE homozygosity results in a genetic predisposition that requires the concurrence of host-related or environmental factors to produce disease ( Fig. 4.2 ). Co-inherited mutations in other HC genes, such as HAMP and HJV , may have a role in disease penetrance of HFE -HC, but they are rare.
The clinical presentation in patients who are homozygous for C282Y is highly variable, with general signs of weakness (60% of patients), arthralgia/arthritis (30–40%), hepatomegaly/cirrhosis (13–60%), diabetes mellitus (10–30%), sexual dysfunction (10–40%) and cardiac symptoms with arrhythmia (20–29%) and cardiac failure (15–35%). Skin pigmentation is estimated to occur in 47% of proband cases. However, extrahepatic disease tends to be less prominent in modern studies, including diabetes mellitus, arthritis, and heart disease. The initial presentation is often vague and nonspecific in many patients, and a high index of suspicion is required to diagnose the condition.
Furthermore, whereas patients with defined HFE -HC will have C282Y mutations, a significant proportion of patients with mutation do not develop significant hepatic iron overload, thus gene penetrance is not obligatory. Large population-based studies confirm that penetrance is low in HFE -hereditary HC, implying that C282Y homozygosity is necessary but not sufficient on its own to cause clinically manifesting disease , because half of C282Y homozygotes will not develop iron overload and two-thirds will not develop HC-associated morbidity. Ethnicity, environmental factors such as blood loss (an important modifier in females), dietary iron intake, alcohol, diabetes mellitus and other as-yet unknown genetic modulators may play a part in this differential expression. , , It is likely that these disease frequencies will change further with increased awareness of the disease and the development of screening techniques.
In proband patients in whom the diagnosis of HC is suspected, serum testing for iron overload forms the cornerstone of initial identification: Tf saturation, unbound iron binding capacity (UIBC) and serum ferritin. Consensus is that Tf saturation of >45%, UIBC <28 μmol/L and ferritin >300 μg/L (>200 in women) identify hereditary HC, although these values may not be appropriate for all populations. Ferritin in isolation is highly sensitive but not specific, and its normality excludes iron overload, but because ferritin is also an acute-phase protein, it is elevated in inflammatory states as a nonspecific finding.
Before the discovery of HFE gene mutations, liver biopsy confirmed the histological diagnosis of HC and allowed for grading of iron, staging of fibrosis and determining hepatic iron index (HII) (the ratio of hepatic iron concentration divided by age), as discussed later. However, subsequent with the development of genotyping, the necessity for liver biopsy now depends on clinical status. If inflammation and other confounding factors are excluded, serum ferritin correlates well with the level of iron excess within organs. Magnetic-susceptibility measurement or magnetic resonance imaging (MRI) may act as a second estimation of iron overload. In C282Y HFE homozygotes, if iron load is moderate and other diagnostic parameters are normal (e.g. serum aminotransferases, fasting blood glucose, hormonal evaluation, cardiogram, and joint/bone x-rays), then therapy by venesection (phlebotomy) may commence without liver biopsy. In cases of heavy iron overload, when one or more of these parameters are abnormal, then liver biopsy is mandatory, in particular to evaluate the presence of cirrhosis, other hepatic pathology or iron-free foci, and at this stage, the biopsy is performed as a prognostic marker. , The current gold standard in the case of hereditary HC is thus genetic testing. The relevance of the H63D mutation as well as C282Y/H63D compound heterozygosity is still uncertain as a small percentage may develop clinically significant HC, normally in the presence of damaging cofactors.
In rare patients, combined mutations of HFE and other iron-related genes, such as HAMP , HJV and TfR2 or polymorphic variants of BMP2, are also reportedly associated with a higher penetrance of HFE -HC, as reviewed by Brissot et al., Piperno et al. and Pietrangelo. The clinical spectrum of a fully expressed HFE -HC includes liver disease, diabetes, endocrine failure, joint inflammation, heart disease and bronze skin. Cirrhosis is part of the classic clinical manifestations and relates to the degree of iron loading. A critical threshold of hepatic iron concentration greater than 236/283 μmol/g dry weight (normal, 0–35 μmol/g) has been proposed for cirrhosis in HFE -HC, but higher liver iron concentrations (LICs) may be also found in the absence of cirrhosis.
