Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Iron is an essential nutrient for every cell of the body. Both decreased and increased total body iron, as well its inappropriate tissue distribution may be clinically important. In iron deficiency, limitation of the synthesis of physiologically active iron-containing compounds can have harmful consequences. In iron overload when iron exceeds the body’s capacity for safe transport and storage, iron toxicity may produce widespread cell (and organ) damage. Altered distribution may lead to selected organ deficiency. The body has no effective means to excrete excess iron and relies upon control of iron absorption to maintain homeostasis. This chapter focuses on clinical disorders arising from derangement of the molecular mechanisms that preserve iron balance.
Systemic iron homeostasis is maintained by the hepcidin-ferroportin axis. Hepcidin is the iron regulatory hormone that controls iron absorption, use, and storage by binding to, blocking, and inducing the degradation of the cellular iron export channel ferroportin, limiting iron entry into circulation from stores and absorption sites (see Chapter 36 ). Hepcidin expression is suppressed by iron deficiency, hypoxia, and increased erythropoiesis, while it is stimulated by iron, inflammation, and infection. Genetic and acquired disorders leading to hepcidin deficiency or ferroportin resistance to the hepcidin effect result in iron overload. Hepcidin excess due to genetic causes leads to iron-deficiency anemia, while acquired forms, such as those associated with inflammatory disorders, result in iron sequestration in stores, iron-restricted erythropoiesis, and anemia.
In normal adults total body iron content approximates 3 to 4 g, most present as heme iron in hemoglobin of red blood cells and erythroid precursors (>2.0 g). Myoglobin in skeletal muscles and cardiomyocytes accounts for an other 300 mg. Functional iron represents a minimal amount in all cells, but those minute amounts are essential for mitochondrial metabolism and enzyme-based processes, such as DNA synthesis and repair. Circulating iron bound to transferrin accounts for only 3 to 4 mg, and turns over several times to supply about 25 mg iron daily to tissues, about 80% of which is used to sustain erythropoiesis. More than 20 mg iron are provided daily by macrophages that recycle the metal derived from the breakdown of senescent and damaged erythrocytes, while the contribution of iron absorption is small (1 to 2 mg/day). Release of iron to tissues occurs through the interaction of diferric transferrin (transferrin bound to two iron molecules) with the ubiquitous cell iron importer transferrin receptor (see Chapter 36 for details). Iron is stored in hepatocytes and macrophages by ferritin that with time aggregates into hemosiderin, a complex of molecules of denatured ferritin that can be visualized with Perls’ Prussian blue stain on histological preparations. The amount of stored iron is variable from 750 to 1000 mg in adult males to much lower amounts (200 to 300 mg) in females. Daily iron losses balance the amount of absorbed iron (1 to 2 mg) and occur mainly through cell desquamation from skin, genitourinary system, gastrointestinal tract, and menses in females. The distribution of body iron is schematically illustrated in Fig. 37.1 .
Since there is no active mechanism to excrete excess iron, iron absorption and recycling are tightly regulated. Disruption of this regulation leads to disorders of iron homeostasis, broadly classified as iron deficiency and overload that can be either genetic or acquired. Other processes such as inflammation that modify iron homeostasis are described in Chapter 36, Chapter 38AU: Please check and confirm cross reference to Chapters 36 and 38. . Diseases causing local alterations of iron distribution are briefly mentioned, but not discussed in detail in this chapter.
Absolute or true iron deficiency is the reduction of total body iron, with depletion of iron stores, a condition that can be present with or without anemia, respectively indicated as iron deficiency anemia and isolated iron deficiency. Functional iron deficiency, that is, the reduced iron availability for tissues, especially erythropoiesis in the presence of replete stores, frequently occurs in acute and chronic inflammation (see box on Definitions and Acronyms Used in Iron Deficiency ) (see also Chapter 38 ). Treatment with erythropoiesis stimulating agents (ESA) may increase erythroid demand beyond iron supply causing functional iron deficiency that is also referred to as iron restricted erythropoiesis .
Iron deficiency: decrease of total body iron, including iron stores in the absence of anemia.
Iron deficiency anemia: decrease of total body iron including iron stores, in the presence of anemia.
Iron-refractory iron deficiency anemia (IRIDA): a rare genetic disease caused by TMPRSS6 mutations resulting in high hepcidin levels and iron deficiency anemia unresponsive to oral iron.
Functional iron deficiency: insufficient iron supply in face of increased iron needs (usually referred to erythropoiesis: it could also apply to other organs).
Iron-restricted erythropoiesis: reduced iron supply to erythropoiesis irrespective of stored iron.
Anemia of inflammation or anemia of chronic disease: anemia associated with up regulation of hepcidin by inflammatory cytokines, leading to iron sequestration in stores and functional iron deficiency.
Most transferrin iron is utilized for heme synthesis and hemoglobinization of erythroid precursors; for this reason, anemia is the more evident and easily recognizable manifestation of iron deficiency and the terms iron deficiency anemia and iron deficiency are sometimes used as synonyms. However, defective iron supply may affect every tissue that needs a minimal but essential amount of iron for the function of iron-containing proteins and enzymes. Thus the two conditions should be kept distinct, since in iron deficiency tissues other than erythropoiesis may be affected, even in the absence of anemia .
Iron deficiency anemia is the most frequent anemia worldwide, estimated to affect over 1.2 billion individuals, and is among the five top causes of years lived with disability burden, according to the Global Burden of Disease Study . About 40% of pre-school children and pregnant women and 32% of young women are anemic worldwide, although variations among countries are huge. More than 50% of the populations in low-income African and Asian countries are estimated to be iron deficient, with the highest prevalence among individuals whose diet contains low bioavailable iron, have helminthic infection resulting in gastrointestinal blood loss, or both. A significantly lower prevalence (around 10% to 15%) is recorded where the diet is adequate as in Western Europe and even lower in the United States because of food fortification and the use of iron supplements. The prevalence of isolated iron deficiency is estimated to be higher than that of iron deficiency anemia, but epidemiological data are lacking. There is increasing awareness that absolute iron deficiency may coexist with anemia of inflammation in several common chronic disorders, causing a mixed type of iron deficiency-inflammation anemia (see Chapter 38 ). This further increases the relevance of iron deficiency that, irrespective of its prevalence, often remains undiagnosed and/or undertreated.
