Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
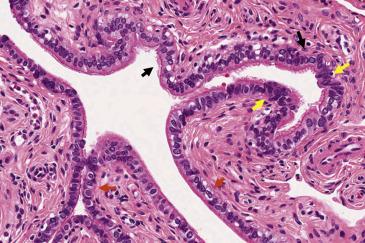
The fallopian tubes comprise the most upper portion of the Müllerian tract bilaterally. They are attached proximally to the uterus (intramural portion), whereas the isthmus and the ampulla extend outside of the uterus and are covered by peritoneum (serosa). In routine histologic preparations, the wall of the fallopian tube is seen as a hollow tubal structure with a lumen, mucosa, muscularis propria, and serosa. The most distal aspect, the fimbriated end, represents an opening of the tubal lumen directly into the peritoneal cavity, lined by Müllerian tubal epithelium and closely juxtaposed to the ovaries. The fimbrial portion of the tube is composed of multiple finger-like projections of loose connective and fibromuscular tissue. The overlying epithelium, like in the rest of the tube, is simple (nonstratified) and comprised of three morphologically distinct populations: (1) the ciliated cells with a characteristic apical eosinophilic terminal bar and a brush-like luminal border containing cilia; (2) secretory cells, which are nonciliated, have variable amounts of cytoplasm depending on the menstrual phase and ovoid nuclei; and (3) intercalated (peg) cells, which have small round nuclei and scant clear cytoplasm and are believed to represent either reserve cells or secretory cells after extrusion of the cytoplasmic secretions ( Fig. 12.1 ).
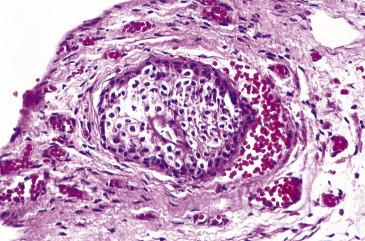
Walthard nests, seen in the tubal serosa, fimbriated end, and mesosalpinx, are composed of small solid or cystic (up to 2 cm) nests of bland stratified, transitional-like epithelium. The nuclei are ovoid to elongated and may display grooves ( Fig. 12.2 ). Walthard nests are thought to represent transitional metaplasia of the tubal peritoneum. They are typically incidental findings but on occasion may resemble granulomas intraoperatively.
The fallopian tube epithelium is of Müllerian derivation and therefore is subject to the same range of metaplastic change seen in other gynecologic sites (cervix, endometrium), although in this site, they are less frequent. Mucinous metaplasia is composed of cells with an endocervical or gastrointestinal mucinous profile, which replace the normal tubal epithelium but retain a simple architecture without stratification ( Fig. 12.3A ). Some lesions are seen in the context of Peutz-Jeghers syndrome , and search for clinical and/or pathologic features of this entity may be warranted. Another important consideration is mucinous neoplasia secondarily involving the tubal mucosa , with the ovary, endometrium, appendix, and gastrointestinal tract being the most common primaries. Any degree of cytologic atypia or architectural complexity should raise concern for metastases, and a primary tumor needs to be excluded clinically. Bland lesions are more challenging; in the context of previous malignancy or a concomitant mass in the ovary, endometrium, or gastrointestinal tract, the possibility of metastasis to the tubal mucosa should be considered. The value of immunohistochemistry in this scenario has not been fully explored.
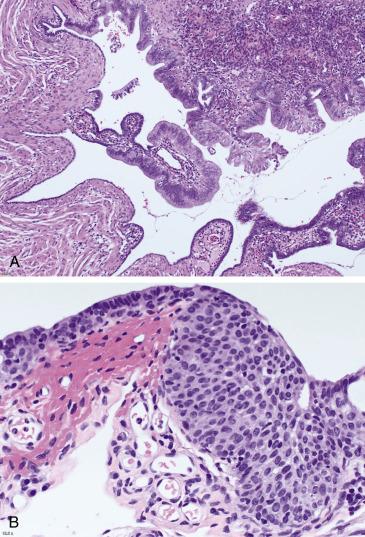
Fallopian tube metaplasias of the transitional and squamous cell type are rare and incidental. They can develop in the context of acute and/or chronic salpingitis or tubo-ovarian abscesses. These forms of metaplasia are microscopic and morphologically bland, being composed of stratified cells with ovoid nuclei containing nuclear grooves ( Fig. 12.3B ). The main differential diagnosis is tubal spread of a carcinoma with squamous differentiation , more commonly endometrial endometrioid carcinoma or cervical squamous cell carcinoma. The presence of complex glandular elements in the former and overt dysplastic cytologic features in the latter will help in the diagnosis.
By far, decidual change is the most common metaplastic change observed in the tube, being documented in 5%–12% of intrauterine pregnancies and in up to 80% of ectopic pregnancies. It can also occur after exogenous hormonal (progestin) therapy. It can be seen intraoperatively as white plaques or nodular peritoneal aggregates, although most commonly is incidental and microscopic only. Histologically, decidual change is composed of polygonal cells with abundant pale cytoplasm and uniform, round nuclei with smooth contours ( Fig. 12.4 ). These cells can form mildly expansile aggregates underneath the tubal serosa or be distributed in more haphazard sheets surrounding tubal mucosa and peritoneal infoldings. When florid, decidual reaction requires distinction from deciduoid mesothelioma and metastatic carcinoma to the fallopian tube. Unlike these conditions, decidual change does not efface the normal elements of the mucosa and lacks cytologic atypia and proliferative activity. Deciduoid mesothelioma will express markers such as keratins, CK5/6, and calretinin and will often show loss of BAP1. Metastatic carcinomas with a deciduoid appearance include breast carcinoma, urothelial carcinoma, and squamous cell carcinoma (cervix, vagina); all these lesions will show cytologic atypia as well as strong and diffuse keratin expression, unlike decidual cells. Decidual change usually regresses weeks after gestation or cessation of hormonal therapy.
Torsion of the fallopian tube usually occurs at the level of the vascular pedicle and therefore involves the ipsilateral ovary. Isolated fallopian tube torsion is, in turn, infrequent. It is more common in premenopausal women, who typically present with severe acute abdominal and/ or pelvic pain. Temporary (self-limited) torsion is usually subclinical and manifests with intermittent pelvic pain. Torsion is more likely to occur after tubal enlargement secondary to hydrosalpinx, pyosalpinx, abscess, or occlusion (e.g., ligation).