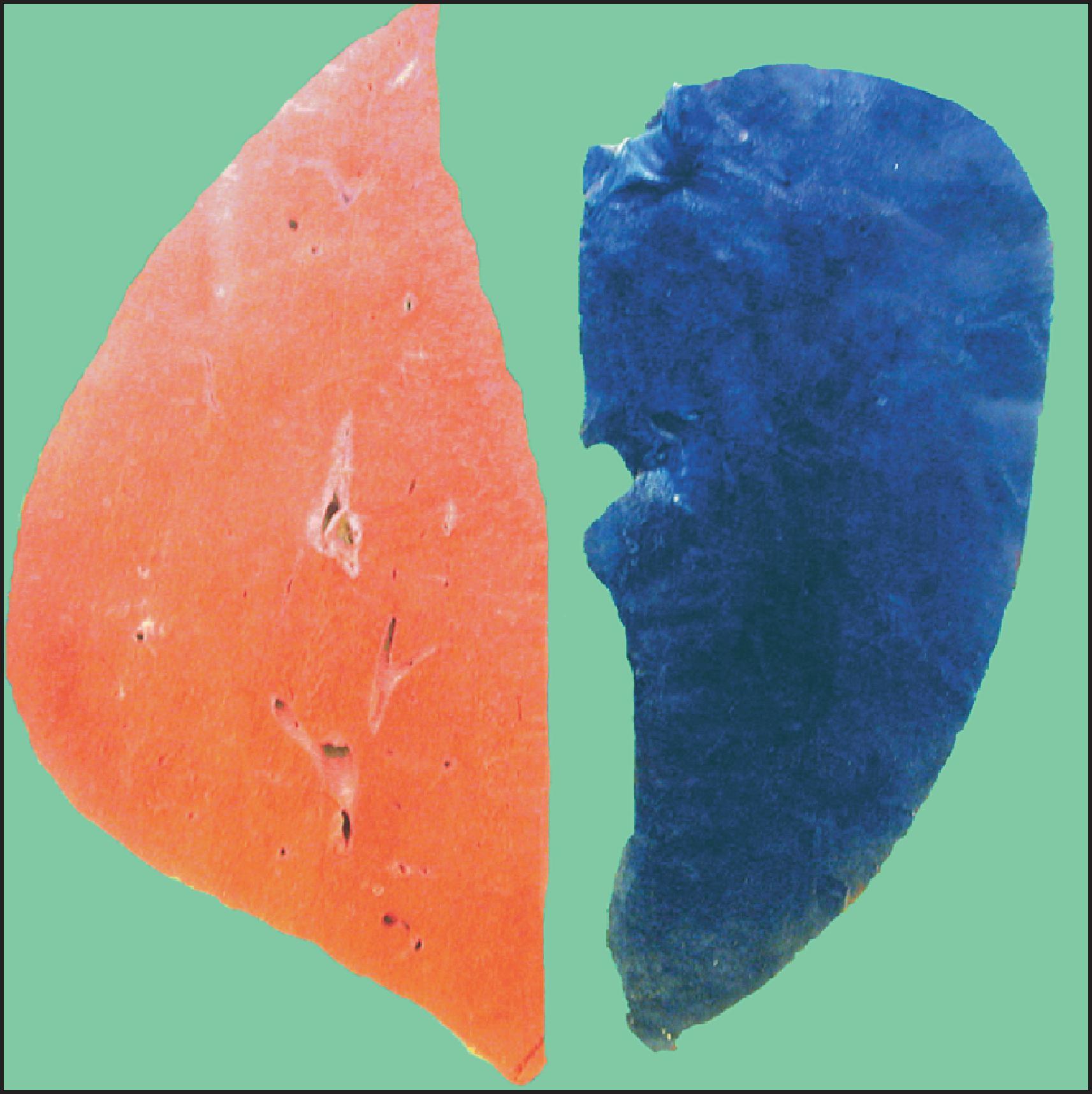
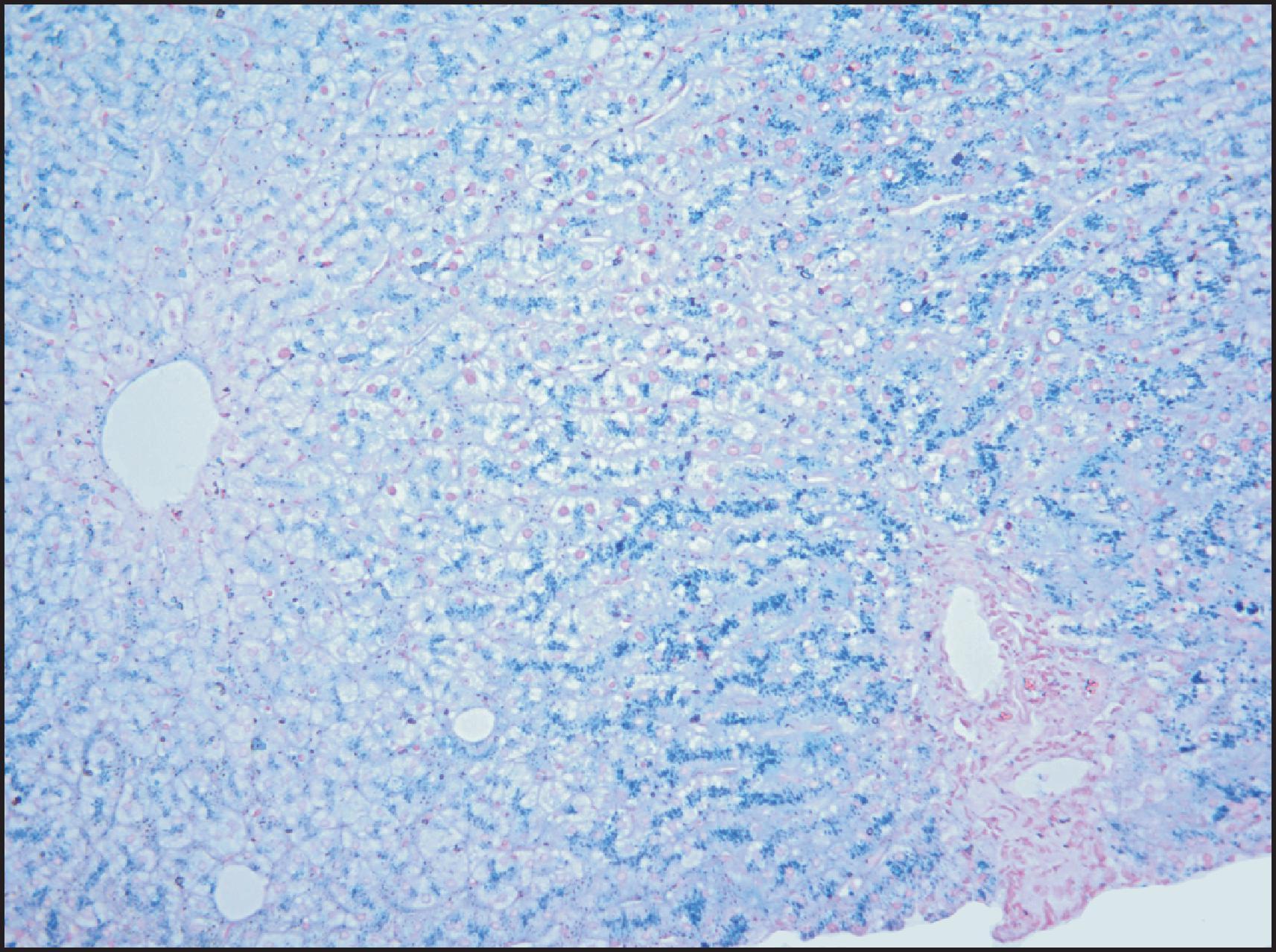
The macroscopic and microscopic features of penetrant HFE -HC are highly characteristic in the liver. The classic gross description is of a brown-to-rusty colour, staining intense blue with Perls ( Fig. 4.3 ), with or without the appearance of cirrhosis. Histologically, the progression of the disease is again characteristic. Iron deposition begins in the periportal hepatocytes (zone 1) ( Fig. 4.4 ) and extends progressively to involve all zones of the liver ( Fig. 4.5 ). The iron has a characteristic pericanalicular pattern when observed under high power ( Fig. 4.6 ). With progression of the disease, there can be deposition of iron in the biliary epithelium ( Fig. 4.7 ) and transfer into Kupffer cells and portal macrophages with sideronecrosis ( Fig. 4.8 ). Of note, none of these characteristic findings (zonal distribution of iron, pericanalicular pattern of iron on high-power examination or iron in bile duct epithelium) are pathognomonic for HFE -HC disease and can be found in non- HFE causes of severe iron overload, including secondary causes. Progressive portal-based fibrosis may evolve to cirrhosis ( Fig. 4.9 ). Of importance is the identification of iron-free foci in histologically advanced liver disease, which may foretell the development of HCC, as discussed later.