Everywhere in the world, even in the high-income countries, iron deficiency affects mainly children and young women because of their increased iron requirements. Physiologic variations in iron demand during life explain differences of age- and gender-related prevalence. Preterm and low-birth-weight newborns have low iron stores and because of rapid postnatal rate of growth—the body weight normally triples in the first year of life—are at high risk of iron deficiency. Infants fed cow’s milk need iron supplementation because of the small bioavailable iron content of cow’s milk. Preschool children are at risk because of rapid growth. Iron requirements decline into childhood but rise again (around 1.5 mg/day) with the adolescent growth spurt and with menarche in females. Iron need in men and postmenopausal women is about 1 mg/day while in women of childbearing age is approximately double, because of menses. Pregnancy enhances iron demands up to 3 and 5 mg daily in the second and third trimester respectively for the expansion of maternal and fetal erythroid mass. The global iron need for pregnancy, including the amount to replace partum blood loss, ranges from 600 to 800 mg. In all these cases the high iron need may be unmet by limited dietary supply, especially in low-income countries, where hypoferremia may also be sustained by concomitant infections.
Bioavailability of non-heme iron is influenced by other diet components that inhibit iron absorption, such as tannins of tea and coffee and phytates of cereals. Reduced intake and dietary habits, such as vegetarians and vegans, who refuse heme-containing food, cause iron deficiency. The other causes are pathological and related to disorders of iron absorption or chronic blood loss as detailed in Table 37.1 . Search for the cause/s is a fundamental step in the diagnostic workup of iron deficiency both with and without anemia. It is especially important in individuals who do not belong to a risk category, because it may lead to diagnose a relevant gastrointestinal pathological process such as peptic ulcer or even colon cancer.
| “Physiological” High Iron Requests |
|
| Insufficient Intake of Bioavailable Iron |
|
| Disorders of Iron Absorption |
|
| Chronic Blood Loss |
|
| Functional iron deficiency |
Reduction of chloride production in atrophic gastritis or after gastric surgery impairs the concentration of ferrous iron available for absorption. Bariatric surgery to correct obesity causes iron deficiency and refractoriness to oral iron therapy by reducing gastric size or by passing the duodenum, the key site of iron absorption. Obesity, because of a mild chronic inflammation and increased hepcidin, is itself a cause of iron deficiency (see Table 37.1 ). The widespread Helicobacter pylori infection may decrease iron absorption by competing for dietary iron or causing bleeding from gastric microerosions. Celiac disease is a common gluten mediated enteropathy that should be searched for in iron-deficient children; in adults, who are affected by milder forms, the disease is often undiagnosed and revealed by iron deficiency. Gluten-free diet promotes mucosal recovery and restored iron absorption. In inflammatory bowel diseases anemia may be multifactorial, resulting from combination of blood losses, iron and vitamin deficiency, inflammatory cytokines, and sometimes treatment secondary effects. Anemia of inflammation prevails in flare up, while true iron deficiency becomes manifest in remission.
Young females are frequently iron deficient because of voluntary dietary restriction, heavy menstrual bleeding, or bleeding from fibroid. Common defects of hemostasis such as mild von Willebrand disease, inherited platelet disorders, and other rare bleeding disorders may cause heavy menstrual bleeding and postpartum hemorrhages and may present as iron deficiency anemia in young women. Bleeding lesions of the gastrointestinal tract may occur at any age but are the main cause of iron deficiency anemia in males and postmenopausal females. Bleeding may be overt or occult and the responsible lesion may be evident as in the case of hemorrhoids or a symptomatic peptic ulcer or silent as a colonic polyp or a malignant tumor. Gastrointestinal bleeding is of special concern in the elderly because of frequent mucosal lesions such as angiodysplasia, or even colon cancer. The elderly do not have increased iron needs but are at risk of deficiency because of reduced iron intake, multiple comorbidities, and the chronic use of drugs that interfere with iron absorption, such as proton pump inhibitors, or contribute to blood loss, such as anticoagulants (see Table 37.1 ). Chronic recurrent epistaxis, hemoptysis, and hematuria are uncommon causes of iron deficiency. In low-income countries hookworm and other helminthic ( Schistosoma ) infections may cause chronic blood losses, adding this effect to the widespread low iron diet.
Among healthy individuals frequent blood donors are at risk of iron deficiency and female donors often require iron supplementation. Since 1 mL of blood contains about 0.4 mg iron, each donation removes about 200 mg. Athletes may be at risk of iron deficiency because of blood loss or intravascular hemolysis and consequent urinary iron loss during strenuous exercise and often have elevated levels of hepcidin because of training-induced inflammation.
Absolute iron deficiency may coexist with anemia of chronic disorders characterized by either overt or silent inflammation, as in rheumatoid arthritis, inflammatory bowel disease, colon cancer, chronic kidney disease, obesity, and diabetes. In chronic infections such as malaria iron deficiency increases the severity of anemia caused by the parasite. Multiple causes often concur to decrease body iron. An example is chronic heart failure, where iron deficiency anemia is reported in 30% to 50% of patients due to decreased iron absorption, decreased renal function, blood loss, and inflammation; importantly, both anemia and iron deficiency are independently associated with increased mortality in heart failure.
Iron deficiency is usually acquired. However, research on hepcidin regulation led to identification of a genetic form called “iron- refractory iron deficiency anemia IRIDA)” (see box on Definitions and Acronyms Used in Iron Deficiency ). It is a rare condition caused by recessive mutations in TMPRSS6 , a gene encoding a liver transmembrane protease that acts as the main hepatic inhibitor of hepcidin expression (see Chapter 36 ). In subjects with iron deficiency TMPRSS6 suppresses hepcidin production to facilitate iron entry to the circulation. Patients homozygous or compound heterozygous for TMPRSS6 mutations have constitutionally high hepcidin levels that, blocking iron absorption, lead to iron deficiency anemia refractory to oral iron treatment since childhood. According to several genome- wide association studies TMPRSS6 genetic variants associate to variant erythroid and iron traits. By modulating hepcidin production, they might influence the mucosal dietary and pharmacological iron absorption in normal subjects, contributing to a variable susceptibility to iron deficiency.