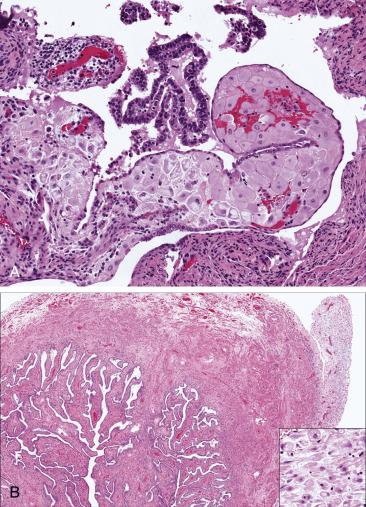
Macroscopic changes vary depending on the duration and the severity of blood flow obstruction. The tube usually appears enlarged and edematous in initial phases and frankly congested or hemorrhagic with severe and prolonged torsion ( Fig. 12.5A ). In massively infarcted tubo-ovarian specimens, it may be difficult to identify the fallopian tube and separate it from the engorged and hemorrhagic tubo-ovarian soft tissue. Generous sampling may be required.
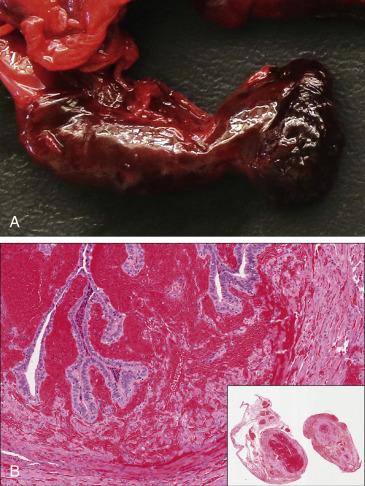
In early torsion, the tube will show edema, venous congestion, patchy hemorrhage, and acute inflammation ( Fig. 12.5B ). Established torsion will manifest microscopically as extensive hemorrhagic necrosis of the tubal wall, usually transmural. The adnexal vessels (particularly the veins) are significantly dilated and frequently contain recent or organizing thrombi. Hydrosalpinx or hematosalpinx may be seen.
Although the diagnosis is straightforward on clinical and pathologic examination in most patients, it is important to exclude a neoplasm causing tubal enlargement and secondary torsion. Even if the tissue is massively infarcted, a neoplastic proliferation will be identified microscopically in most cases. Extensive hemorrhage can also be due to trauma , endometriosis , or ectopic gestation . Unlike torsion, in these scenarios the adnexal vessels are not congested or occluded, and the tubal wall is viable.
Intermittent torsion, which is diagnosed clinically, can be treated with surgical fixation of the adnexa to the pelvic wall to prevent further episodes. Complete tubal torsion with secondary infarction requires urgent surgical excision of the tube with or without the ovary. Complications include auto-amputation of the adnexa, rupture with hemoperitoneum, and superimposed infection.
Twisting of fallopian tube around its vascular pedicle with secondary blood flow obstruction
Typically involves tube and ipsilateral ovary; isolated tubal torsion rare
Intermittent torsion causes recurring pain
Complete torsion leads to autoamputation of adnexa, rupture, hemorrhage, and superimposed infection, which can be fatal.
Predominantly seen in reproductive-age women
Acute and severe low abdominal pain
Urinary frequency
Suspension of adnexa in cases of intermittent torsion
Emergency surgery (salpingectomy or salpingo-oophorectomy) if complete torsion complicated with infarction
Edematous, congested, or frankly hemorrhagic tube
Tube may be difficult to identify if infarcted.
Edema, congestion, patchy hemorrhage, and acute inflammation in early phases
Transmural hemorrhagic necrosis, marked vascular congestion, and thrombosis if severe torsion with complete vascular obstruction
Tubal neoplasm with secondary infarction
Hematosalpinx (due to ectopic pregnancy, endometriosis, trauma)
Salpingitis isthmica nodosa (SIN) is a disorder characterized by nodular swelling of the isthmic portion of the fallopian tube caused by diverticula of tubal epithelium into the muscular wall. The pathogenesis and etiology of this disease remain unclear; some authors have postulated a congenital origin, whereas others favor an acquired process, possibly postinflammatory. SIN most commonly occurs in women between 25 and 60 years of age (average age at diagnosis is 30 years) and is more frequent in black women. The incidence of SIN in the general population ranges from 0.6% to 11%; however, in tubes with ectopic gestation, its prevalence has been reported as high as 49%. The association of SIN with ectopic pregnancy and infertility is well documented; in the former, SIN is believed to trap the fertilized ovum within the fallopian tube, whereas in the latter, it interferes with upward sperm migration. The lesion is otherwise asymptomatic and discovered incidentally during work-up for infertility or ectopic gestation.
Hysterosalpingography, a common technique in the work-up of infertility, is reliable in the diagnosis of SIN, which is seen as small globular collections within the tubal wall, either discontinuous or in continuity with the tubal lumen. Tubal obstruction and hydrosalpinx are commonly seen as well. Other techniques include laparoscopic chromopertubation, salpingoscopy, and transvaginal hydrolaparoscopy (the latter allows visualization of the tubal mucosa).
SIN is typically located in the isthmus but can, on occasion, involve the ampulla or the interstitial portions of the tube. Two-thirds of the cases occur in the right tube and one-third in the left; only ∼4% are bilateral. SIN appears as nodular protrusions on the tubal serosa (which is otherwise smooth), each measuring up to 2 mm and usually spanning an area of up to 2 cm. On cut section, visibly dilated diverticular structures can be observed within the wall. Hydrosalpinx is also commonly seen.
Diagnosis of SIN can be confirmed only on histopathologic examination, which requires a well-oriented complete cross-section of the isthmic region of the tube. On scanning magnification, SIN is characterized by glandular “outpouchings” of varying sizes within the tubal muscularis propria, with a distribution resembling adenomyosis of the uterus or even an infiltrative glandular proliferation ( Fig. 12.6 ). On high-power examination, the glandular structures are lined by benign tubal epithelium, and connection of the intramural epithelial diverticuli to the mucosa can be sometimes appreciated. The surrounding smooth muscle can be hypertrophic. The tubal serosal surface is uninvolved.