![Figure 4.4, Male of 38 years with deranged liver function tests. He was subsequently identified as homozygous for the C282Y mutation on the basis of the biopsy finding. (A) Liver biopsy is histologically unremarkable on routine staining. (Haematoxylin and eosin [H&E] stain.) (B) However, grade 1 iron deposition is present in periportal hepatocytes. (Perls stain.) Figure 4.4, Male of 38 years with deranged liver function tests. He was subsequently identified as homozygous for the C282Y mutation on the basis of the biopsy finding. (A) Liver biopsy is histologically unremarkable on routine staining. (Haematoxylin and eosin [H&E] stain.) (B) However, grade 1 iron deposition is present in periportal hepatocytes. (Perls stain.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersofIronOverload/3_3s20B9780702082283000041.jpg)
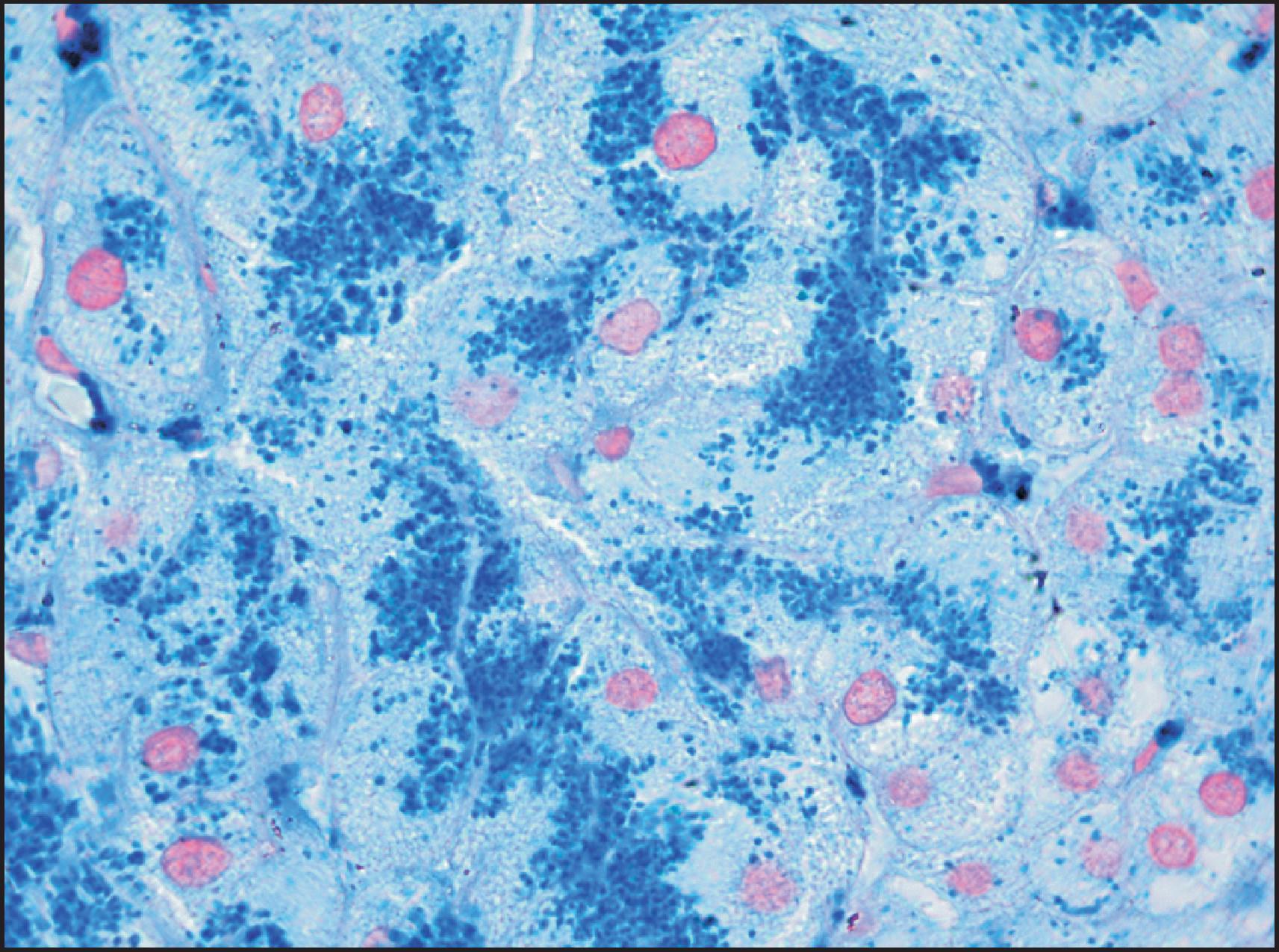
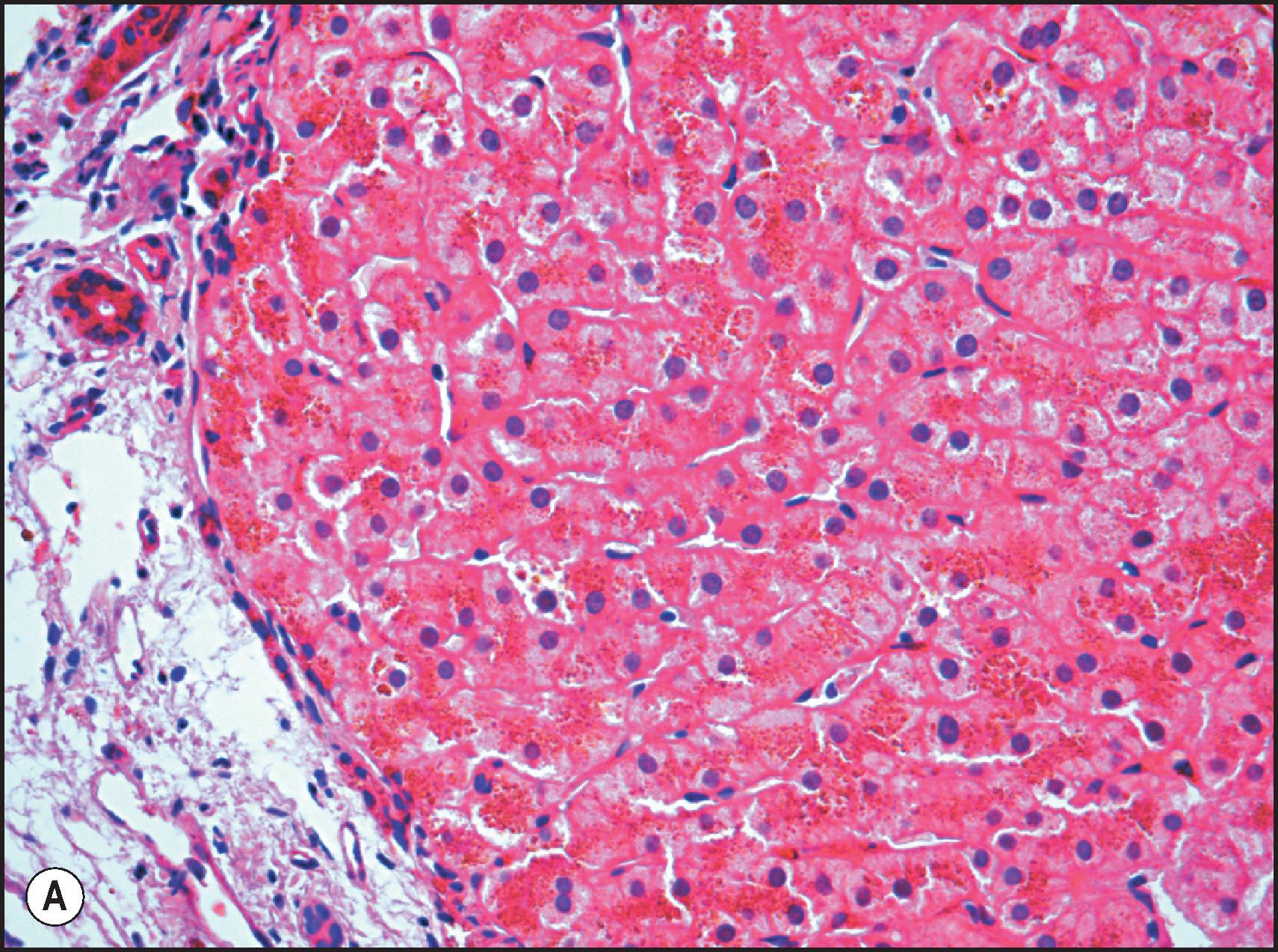
![Figure 4.8, Male of 45 years confirmed to be homozygous for the C282Y mutation and with a history of alcohol abuse. Cirrhosis with grade 4 iron overload was confirmed histologically. Sideronecrosis is identified by large globules of haemosiderin associated with aggregates of heavily iron-laden Kupffer cells (Periodic acid-Schiff [PAS]-positive), both periportal and within the lobular parenchyma. (PAS stain.) Figure 4.8, Male of 45 years confirmed to be homozygous for the C282Y mutation and with a history of alcohol abuse. Cirrhosis with grade 4 iron overload was confirmed histologically. Sideronecrosis is identified by large globules of haemosiderin associated with aggregates of heavily iron-laden Kupffer cells (Periodic acid-Schiff [PAS]-positive), both periportal and within the lobular parenchyma. (PAS stain.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersofIronOverload/7_3s20B9780702082283000041.jpg)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here