Cellular and systemic mechanisms are active in responses to iron deficiency to compensate for the lack of iron. When compensatory mechanisms are overwhelmed depletion of total body iron and iron deficiency anemia occur. The cell iron sensor and regulatory proteins (IRPs) increase the synthesis of the iron importer transferrin receptor to take up more iron, while the translation of the iron store ferritin and iron exporter ferroportin is suppressed (see Chapter 36 for detailed coverage of the mechanisms described herein). “Ferritinophagy,” a process of degradation of ferritin in lysosomes, mediated by the cargo protein Nuclear Receptor Coactivator 4 (NCOA4), is activated to recover iron stored in ferritin. Suppression of hepcidin production to increased iron supply is a key mechanism to compensate for iron deficiency. With increased ferroportin activity macrophages recycle more iron to transferrin and iron absorption is enhanced. Inhibition of hepcidin expression is more marked in iron deficiency with than without anemia because the tissue hypoxia accompanying anemia further contributes to hepcidin suppression.
In the duodenal mucosa the effect of hepcidin on ferroportin is coupled to the function of hypoxia inducible factor-2alpha (HIF-2α). In the presence of anemia tissue hypoxia stabilizes HIF-2α, which controls the expression of iron transporters divalent metal transporter 1 (DMT1) and duodenal cytochrome B (DCYTB) on luminal and ferroportin on the basolateral membrane, increasing the flux of iron into the circulation. Microorganisms compete with the human host for iron, leading to the hypothesis that gut microbiota might contribute to systemic iron homeostasis. Studies in mice suggest that some Lactobacillae species increased in iron deficiency produce metabolites that inhibit HIF-2α function in duodenal mucosa to favor bacterial iron acquisition when availability is limited. Whether a similar competition occurs also in humans has to be verified.
The synthesis of erythropoietin (EPO) is modulated not only by hypoxia but also by iron. In iron deficiency without anemia EPO synthesis is attenuated because high IRPs partially suppressed translation of HIF-2α; this limits erythropoiesis, saving iron for other vital functions. In the presence of anemia, the need for oxygen delivery by erythroid cells prevails, HIF-2α is fully stabilized, and EPO is strongly increased. The sensitivity of erythroid cells to EPO may be iron-modulated also via transferrin receptor 2, which contributes to hepcidin activation in the liver and is a partner of the EPO receptor in erythroid cells. In absolute iron deficiency, erythroblasts and erythrocytes release excess iron to plasma through ferroportin, a mechanism of protection from oxidative stress that likely becomes a compensatory mechanism in iron deficiency.
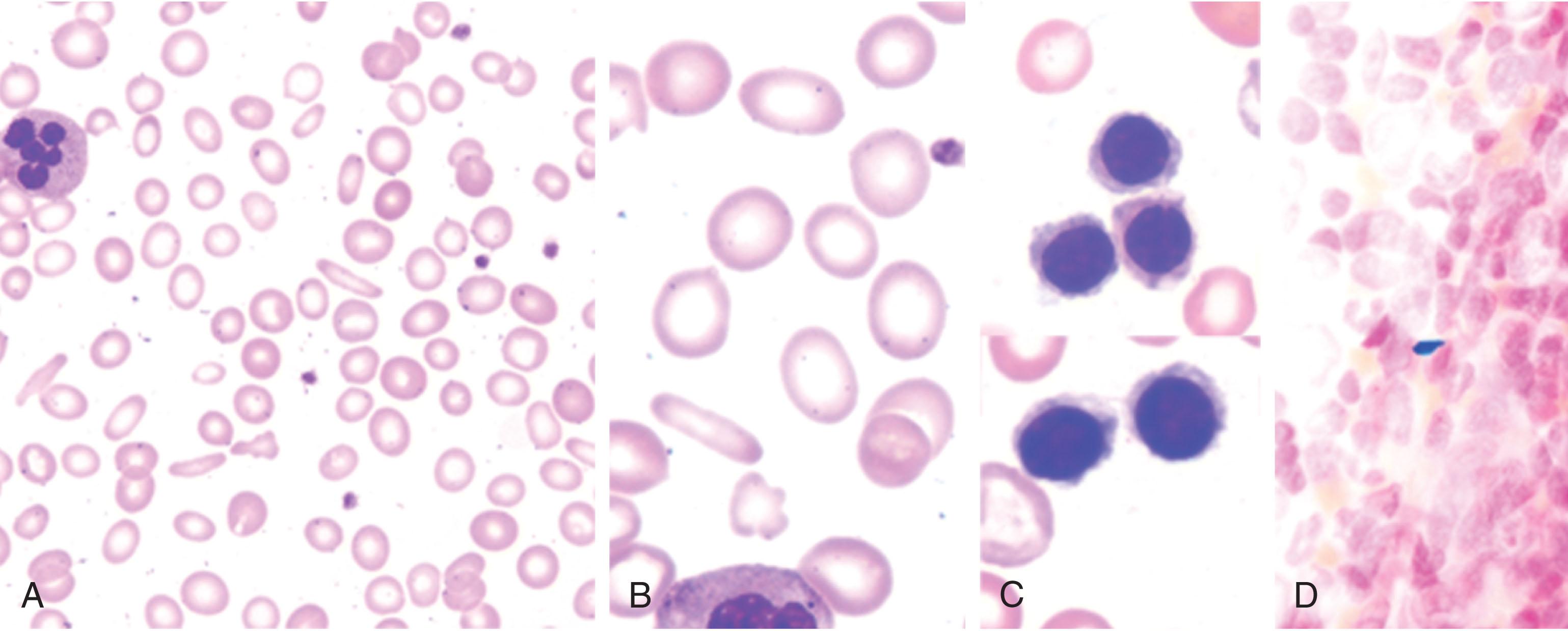
Deficiency of iron for mitochondrial function is likely the most important event that affects skeletal muscle, cardiomyocytes, and brain. The hematological effects of iron deficiency are easily recognizable. Reduction of transferrin bound iron impairs the iron uptake by maturing erythroblasts and anemia develops, paralleling the severity of iron deficiency. Initially red cell count is preserved, although erythrocytes become smaller with reduced Hb content ( Fig. 37.2 ).
Signs of iron deficiency may be evident in epithelial cells that require iron for continuous renewal; the recognition of the consequences of iron deficiency in other organs is more difficult in the lack of specific laboratory tests.