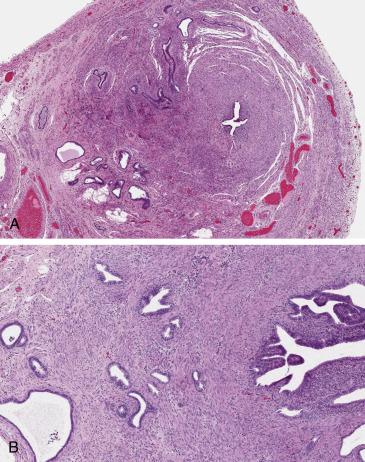
The differential diagnosis of SIN, given mostly its architectural low-power characteristics, includes chronic follicular salpingitis, endometriosis, and adenocarcinoma. Follicular salpingitis produces small glandular spaces in the submucosa, separated by lymphoplasmacytic inflammatory infiltrates and reactive fibroconnective tissue; however, there is no smooth muscle in between glands. Tubal intramural endometriosis can mimic SIN since it can be extensive and haphazardly distributed throughout the wall; however, the diagnosis of endometriosis requires the presence of endometrial-type stroma, which is absent in SIN. Adenocarcinoma involving the tube, primarily or secondarily, can mimic SIN; adenocarcinoma will show a frankly infiltrative and irregular proliferation, desmoplastic stromal reaction, and overt malignant cytologic features.
Once suspected clinically and radiologically, patients with infertility and SIN can be managed with segmental resection with tubo-cornual anastomosis, and recanalization if tubal obstruction is detected. Success with gonadotrophin-releasing hormone analogues (GnRH-a) has been documented in terms of remission of nodularity and tubal patency. If fertility preservation is not desired, salpingectomy is recommended.
Nodular enlargement of proximal fallopian tube due to diverticuli
Uncertain etiology
0.6%–11% in healthy women
Up to 49% with ectopic pregnancy
Preference for isthmic portion
More common in right tube
Predisposes toward ectopic gestation and infertility
Marked predilection for black women
25 to 60 years (average 30 years)
Found incidentally, during work-up for infertility or at time of surgery for ectopic pregnancy
Hysterosalpingography shows diverticular outpouchings of mucosa into the muscularis.
Surgical excision with re-anastomosis, recanalization, and medical treatment (GnRH-a) are options for infertile patients.
Resection (salpingectomy) if fertility no longer desired
One or more nodules 1–2 mm, spanning up to 2 cm
Smooth serosa
Glandular epithelium within tubal muscularis propria, in continuation with mucosa or (more commonly) discontinuous
Haphazard distribution (akin to adenomyosis) or pseudoinfiltrative
Banal epithelium with tubal differentiation
Chronic follicular salpingitis
Endometriosis
Invasive adenocarcinoma
Early gestations implanting outside the uterus can involve the ovary and other pelvic or abdominal organs. However, the fallopian tube is the site of the vast majority (∼95%) of ectopic pregnancies. Most cases involve the ampulla (∼80%), followed by the isthmus (∼10%) and infundibulum (∼5%). Abnormalities impairing the passage of the fertilized ovum to the uterine cavity, and therefore predisposing to ectopic pregnancy, include salpingitis and pelvic inflammatory disease (PID), endometriosis, prior tubal surgery, as well as structural tubal abnormalities such as SIN or congenital defects. Another mechanism is retrograde transit of the fertilized egg from the uterus into the tube, supported by the observation that in some cases the ectopic gestation occurs contralateral to the ovary with the corpus luteum of pregnancy. A significant number of ectopic pregnancies resolve spontaneously and are usually silent clinically. If the implanted gestation secures enough blood supply from the tube, it will grow causing hemorrhage, progressive distention, and eventual wall rupture. Thus, symptomatic ectopic gestation manifests with amenorrhea, shortly followed by abnormal vaginal bleeding and acute pelvic or lower abdominal pain. In ectopic pregnancy, β-hCG serum levels are lower and rise at a slower rate compared to uterine gestations.
Transvaginal ultrasound is used to evaluate the uterine cavity in search for a gestational sac, or in turn for the presence of an adnexal mass. Well-developed ectopic gestations will appear as a sac, sometimes with a visible embryo, associated with a thick or irregular tubal wall. If rupture has occurred, fluid in the cul-de-sac can be apparent.
The fallopian tube will show an area of dilation associated with attenuation of the wall, congestion, and hemorrhage. In some cases, blood clot is present either on the external surface of the tube or detached. Sometimes, a disruption of the wall with irregular hemorrhagic edges is seen, which needs to be documented as it may represent the point of rupture. Blood oozing from the distal (fimbriated) end can be apparent. On cut surface, the distended area corresponds to hematosalpinx, in which placental and/or fetal tissues may be observed ( Fig. 12.7A ). Sampling needs to incorporate all abnormal areas of the tube, including possible sites of rupture, as well as detached blood clot, as it may signify an ectopic gestation extruded from the tube after rupture or intraoperatively. Submitting the entire specimen may be required, particularly if grossly normal.
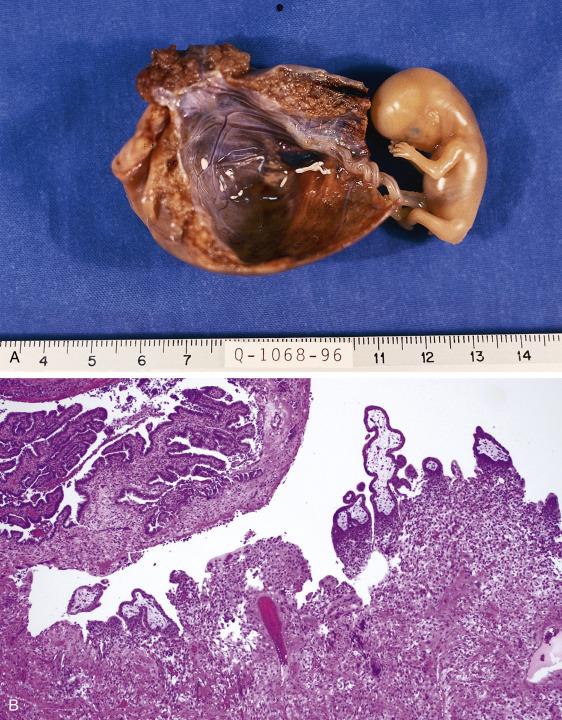
The diagnosis of ectopic tubal pregnancy requires the presence of products of conception within the fallopian tube. Chorionic villi and/or (rarely) embryonic tissues are mostly seen within the lumen embedded in blood. The presence of implantation site trophoblast involving the tubal mucosa, muscularis, and/or serosa is confirmatory ( Fig. 12.7B ). Trophoblastic infiltration can be prominent, as in the absence of decidua, implantation site trophoblast will continue proliferating through the wall. If rupture has occurred, the wall will be markedly distended, thinned, and effaced by trophoblast, often associated with hemorrhage, necrosis, and fibrin deposition. Attention to the villous morphology is important, as molar gestations can also occur ectopically (see Chapter 19 ).