In inflammation the enhanced expression of hepcidin induced by pro-inflammatory cytokines like IL-6 withholds iron in macrophages and decreases plasma iron. Hypoferremia coupled with inappropriate EPO production impairs erythroid activity (see Chapter 36 ). When true iron deficiency develops in the context of inflammation, adaptation to iron deficiency prevails and hepcidin decreases, provided the inflammatory trigger is not too powerful.
Patients with iron deficiency anemia or isolated iron deficiency are often asymptomatic or show non-specific symptoms and come to medical attention because of abnormal blood tests. In other cases symptoms are directly related to anemia or to the underlying disorder responsible for iron deficiency. In young individuals iron deficiency anemia may go unnoticed until severe, because slow development allows cardiovascular adaptation mechanisms, promoting increased cardiac output and thus maintaining tissue oxygenation. On the contrary, anemia in the elderly may trigger dyspnea, palpitations, or angina due to ischemic cardiomyopathy, heart failure, and/or arrhythmias.
Complaints such as pallor, fatigue, weakness, headache, and dizziness are common but nonspecific. Epithelial signs of iron deficiency such as frail and broken nails, koilonychia, alopecia, atrophic glossitis, angular cheilitis, and rarely dysphagia, due to Plummer-Vinson esophageal webs, are more specific but occur almost exclusively in severe longstanding deficiency. Pica is a compulsive behavior to eat non-nutritive substances such as clay, paper, and ice (pagophagia); this rare, suggestive symptom may worsen iron deficiency. Restless leg syndrome, a distressing condition of uncontrollable leg movements, likely reflects a neurological iron deficiency. Elderly patients may present with tachycardia, increased cold sensitivity due to peripheral vasoconstriction, high systolic with low diastolic blood pressure in the context of a high-output syndrome when anemia is severe. Cardiac ejection murmurs may be present at heart auscultation.
Decreased attention and concentration ability is common in school-age children and adolescents, while in the elderly iron deficiency may accelerate cognitive decline. Decreased physical performance, exercise tolerance, and work productivity ascribed to iron deficiency in adults have important socioeconomic impact. Iron deficiency in pregnancy may have deleterious effects on both mother and fetus, with adverse outcomes including increased risk of preterm labor and low neonatal weight.
A correct diagnosis of iron deficiency and iron deficiency anemia should always include the investigation of the cause. The cause can be “physiologic” as in case of high, unmet iron needs of children and young menstruating or pregnant women. However, even in these cases a careful history and physical examination should be taken to exclude unlikely pathological causes. In males, postmenopausal females, and the elderly it is mandatory to exclude gastrointestinal blood losses; in young females a search for a gastrointestinal cause is recommended in symptomatic, iron unresponsive or relapsing cases. In the presence of comorbidities and especially in old individuals, discontinuation of drugs that interfere with iron absorption or favor blood loss has to be considered whenever possible.
The detection of iron deficiency superimposed on the anemia of inflammation may be challenging because symptoms of the underlying disorder may prevail, mask iron deficiency anemia, or cause fatigue, the same cardinal symptom of anemia. Usually the diagnosis is based on laboratory tests.
Anemia, defined by WHO criteria as Hb < 13 g/dL in males, < 12 g/dL in females, and < 11 g/dL in pregnant women, is absent in isolated iron deficiency; when present it may be mild, moderate, or severe. The absolute reticulocyte count is low, consistent with decreased erythrocyte production.
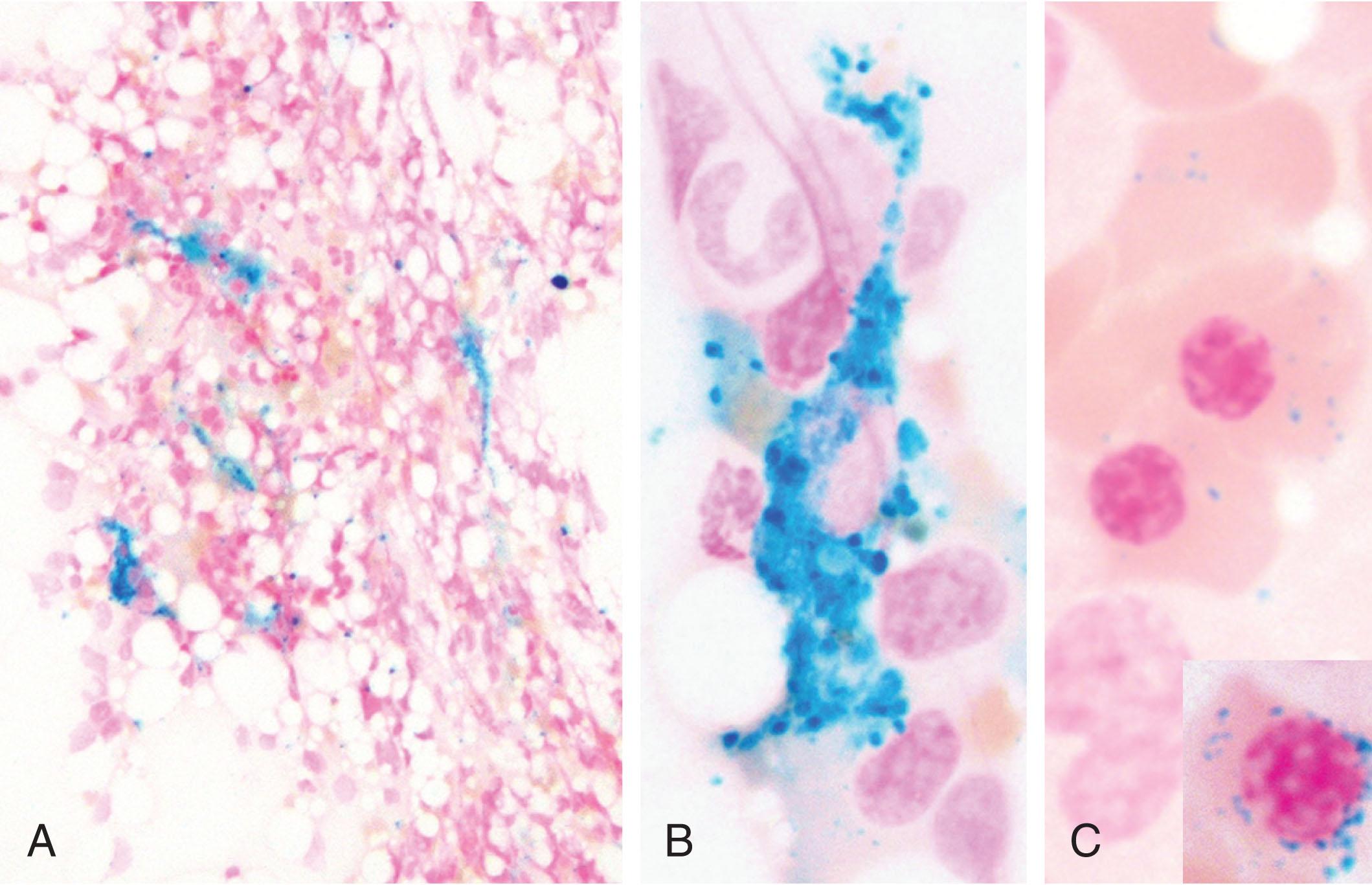
Circulating ferritin is an indirect estimate of body iron stores; it is an important tool to evaluate iron disorders in general, and especially iron deficiency. In the absence of inflammation serum ferritin <30 μg/L appears to be the most reliable and cost-effective test for the diagnosis of iron deficiency. The cut-off of 30 μg/L has 92% sensitivity and 83% specificity, as established comparing ferritin levels with bone marrow Perls’ staining ( Fig. 37.3 ), the recognized gold standard for iron deficiency, although these results were obtained in a small study. Isolated iron deficiency without anemia is defined by serum ferritin <30 μg/L and normal Hb levels. Serum iron is reduced, while total iron binding capacity (TIBC), which measures the functional capacity of transferrin as iron carrier, or transferrin levels are increased, resulting in decreased transferrin saturation (the ratio of iron on TIBC). However, as discussed below transferrin saturation may be low also in anemia of inflammation. Thus for a precise evaluation of an individual case and for the differential diagnosis with other conditions characterized by altered iron parameters it is important to assess both serum ferritin and transferrin saturation.
When anemia develops serum iron and transferrin saturation are even lower than in isolated iron deficiency. Usually transferrin saturation is <16 % and serum ferritin <12 μg/L. However, the use of ferritin <30 μg/L as the lower limit of normal captures most cases with the exception of some situations in the elderly. Thresholds of serum ferritin to diagnose iron deficiency in the elderly should be higher, considering concomitant morbidities and inflammation.
Erythrocyte morphology on peripheral blood smear inspection is abnormal, with small, hypochromic erythrocytes showing the pale central area larger than usual (see Fig. 37.2 ). Automated counters better assess microcytosis and hypochromia by providing mean MCV (mean corpuscular volume), MCH (mean corpuscular hemoglobin), and MCHC (mean corpuscular hemoglobin concentration) values. Erythrocyte indexes are not altered in all cases. Reduction of MCV (<80 fl), MCH (<27 pg), MCHC (<32 g/L) and increased hypochromic red cells (HYPO, with MCH < 28 pg) are relatively late changes compared to iron parameters, due to the long erythrocyte lifespan. The same applies to the increased red cell distribution width (RDW), an index of anisocytosis, indicating the presence of erythrocytes of different size ( Table 37.2 ). Iron deficiency anemia of recent onset is usually normocytic and normochromic and only Hb content of reticulocytes (CHr) may be reduced (<27 pg). This early indicator of iron deficiency may signal the need of iron supplementation after treatment with ESA, while an early increase in CHr (day 4) indicates a positive response to iron treatment. The percentage of circulating hypochromic red cells (>6%) is a sensitive marker of iron deficiency in patients with chronic kidney disease; experience in other conditions is limited. Erythrocyte indexes can be deceitfully normal in case of combined iron and vitamin B 12 or folic acid deficiency.
| IDA | IRDA | AI | IDA+AI | Normal Values | |
|---|---|---|---|---|---|
| Serum iron (μMol/L) | Low | Low | Low | Low | 10–30 |
| Tsat (%) | <16 | <10 | N/<20 | <20 | 16–45 |
| Serum ferritin (μg/L) | <10 | N/low | >100** | <100** | 30–200(F) 40–300(M) |
| Hb (g/dL) | Low | Low | Low | Low | >12 (F) > 13 (M) |
| MCV (fL) | <80 | <75 | N/<80 | <80 | 90 ± 10 |
| MCH (pg) | <27 | Very low | N/low | Low | 27–34 |
| CHr (pg) | Low | Low | Low | Low | 31.2 ± 1.6 |
| ZnPP (μmol/mol heme) | >80 | >80 | 60–80 | >80 | <60 |
| sTFR | High | High | N/low | N/high | a |
| sTFR/log ferritin | >2 | - | <1 | >2 | 1 |
| Serum hepcidin | Very low | N/high | High | N | a |
| Iron in BM Perls staining | Absent | + | +++ | + | +/++ |
In all cases of iron deficiency, zinc is preferentially chelated into protoporphyrin-IX (PPF). Elevated ZnPPF levels (>80 μg/dL) persist throughout the lifespan of erythrocytes, providing a screening test for iron deficiency. However, ZnPPF levels may be elevated in other conditions of low iron utilization, such as anemia of inflammation, hemolytic and sideroblastic anemia, and in chronic lead and other heavy metal poisoning.
Iron deficiency increases transferrin receptor (TFR) synthesis in all cells. However, when TFR is not stabilized by its ligand because diferric transferrin is low/absent, its extracellular domain is shed from the membrane and released into the circulation as a soluble component (sTFR). Erythroid precursors that express the largest number of receptors are the major contributors to sTFR concentration. Although its function remains unknown, serum sTFR levels measured by ELISA provide a sensitive and quantitative measure of iron deficiency (see Table 37.2 ) in the absence of erythroid hyperplasia. Unfortunately the use of this test is limited by the scarce availability and the absence of standardization and harmonization in different laboratories.