In patients with clinically suspected ectopic pregnancy, abundant placental and/or embryonic tissue should be identified in the tube. If these are absent or only scant and there is no trophoblast infiltrating the wall, the possibility of intrauterine gestation or ectopic pregnancy outside of the tube must be considered and clinically excluded. A largely distended and hemorrhagic fallopian tube may also be seen in tubal torsion and endometriosis ; in these scenarios, gestational tissue is absent and the clinical features of ectopic pregnancy, such as abnormally rising β-hCG, are absent.
Complications (rupture, bleeding, or placental invasion of pelvic organs) can be life-threatening; however, early diagnosis can be currently achieved clinically and radiologically in most cases, resulting in very low mortality rates (<0.1% in the last 30 years in the United States). Treatment of patients with small, noncomplicated tubal gestations is medical with methotrexate and close observation. Those who progress despite pharmacologic treatment or present with imminent or established rupture and hemoperitoneum require salpingectomy. Since predisposing factors are mostly unilateral, most women can achieve a normal pregnancy after having an ectopic tubal gestation.
Pregnancy in which the fertilized egg implants in fallopian tube
>95% cases occur in fallopian tube.
80% occur in ampulla, 10% in isthmus, and 5% in infundibulum.
Some ectopic tubal gestations regress and abort spontaneously.
If persistent, they almost invariably lead to hemorrhage and perforation, which can be life-threatening.
Sexually active, reproductive age women (15–24 years)
Amenorrhea followed by vaginal bleeding
Acute lower abdomen or pelvic pain
Transvaginal ultrasound shows adnexal mass/sac and absence of intrauterine gestation.
Most cases diagnosed early and managed successfully with methotrexate or surgical excision (salpingectomy)
Area of distention, thinning of wall, congestion, and hemorrhage
Luminal blood mixed with placental tissue on cut section
Point of rupture may be seen as irregular disruption
Intraluminal products of conception (chorionic villi, extravillous trophoblast, and rarely embryonic tissue)
Trophoblast infiltration into tubal wall, associated with hemorrhage and effacement
Products of conception may be present within clotted blood (which can be detached)
Gestation can be normal or aneuploid (molar)
Intrauterine gestation
Ectopic pregnancy outside of tube (ovarian, peritoneal)
Tubal torsion
Tubal endometriosis
Endometriosis frequently involves the fallopian tube: it is seen in up to 10% of tubal resection specimens. It predominantly affects women of reproductive age. In this population, it is a prevalent cause of dysmenorrhea, chronic pelvic pain and infertility, which are related to chronic bleeding with subsequent inflammation, scarring, adhesion formation, and progressive tubal obstruction. It is postulated that endometriosis is caused by retrograde flow of endometrial cellular components and blood during menstruation. The pathogenesis of this disease is, however, still uncertain.
Intraoperatively, endometriosis commonly appears as dark blue to brown nodules or patches involving serosal surfaces and connective tissues. They range in size from 1 mm or less to 1–2 cm in diameter. Large lesions can be cystic or have a solid polypoid appearance. Tubal endometriosis is more commonly seen in the distal end.
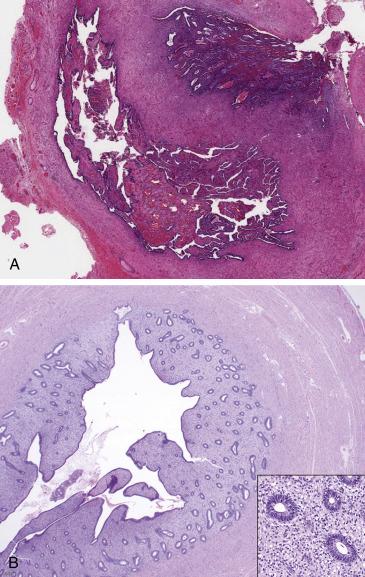
Tubal endometriosis is seen in the form of endometrial elements (glands and stroma ± signs of hemorrhage) present in the fallopian tube serosa or muscularis ( Fig. 12.8A ). Involvement of the tubal mucosa by endometrium can also be seen as a form of endometriosis, although it may also represent colonization of the tubal epithelium, which is more common in the proximal aspect of the tube and has been associated with tubal ligation and complete occlusion of lumen ( Fig. 12.8B ). Endometriosis can undergo decidualization or Arias-Stella reaction depending on the hormonal background.
Macroscopically, diffuse involvement of the tube by endometriosis or a large endometriotic cyst can be confused with tubal torsion, abscess, or ectopic gestation, if the clinical context is unknown. Correlation with the clinical and histopathologic findings will clarify the diagnosis. It is important to note that endometriosis can be complicated with superimposed infection and abscess formation. The distinction between endometriosis and SIN was discussed earlier. Other important entities in the differential include endometrioid adenocarcinoma involving the tube and Müllerian adenosarcoma. Well-differentiated endometrioid carcinoma of either endometrial or primary tubal origin may resemble endometriosis; most cases, however, will display significant expansile or infiltrative growth and architectural complexity indicative of a malignant process (glandular confluence, cribriform, microacinar, or papillary architecture). Müllerian adenosarcoma should be considered if the lesion is predominantly solid and polypoid; attention to the classic architecture (leaf-like appearance, periglandular stromal condensation) and the presence of stromal cytologic atypia will aid in the diagnosis.
Endometriosis is a morbid disease; infertility, chronic pelvic pain, and endometriosis-associated malignancy are its most important complications. Hormonal treatment is the mainstay of therapy, which has variable long-term responses. Surgical approach may be indicated in order to determine the extent of the disease and in the setting of large or refractory lesions. In the management of infertility, resection of endometriosis with lysis of tubal adhesions and intraluminal probing to assess for patency also has a role.