The diagnosis of iron deficiency in the setting of chronic inflammatory disorders is challenging since ferritin may increase as an acute phase protein. No specific test assesses tissue iron deficiency when ferritin levels are unreliable. It is generally agreed that iron deficiency can be ruled out only when ferritin levels are >100 μg/L; a higher cut-off to diagnose iron deficiency (<300 μg/L) is accepted in special conditions, such as chronic heart failure, provided that transferrin saturation is low (<20%). Inflammation is characterized by hypoferremia, hyperferritinemia, and normal/decreased levels of sTFR (see Table 37.2 ). The shedding of the receptor persists at an elevated level when inflammation is associated with iron deficiency. The discrepancy between levels of sTFR (high in iron deficiency and normal/low in inflammation) and serum ferritin (low in iron deficiency and normal/high in inflammation) can be utilized to calculate the ratio between sTFR and log-transformed serum ferritin. This ratio is 1 when iron is normal, <1 in anemia of inflammation, and >2 in iron deficiency and in anemia of mixed type due to both iron deficiency and inflammation (see Table 37.2 ), representing a valuable diagnostic tool. Unfortunately, the reported limitations with regard to standardization present an obstacle to routine use.
Prussian blue (Perls’) staining directly assesses iron stores revealing the presence/absence of iron in bone marrow macrophages (see Fig. 37.3 ) and offering a semi-quantitative grading of iron. Absence of bone marrow iron at Perls’ staining is still considered the reference test for iron deficiency. However, the test is rarely used nowadays, unless other disorders that require bone marrow aspiration are suspected. Hepcidin levels are low/undetectable in absolute iron deficiency, except in patients with genetic IRIDA. The latter have low transferrin saturation, strikingly decreased MCV and MCH, and normal/high hepcidin and ferritin levels. Serum hepcidin levels are high in inflammation as are serum ferritin and C-reactive protein (CRP). Whether the hepcidin concentration may help in assessing mixed anemia (due to both iron deficiency and inflammation) is still under investigation. Table 37.2 reports the results of tests currently used to differentiate iron deficiency from other conditions characterized by altered iron parameters. In individual patients it is important to evaluate the global clinical and laboratory picture rather than relying on single tests. The efficiency of iron absorption might be tested by an oral iron challenge (see the online chapter for additional text on Iron Challenge to Test Iron Absorption ). Finally the suspected diagnosis of iron deficiency may be retrospectively confirmed by the positive outcome of a therapeutic trial of iron supplementation.
The differential diagnosis of iron deficiency anemia should take into account other microcytic hypochromic anemias and/or other conditions of altered iron tests.
Apart from lack of iron supply to erythropoiesis, microcytic erythrocytes may result from defective globin or heme production in thalassemia syndromes and congenital sideroblastic anemias respectively. Red blood cell abnormalities in iron deficiency are less severe than in thalassemia where typical target cells are present on the peripheral blood smear. MCV and MCH are less decreased and RCDW wider in iron deficiency than in thalassemia. Heterozygous carriers of β-thalassemia usually have mild or no anemia but severe MCV and MCH reduction and compensatory Hb A 2 increase at hemoglobin electrophoresis. Carriers of deletion or non-deletion α-thalassemia may be silent or have microcytic red cells depending on the severity of the molecular defect. Only tests based on α-globin gene sequencing can establish the correct diagnosis. Diagnosis of thalassemia syndromes is discussed in Chapter 41 .
Congenital sideroblastic anemia may be microcytic and hypochromic especially in males with X-linked sideroblastic anemia due to delta-aminolevulinate synthase 2 (ALAS2) mutations and in recessive forms due to mutations in the glycine mitochondrial importer SLC25A38 gene (see Chapter 39 ). However, serum iron and ferritin are high, ringed sideroblasts are present at bone marrow Perls’ staining, and siderocytes may be observed in the peripheral blood smears (see Fig. 37.2 ). Precise molecular diagnosis of sideroblastic anemia requires genetic testing. Rare atypical microcytic anemias such as atransferrinemia, mutations of DMT1, and aceruloplasminemia are associated with increased body iron (see iron overload).
Anemia of inflammation is usually normocytic unless the underlying disorder is severe, longstanding, or complicated by absolute iron deficiency. As discussed above, transferrin saturation may be normal or decreased (<20%), total iron-binding capacity low-to-normal, while ferritin is normal/high (>100 μg/L) (see Table 37.2 ). The differential diagnosis relies on the discrepancy between low transferrin saturation and high ferritin. Usually the diagnosis is suggested by both the underlying disorder and elevated CRP or erythrocyte sedimentation rate (see Chapter 38 ).
The prognosis of iron deficiency/iron deficiency anemia treated with iron compounds is excellent: most cases are responsive to iron treatment and both anemia and abnormalities of iron tests revert to normal. The ultimate outcome depends on the underlying cause and can be of concern when iron deficiency is the first symptom of a gastrointestinal cancer.
The patient’s diet has to be integrated with heme-rich food whenever possible. However, due to the low percentage of dietary iron absorbed (5% to 15% in normal subjects and about 25% to 30% in iron deficiency), the diet alone is insufficient to overcome iron deficiency and pharmacological iron has to be supplemented. Iron preparations are available for either oral or intravenous therapy.
Oral iron is the first-line therapy in stable patients with isolated iron deficiency or mild-moderate anemia. A plethora of compounds are available: the most effective and inexpensive are iron salts, such as ferrous gluconate, sulfate, and fumarate (containing from 36 to 100 mg elemental iron/tablet). Traditionally, the recommended therapy was 100 to 200 mg elemental iron daily supplemented as iron salts in divided doses at fast and between meals. However, a modified schedule may increase the efficacy and reduce the side effects of oral iron treatment. Several requirements have to be met for oral iron to be efficacious. Patients must be compliant to the treatment schedule, gastric chloride production should be adequate, duodenal mucosa should be intact, and hepcidin levels should be suppressed to allow iron export by ferroportin into plasma. Unfortunately compliance to oral iron treatment is low, because of gastrointestinal side effects and because of the treatment duration. To correct anemia and replete the exhausted stores—that means reaching a target ferritin of 50 to 100 μg/L—may require 3 to 6 months of treatment and few patients comply with such a long schedule. Nausea, epigastric discomfort, vomiting, constipation, dark stools, and metallic taste occur in 30% to 70% of the cases according to different studies, often leading to premature therapy discontinuation. Side effects are ascribed to the toxicity of non-absorbed iron on intestinal mucosa through generation of reactive oxygen species (ROS). Dose reduction or iron administration at meals may induce a better tolerance, although prolonging treatment. A further problem of non-absorbed iron concerns the modification of the intestinal microbiota, with reduction of Lactobacillae and Bifidobacteria and enhanced growth of pathogens such as enterobacteria, causing dysbiosis.
Slow-release iron formulations are better tolerated than iron salts, but less effective since beyond the duodenum iron absorption is negligible. Ferric iron is insoluble and compounds such as ferric saccharide complexes are less efficacious than iron salts. Ferric citrate, an intestinal phosphate binder, is useful in chronic kidney disease patients to correct both anemia and hyperphosphatemia. Preparations of iron plus multivitamins are useless, the exception being ascorbic acid that increases iron salts absorption.
Recent studies suggest a modified schedule of iron salts administration to increase the therapeutic efficacy and decrease adverse effects. Short-term trials with stable iron isotopes administered as iron sulfate at variable doses (from 60 to 240 mg) to iron-deficient non-anemic women showed that each dose acutely increases serum hepcidin levels, thus decreasing the fractional iron absorption of the consecutive doses up to 48 hours. The conclusion is that in iron deficiency without anemia a low iron dose (60 mg) administered as a single dose on alternate days is more effective and tolerated than higher refracted doses. Studies in iron deficiency with anemia are limited and less clear-cut; more intensive dosing (up to 100 mg/day or 200 mg on alternate days) is needed to reach adequate absorption. Thus in the presence of anemia the schedule choice may consider the rapidity of the desired response, the prolonged course in case of alternate day administration, the common adverse effects when high doses are prescribed, and in some cases also the patient’s preference.
Response to oral iron therapy is indicated by raised reticulocyte count in 1 week, enhanced reticulocyte Hb content even earlier, and increased Hb by the second week. In non-responding patients possible causes of iron refractoriness must be investigated (see box on Refractoriness to Oral Iron ).
Refractoriness to oral iron therapy is classically defined as Hb increment < 1 g/dL after 4 weeks of therapy. For practical purposes lack of Hb increase in the second week (or lack of reticulocyte Hb content increase after one week) indicates inadequate response. In non-responding patients the schedule of drug administration should be reviewed to distinguish between inappropriate treatments (compound type, insufficient iron dose, modalities of administration, premature discontinuation), real intolerance for important side effects, and real inefficacy due to reduced intestinal absorption, high hepcidin levels, and/or concomitant inflammation. In some cases it is important to reassess the diagnosis in order to exclude disorders of iron absorption, excessive bleeding, or other causes of microcytic anemia. Helicobacter pylori infection should be eradicated in iron-resistant cases to improve iron absorption. Iron refractoriness in celiac disease may revert after gluten-free diet and villi normalization. Refractoriness to oral iron in IRIDA, due to excessive hepcidin levels, is a model of lack of oral iron response in inflammation; both require parenteral iron treatment.
In severe anemia or when oral iron treatment is inconvenient for whatever reason, the alternative option is parenteral, intravenous, iron administration.
Indications to intravenous iron therapy have been limited in the past by the risk of severe hypersensitivity reactions with old iron compounds, especially high-molecular-weight iron dextran that is no longer available. True refractoriness, poor intestinal iron absorption due to medical or surgical pathological conditions, excessive needs not met by oral therapy (e.g., chronic bleeding not compensated by oral iron therapy), severe anemia, and need of quick recovery are established indications. The increased safety of currently available formulations has broadened the indications to parenteral iron therapy ( Table 37.3 ). Intravenous iron is more effective than oral iron in conditions of mixed anemia (iron deficiency and inflammation), such as in inflammatory bowel disease, chronic heart failure, and chronic kidney disease (CKD). In acute flares of inflammatory bowel disease iron deficiency should be exclusively treated by intravenous iron, considering the high hepcidin levels, and to avoid further mucosal damage and microbiota alteration. Patients in remission phase may respond to oral iron. Intravenous iron improves symptoms and physical performance, such as the 6-minute walk distance test, and quality of life in patients with iron deficiency and chronic heart failure with reduced ejection fraction. This occurs even independently from correction of anemia suggesting a direct effect of iron on cardiomyocyte function. Parenteral iron is more effective than oral iron in combination with erythropoiesis stimulating agents in CKD and has blood transfusions and ESA dose sparing properties that are relevant considering the negative ESA-induced cardiovascular side effects.
| Established Indications |
|
| Extended Indications |
|
The use of intravenous preparation is increasing because of the increased safety profile of available preparations. The contemporary generations of compound have a structure ( Table 37.4 ) based on a carbohydrate shell surrounding the iron core, which confers increased stability, preventing premature iron release. On one hand this reduces the acute adverse effects ascribed to labile free iron released in the circulation and on the other it allows high-dose infusion in a single injection.
| Compound | Single Dose (mg) | Total Replacement in One/Two Infusions | Maximum Dose (mg) | Infusion Time (min) |
|---|---|---|---|---|
| Iron gluconate | 125 | No | 125 | 60 |
| Iron sucrose | 200 | No | 200 | 15–60 |
| LMW dextran iron | 100 | Yes | 1000 | 60 |
| Ferumoxytol | 510 | Yes | 1020 a | 15 |
| Ferric carboxymaltose | 750–1000 | Yes | 750 –1000 b | 15 |
| Ferric derisomaltose c | 1000 | Yes | 1000 | 15 |
a Alternatively two infusions of 510 mg one week apart.
b Up to two 750 mg or 1000 mg doses, according to body weight at least one week apart.
The currently available preparations have equivalent efficacy. The most important difference is between low-dose compounds that require repeated infusions and high-dose compounds. The latter have gained popularity because the total iron amount needed is replaced in one/two infusion(s) with a good safety profile (see Table 37.4 ). The dose of iron to be replaced with multiple infusions is calculated based on body weight, the Hb deficit plus the amount (calculated in 500 mg) needed to restore iron stores. For the high-dose compounds a simplified formula is used based on Hb levels and body weight (see the online chapter for additional text on Intravenous Iron Treatment: How to Calculate the Correct Dose ). Since Hb increase and stores reconstitution occur more promptly with high-dose preparations these compounds are convenient when a rapid recovery is necessary or in patients with limited hospital access; the main disadvantage is the high cost, which is nevertheless balanced by reduced hospital accesses. In practice the choice of the drug, besides what is approved/available at different centers, often depends on the clinical status such as age, sex, longstanding vs recent onset or recurrent anemia, the reversibility of the underlying cause, cost evaluation, and ultimately also patient’s preference.