Endometrial-type glands and stroma in fallopian tube mucosa, muscularis, or serosa
Occurs in approximately 10% of resected fallopian tubes
Often distal end of fallopian tube
Reproductive years
Major cause of infertility and chronic pain related to scarring, obstruction and adhesions
Potential for development of Müllerian neoplasia
Dysmenorrhea
Pelvic pain
Infertility
Hormonal suppressive treatment
Surgery in patients with large or refractory lesions
Small dark blue/brown serosal nodules
Large lesions can be solid or cystic (hemorrhagic/chocolate cyst).
Endometrial-type glands and stroma ± hemorrhage
Serosal, intramural, or mucosal location (latter regarded as direct extension of endometrium into tube)
± decidual or Arias-Stella reaction
Salpingitis isthmica nodosa
Endometrioid adenocarcinoma
Low-grade Müllerian adenosarcoma
Tubal epithelial hyperplasia has been reported in a significant proportion (∼18%) of fallopian tubes, although in the vast majority, the hyperplasia is mild (and likely largely unnoticed in routine practice). When florid, hyperplasia can mimic fallopian tube carcinoma, hence the term “pseudocarcinomatous hyperplasia” used by some authors. Florid hyperplasia is associated with exogenous or endogenous estrogenic stimulation, concurrent ovarian neoplasms and salpingitis. The latter has been documented in ∼50% of tubal hyperplasias and is a consistent feature of cases at the higher end of the spectrum (pseudocarcinomatous hyperplasia), in which the inflammation tends to be severe and related to PID. Patients are typically young and premenopausal (mean 28.6 years, range 27 to 40 years).
The fallopian tubes are either grossly unremarkable, mildly enlarged or, in patients with severe salpingitis and PID, complicated with tubo-ovarian adhesions and abscess formation. The lumen is usually indistinct but can be dilated. There is no distinct mass or tumor formation.
Florid epithelial hyperplasia has a striking low-power appearance with numerous plicae occupying the tubal lumen and fusing together, which results in complex (cribriform, pseudoglandular, or sieve-like) patterns. Moreover, the epithelial proliferation may distend the lumen and compress the surrounding muscularis. Cytologically, the hyperplastic epithelium is bland or shows at most moderate nuclear atypia and pseudostratification as well as only occasional mitotic figures ( Fig. 12.9A ). A dense and diffuse chronic inflammatory infiltrate admixed with the epithelium is consistently present ( Fig. 12.9B ). Associated features of PID (acute salpingitis, tubo-ovarian abscess) can be observed.
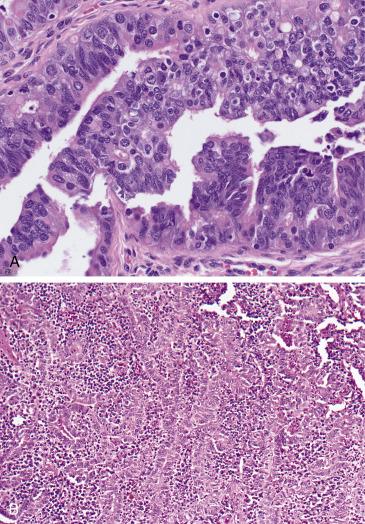
The most important differential diagnosis of pseudocarcinomatous hyperplasia is carcinoma , particularly endometrioid or serous. Patients with tubal carcinoma tend to be older and present with a mass. Histologically, overt and destructive invasion into the wall will also be in keeping with a malignant process. High-grade serous carcinoma (HGSC) will show significant stratification and micropapillary tufting, loss of polarity, severe nuclear atypia, and conspicuous mitotic activity. Although the immunohistochemical profile of florid hyperplasia has not been thoroughly documented in the literature, an abnormal p53 expression pattern is more consistent with a malignant process. Endometrioid carcinoma may display villoglandular and cribriform architecture similar to tubal hyperplasia; however, it will also display endometrioid features (squamous, mucinous or secretory differentiation) and some degree of solid growth, which are all absent in florid hyperplasia.
By itself, florid tubal epithelial hyperplasia is a benign process. It is important to identify and report any associated pathology that may require treatment (salpingitis, abscess).
Reactive proliferation of tubal mucosa
Associated with salpingitis, PID, estrogenic stimulation, and concomitant ovarian tumor
Related to underlying process (chronic pain, discharge, systemic symptoms)
27 to 40 (mean 28.6) years
Incidental finding
Benign reactive condition
Treatment of associated pathology (e.g., infection) may be necessary
Tubes are normal or mildly enlarged
No mass lesion
Epithelial hyperplasia with elongation and fusion of plicae (cribriform and pseudoglandular appearance)
Distension of lumen and compression of muscularis can be seen
Epithelium with mild to moderate atypia and rare mitoses
Accompanying dense chronic inflammation
May be associated with tubo-ovarian abscess formation and adhesions
High grade serous carcinoma
Endometrioid carcinoma
Pelvic inflammatory disease (PID) is a clinical diagnosis defined as inflammation and infection of the upper female genital tract, and acute salpingitis is its closest biologic and histopathologic correlate. Chronic salpingitis develops after one or more episodes of acute salpingitis. Infection occurs by ascending spread of microorganisms from the lower genital tract (cervix and vagina). The most common infectious agent is Chlamydia trachomatis , which accounts for up to 60% of cases; Neisseria gonorrhoeae is also frequent and associated with more severe disease, but its incidence has declined. Other important microorganisms include Mycoplasma genitalium and anaerobes like Peptostreptococcus spp. and Prevotella spp. Coinfection with anaerobes plus chlamydia and/or gonorrhoeae can occur and is associated with severe infection.
This disease occurs in young, sexually active women (15–24 years) with risk factors related to sexually transmitted infections including multiple sexual partners, unprotected sex, smoking, and illicit drug use. In turn, oral contraceptive use is associated with lower prevalence. The gold standard in the diagnosis of PID is laparoscopic visualization of the tubes, which appear inflamed and/or suppurative. This diagnosis, however, is first suspected clinically in the presence of acute pelvic or abdominal pain, fever, urinary frequency, nausea, back pain and abnormal bleeding or discharge. Women with these symptoms should undergo pelvic examination; if there is any pelvic organ tenderness (cervical motion tenderness, uterine or adnexal tenderness) or evidence of lower genital tract inflammation (cervicitis, purulent discharge, and/or leukorrhea defined as more than one neutrophil per epithelial cell in vaginal fluid), a clinical diagnosis of PID is made and an empiric course of antibiotics is warranted.
Imaging may be used to confirm the clinical diagnosis and establish its severity. Transvaginal ultrasound is the method of choice, followed by magnetic resonance imaging. These studies will reveal thickened, fluid-filled tubes; alterations in tubal shape; septations; pelvic free fluid; and/or tubo-ovarian abscess.
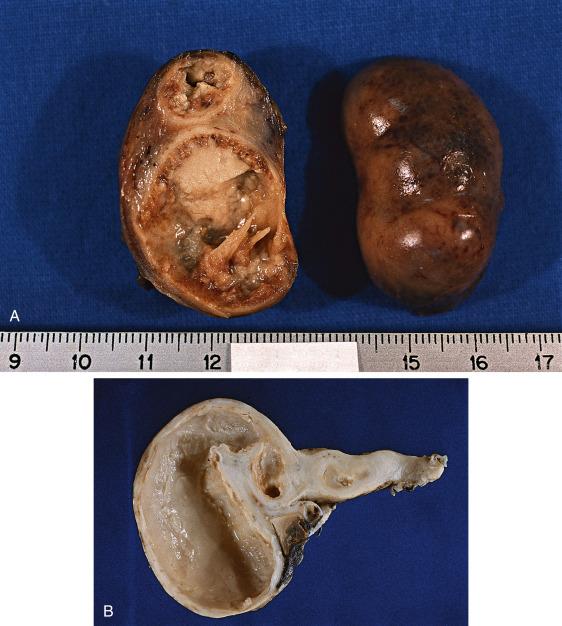
In uncomplicated cases of PID, the tubes are removed only exceptionally. The process is typically bilateral and is characterized by hyperemia of the tubal serosal surface, edema of the wall, and purulent exudate from the surface or the fimbriated ends. Upon sectioning, the wall is thickened and congested, and the lumen is distended by fluid accumulation. In patients with chronic or recurring infective episodes, areas of adhesion and scarring can be noted throughout the tube, often with secondary hydrosalpinx or pyosalpinx ( Fig. 12.10A,B ). Tubo-ovarian abscesses vary in size but can be larger than 10 cm; on cut section, they are comprised of a cavitary lesion with purulent material, thickening fibrosis, and edema encasing the adnexa.
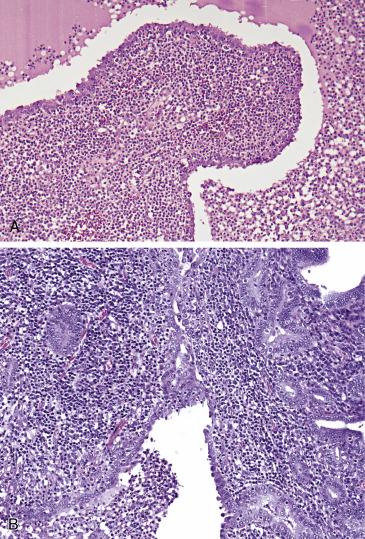
Depending on the severity, acute salpingitis is characterized histologically by neutrophilic inflammation of varying amounts and extent. It tends to be more prominent in the mucosa, associated with edema and enlargement of the tubal plicae ( Fig. 12.11A ). Florid hyperplasia of the epithelium occurs often, sometimes mimicking a malignant proliferation (“pseudocarcinomatous”) ( Fig. 12.11B ). The lumen of the tube is filled with necrotic debris and neutrophilic exudate. Tubo-ovarian abscess is characterized by confluent neutrophilic exudate and necrotic debris occupying a central cavity, in turn surrounded by reactive fibrotic tissue and prominent mixed inflammation. In chronic salpingitis, the inflammatory infiltrate is mixed with plasma cells, lymphocytes, and histiocytes in a background of fibrosis and effacement of the mucosal folds. The plicae are shortened and blunted with secondary fusion.
Endometrial biopsy is sometimes performed as part of the work-up of PID, and chronic (plasma cell) endometritis has been regarded as a supporting finding of PID (and a surrogate for acute salpingitis). As discussed in Chapter 9 , the finding of plasma cells by itself is not specific and may be encountered in endometrial polyps and anovulation; therefore, the diagnosis of endometritis requires correlation with the clinical presentation and the background endometrium; plasma cells must be found in fragments of endometrium functionalis, which often shows metaplastic epithelial changes and other inflammatory cellular components.
Obtaining cultures from tubal exudate or tissue should be considered as the first step in the examination of any surgical specimen received with a clinical impression of acute salpingitis or PID. Special stains (Gram stain) can be performed, although a negative result does not preclude the diagnosis.
Florid reactive epithelial changes associated with acute salpingitis in the setting of PID should be distinguished from in situ and invasive carcinoma . The clinical context, patient’s risk factors, young age, and the absence of a mass forming lesion will help in excluding malignancy (see “Florid Epithelial Hyperplasia” section). Other forms of salpingitis, particularly tuberculous and actinomycotic salpingitis , require appropriate diagnosis as they are treated differently. In the former, the inflammation is characteristically granulomatous, and the microorganism can be identified through AFB stain, culture, immunohistochemistry, or nucleic acid amplification; in the latter, the presence of sulfur granules (aggregates of filamentous bacteria) within the abscess exudate is diagnostic.
The sequelae of acute infectious salpingitis and PID include tubal scarring and adhesion to other pelvic structures and chronic salpingitis, which predispose patients to ectopic pregnancy, infertility, and chronic pelvic pain. The risk of these complications increases with the number and severity of episodes of salpingitis. In developed countries, early access to care and antibiotic treatment has greatly decreased the rate of complications and mortality, which conversely remain high in developing countries.
Empiric antibiotic treatment is the mainstay of therapy and requires prompt initiation along with monitoring with imaging and laboratory tests. Laparoscopy and surgical removal are performed in women with tubo-ovarian abscess not showing clinical improvement after 72 hours of antibiotic treatment and those complicated with sepsis, hemodynamic instability or abscess rupture. Microbiology samples of the tube can be taken at the time of surgery and/or excision, which will help tailor the antibiotic treatment. Addressing risk factors is important to prevent further episodes. Importantly, the disease can be prevented with the treatment of lower genital tract infections and subclinical chlamydia or gonorrhea infection detected on cytologic screening.