In general, intravenous iron supplementation is safe. Minor/moderate infusion reactions (nausea, pruritus, urticaria, flushing, back or thoracic pain) often self-limited with infusion interruption are reported in 1:200 infusions with different preparations. Delayed reactions (headache, myalgia, arthralgia) may occur up to 48 hours after the infusion. Severe reactions (hypotension, dyspnea) are infrequent, estimated around 1:200,000. Guidelines to minimize the infusion risk are available and training of the personnel is important (see the online chapter for additional text on How to Manage Hypersensitivity Reactions to Intravenous Iron ).
Contraindications to intravenous iron are limited. Immediate discontinuation is required in acute infections since microorganisms may use iron as a growth factor. Intravenous iron should not be used in the first trimester of pregnancy because of the lack of safety data and in patients with previous severe hypersensitivity reactions. Of some concern is the incidence of hypophosphatemia in patients treated with ferric carboxymaltose, an incidence significantly higher than with other preparations. Hypophosphatemia alteration is usually mild, transient, and asymptomatic. Occasionally, after repeated doses, it persists and causes osteomalacia with bone pain and risk of fractures. Phosphate control is recommended in the rare patients receiving multiple injections of high-dose ferricarboxymaltose.
Concerns exist about overcorrecting iron depletion with intravenous iron in subjects with heart failure, CKD, or inflammatory conditions. Possible late complications of excess iron in terms of increased risk of oxidative stress and atherosclerosis are not assessed in available trials. Increased susceptibility to infections in CKD patients who receive chronic iron therapy is controversial; a recent large trial of prolonged iron treatment in hemodialysis patients showed that the infection rate was similar irrespective of the high or low iron dose used. In the choice of oral vs intravenous treatment, risks and benefits should be evaluated on an individual basis.
Red blood cell transfusions should not be used to treat iron deficiency anemia unless anemia is life threatening or patients are at risk of cardiac or cerebral ischemia.
Prevention strategies should target populations at risk, especially children and pregnant women. Diet changes implementing heme-iron rich food and avoiding non-bioavailable iron-containing food are ideal, but unrealistic in some contexts. Food (flour, rice, or others) iron fortification proved effective in preventing iron deficiency in children in different world areas. Supplementation with 30 to 60 mg elemental iron for 3 months/year as prevention of iron deficiency in young women and lower adapted doses in children have been recommended by WHO in areas with high prevalence of anemia. These protocols are not recommended in regions endemic for infection, such as malaria, unless supplementation is associated with antimalarial treatment, since iron may potentiate Plasmodium replication.
A type of prevention of iron deficiency anemia involves the screening of anemia before elective surgery and/or the use of intravenous iron to increase Hb and iron stores before major surgical procedures (e.g., orthopedic or abdominal) predicted to induce heavy blood loss. Several centers worldwide are implementing perioperative intravenous iron use under general programs of “Patient blood management.” The aim is to limit both blood losses during surgery and the consequent need for blood transfusions to treat postoperative anemia, because these are associated with infections and negative outcome in high-risk interventions.
Novel iron preparations with high efficacy and low side effects are needed to render prolonged oral treatment more feasible. The efficacy of the alternate day protocol should be explored for extended periods of time and more extensively evaluated in iron deficiency anemia. Extended follow-up of patients treated with high-dose intravenous iron should clarify possible long-term side effects. In patients treated with ESA or in the setting of inflammatory conditions, new compounds able to mobilize endogenous iron sequestered in macrophages could eventually replace intravenous iron. The availability of multiple options for iron therapy will offer the best-personalized treatment to individual iron-deficient patients.
Iron overload is an increase of total body iron resulting from iron supply in excess of iron requirements. Mild body iron increase is clinically silent; clinical complications due to iron toxicity are usually evident when iron exceeds about 4 to 5 g total body content. This may occur in hereditary hemochromatosis, in iron-loading anemias, and in all patients receiving blood transfusions for chronic anemia such as thalassemia major and other congenital or acquired refractory anemias.
In hemochromatosis and iron-loading anemias excessive and deregulated iron absorption causes an increased saturation of transferrin that leads to the appearance of non-transferrin-bound iron (NTBI) in the circulation. This toxic iron species is avidly taken up by parenchymal cells of liver and other organs, while macrophages remain iron-free. In chronically transfused patients excess iron is initially safely stored in macrophages; when the storing capacity is overcome, transferrin becomes fully saturated and the increased NTBI, as in hemochromatosis, damages liver and other organs. The main causes of iron overload are summarized in Table 37.5 .
| Inheritance | Gene | Phenotype | |
|---|---|---|---|
| Genetic Iron Overload | |||
| HFE-HH (type 1) | AR | HFE |
|
| Non-HFE HH (type 2) | AR |
|
|
| Non-HFE HH (type 3) | AR | TFR2 | Low hepcidin. Early or adult-onset iron overload |
| Non-HFE HH (type 4) | AD | Gain of function SCL40A1 mutations |
|
| Ferroportin disease | AD | Loss of function SCL40A1 mutations | Macrophage iron overload |
| Genetic Iron Loading Anemias | |||
|
|
|
Microcytic anemia. Transfusion-independent iron overload |
| Congenital sideroblastic anemia (non-syndromic) |
|
|
|
|
|
|
Anemia, jaundice, splenomegaly, erythroblasts multinuclearity, iron overload |
| Atransferrinemia a | AR | TF |
|
| DMT1 deficiency | AR | SLC11A2 |
|
| Aceruloplasminemia | AR | CP | Iron accumulation in basal ganglia, liver, and spleen; diabetes, neuro-, retinal- degeneration, anemia |
| Acquired Iron Overload | |||
| Chronic blood transfusions (congenital and acquired anemias) | Iron overload requiring chelation therapy (e.g., thalassemia major) | ||
| Intravenous iron administered in excess of needs | Iron accumulation in macrophages | ||
| Acquired Iron Loading anemias | |||
| MDS RS | Clonal disorder with somatic mutations | SF3B1 | Macrocytic anemia. Ringed sideroblasts. Iron overload |
| Regional Iron Accumulation | |||
| Pulmonary hemosiderosis | Idiopathic |
|
|
|
|
|
Brain iron accumulation and neurodegeneration + cardiac iron overload |
a The term atransferrinemia is incorrect. The disease should indeed be an hypotransferrinemia since mutations in all cases do not fully suppress the protein synthesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here