Inflammation of fallopian tube of bacterial nongranulomatous origin
Histopathologic correlate of PID
Chlamydia trachomatis, N. gonorrhoeae , mycoplasma, and anaerobes most frequent agents
Estimated 1.2 million cases of PID in US (2000)
Prevalence is decreasing in developed countries
Long-term complications: infertility, ectopic pregnancy, and chronic pain
Reproductive-age, sexually active women (15 to 44 years)
More frequent and severe in developing countries
Risk factors: multiple sexual partners, unprotected sex, smoking, drug use
Acute salpingitis: acute pelvic or abdominal pain, fever, constitutional symptoms, vaginal discharge or bleeding
Chronic salpingitis: chronic pelvic pain and infertility
Tubal thickening, edema, fluid collection
Prompt empiric antibiotic treatment (based on clinical diagnosis)
Laparoscopy and surgical removal rarely performed (if medical therapy fails, patient becomes unstable, or tubo-ovarian abscess with rupture)
Acute salpingitis: hyperemia, edema, fibrinopurulent serosal or luminal exudate
Tubo-ovarian abscess: cavitary mass encasing adnexal tissue
Chronic salpingitis: distorted tube with adhesions and scarring, hydrosalpinx or pyosalpinx
Acute salpingitis: neutrophilic infiltrates, more often mucosal; ulceration; reactive epithelial changes may be striking
Tubo-ovarian abscess: necrotic debris and confluent acute inflammation surrounded by mounting reactive fibrosis and mixed inflammatory infiltrates
Chronic salpingitis: blunted, shortened plicae, associated with chronic inflammation and fibrosis
Endometrial biopsy may be performed as part of work-up (chronic endometritis regarded as sign of PID/salpingitis).
Carcinoma
Tuberculous salpingitis
Actinomycotic salpingitis
Granulomatous inflammatory reaction in the fallopian tubes is caused by a myriad of infectious and noninfectious causes. Among the former, tuberculosis is the most common type. The vast majority of cases are caused by Mycobacterium tuberculosis ; other mycobacteria ( Mycobacterium bovis ) have been reported. Other infectious agents causing granulomatous inflammation include actinomycosis and parasites (schistosomiasis and Enterobius vermicularis ).
Tuberculous salpingitis tends to affect young women living in areas with endemic tuberculosis, typically in the setting of pulmonary or systemic tuberculosis with hematogenous spread. Over 90% of cases have bilateral tubal involvement, as well as compromise of other gynecologic organs. Common manifestations include subacute or chronic pain, abnormal vaginal bleeding, and infertility (seen in up to 66% of affected women); some patients manifest no genital symptoms.
The tubes may appear normal or mildly edematous and enlarged. About 20% display soft tubercles on the serosal surface. The lumen may contain caseous debris ( Fig. 12.12A ) or serosanguineous fluid. Upon sectioning, the nodular tubercles contain central caseating necrosis with surrounding edema and/or fibrotic thickening.
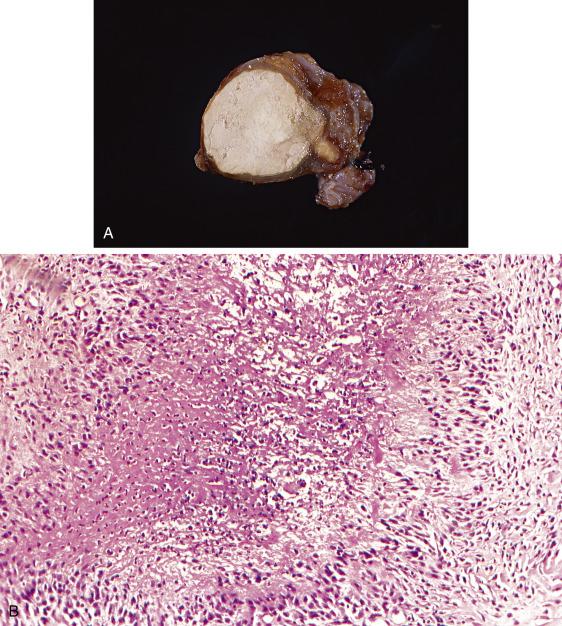
The typical appearance of the tuberculous granulomatous reaction is of a nodular (single or confluent) inflammatory aggregate with central necrotic debris surrounded by giant cells and histiocytes engulfing the bacilli, in turn surrounded by lymphocytic infiltrates and a mounting fibroblastic response ( Fig. 12.12B ). Granulomas are more common in the mucosa, but the lymphoplasmacytic infiltrate may extend beyond the confines of the granuloma into the surrounding wall. Reactive changes in the tubal epithelium are common and can be quite exuberant, mimicking tubal carcinoma (see “Florid Epithelial Hyperplasia” section).
Identification of the microorganism on tissue sections may be achieved using the acid fast bacilli (AFB) stain. Moreover, isolation of the organism in culture can be attempted in fresh tissue if the diagnosis is suspected at the time of surgery and gross examination. These tests are highly specific and confirmatory of the diagnosis. However, AFB stain lacks sensitivity and culture results may take several days to weeks. Detection of the organism using nucleic acid amplification techniques has shown superior detection rates (68%) compared with AFB stain (32%). Immunohistochemistry with a monoclonal antibody against M. tuberculosis has been reported to have even higher sensitivity (96%) in routinely fixed material and aspirates.
In the setting of necrotizing granulomas, infectious agents other than M. tuberculosis need to be considered, particularly in a patient with no known history of tuberculosis. Clinical and epidemiologic history is critical to identify rare agents such as atypical mycobacteria or parasites. The latter are accompanied by a prominent eosinophilic response. In the case of Enterobium vermicularis , worms or their ova (25 × 30 μm in size and covered by a thick shell) can be seen in histologic sections. Schistosoma haematobium and Schistosoma mansoni have been reported in the tube, in some women associated with ectopic pregnancy; their eggs can be identified in the remaining tubal tissue after sodium hydroxide digestion; they are ovoid, approximately 150 μm in size, and have a lateral ( S. mansoni ) or terminal ( S. haematobium ) spine. Lipoidal salpingitis is a granulomatous reaction secondary to lipoidal contrast agents, which are seen in routine preparations as empty spaces of varying sizes within the lamina propria surrounded by histiocytes and foreign-body giant cell reaction. Xanthogranulomatous reaction in the tube is rare but can be seen in endometriosis; it contains large amounts of foamy hystiocytes but typically lacks other inflammatory cellular elements, and unaffected endometriosis is usually seen in the vicinity. Other non-infectious causes of granulomatous salpingitis include systemic conditions such as sarcoidosis, Crohn’s disease and traumatic or iatrogenic implantation of foreign material into the tube. The clinical history and identification of foreign bodies within the tube will aid in the correct diagnosis.
Tuberculous salpingitis is associated with infertility in ∼66% of cases. As a sign of systemic infection, its management requires multidrug antibiotic treatment (6–9 months and may include isoniazid, rifampin, ethambutol, and pyrazinamide). Response is excellent in most patients.
Granulomatous salpingitis caused by M. tuberculosis or M. bovis
Endemic in less developed areas
Occurs in patients with pulmonary or systemic tuberculosis
Vast majority in developing countries
More frequent in adolescents and young adults
Infertility in two-thirds of all women with pelvic tuberculosis
Prognosis good but related to degree of systemic compromise
Antituberculous medication; usually excellent response
Frequent bilateral involvement
Visible serosal tubercles (∼20%)
Luminal or intramural caseous debris
Necrotizing granulomas within tubal wall (often mucosal)
Chronic inflammation and fibrosis in muscularis propria
Florid epithelial hyperplasia common
AFB stain and culture are highly specific
Nucleic acid amplification and immunohistochemistry offer better sensitivity
Parasitic infections
Foreign body reaction (e.g., lipoid salpingitis)
Xanthogranulomatous reaction
This type of infectious inflammatory process is presented separately given its distinct clinical risk factors and presentation. Actinomyces israelii is a filamentous, Gram-positive, anaerobic bacterial microorganism. Spread to the abdomen and pelvis can be caused by surgery, bowel perforation, or trauma. In addition, it can be secondary to colonization of an intrauterine device, which has been reported in 1%–12% of women using this contraceptive method. Not surprisingly, the majority of women affected are of reproductive age. Tubo-ovarian spread manifests clinically with subacute to chronic pelvic pain, bloating, fever, constitutional symptoms, vaginal bleeding, or purulent discharge. Not infrequently, it presents as an adnexal mass associated with pelvic adhesions and fluid collection, sometimes suspicious for a neoplasm clinically and radiologically. Some patients are first diagnosed after identification of the microorganism in cervicovaginal Papanicolaou smear.
Actinomyces infection usually results in a large heterogeneous mass encasing the fallopian tube and ovary; interestingly, the right side is more commonly involved than the left. On cut surface, it can be solid and soft to firm, and show pseudocystic cavitation containing purulent material. Fistulous tract formation with the colon, small bowel, urinary bladder, vagina, or skin may also be present. On occasion, macroscopically visible yellow granules up to 1 mm (“sulfur granules”) will be appreciated within the exudate.
Microscopically, actinomycosis is seen as a symmetrical and round collection of filamentous bacteria arranged in a radiating fashion, which is the histologic correlate of the sulfur granule ( Fig. 12.13A,B ). The inflammatory component is mixed, with neutrophilic and lymphoplasmacytic populations admixed with granulomatous inflammation. Abscess formation appears as cavitary necrosis and confluent acute inflammation surrounded by reactive fibrosis and prominent inflammation ( Fig. 12.13A ).
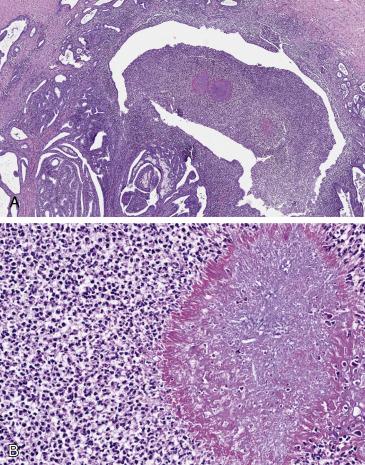
Gram, periodic acid Schiff (PAS), and methenamine-silver stains may be used to highlight the filamentous bacterial colonies.
The diagnosis of carcinoma may be clinically suspected, as most women with actinomycosis present with a complex tubo-ovarian abscess suspicious for malignancy on imaging. In these cases, generous sampling may be advisable; the histomorphology is otherwise typical and suffices to exclude a neoplastic process. Other types of tubo-ovarian abscess (gonorrhea, Chlamydia ) can be considered, particularly if the round radiating bacterial colonies are not apparent; in this scenario, a Gram stain may be of value. If fistulization has ensued, other causes such as inflammatory bowel disease , trauma , or diverticulitis need to be considered; correlation with clinical history and attention to the presence of bacterial granules is important. Pseudoactinomycotic radiate granules bear superficial resemblance to actinomyocytic sulfur granules but, unlike the latter, are nonreactive for the aforementioned special stains.
Most women presenting with persisting or recurrent tubo-ovarian abscess and those with fistula formation require surgical excision and/or drainage followed by antibiotic treatment. Primary antibiotic therapy can be considered if the diagnosis is suspected clinically and there is no adnexal mass formation, and in patients diagnosed with actinomyces in Papanicolaou smear. Prophylactic treatment is considered in patients with intrauterine device, requiring change of the device every 4 years, along with penicillin or doxycycline treatment. Prognosis in treated patients is good; however, complications of PID such as chronic pain and infertility can be seen in these patients.
Chronic suppurative and granulomatous infection caused by A. israelii (Gram-positive, anaerobic to microaerophilic bacteria)
Detected in 1%–12% among intrauterine device users
Risk factors: intrauterine device use and bowel perforation/trauma/surgery
Related to sequelae of inflammation (adhesions, infertility, fistula formation)
No race predilection
Reproductive-age women
Pelvic pain or bloating
Vaginal bleeding or discharge
Adnexal mass simulating ovarian neoplasia
Antibiotic regimen curative
Surgical treatment if persisting abscess or fistula formation, followed by antibiotics
Prophylactic removal or periodic change of intrauterine device
Right side more frequently involved
Large firm mass or abscess involving tube and ovary
Abscesses contain purulent material, occasionally with sulfur granules
Fistulous tracts (bowel, bladder, vagina, or skin)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here