Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
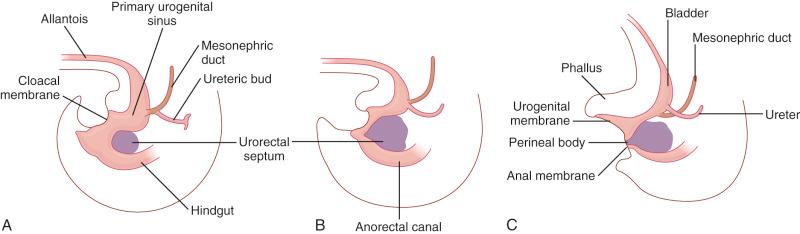
A portion of the distal hindgut forms an expansion called the cloaca, which is a common chamber for the developing intestinal, urinary, and reproductive tracts. The division of the cloaca into separate conduits for these three functioning systems begins in the fifth week of embryogenesis. The urorectal septum, a coronal ridge of mesenchyme, extends caudally to join the urogenital membrane, thereby creating two separate tracts—anteriorly, the urogenital sinus and, posteriorly, the rectum by the end of the eighth week ( Fig. 10.1 ; see Chapter 1 ). Concurrently, the müllerian ducts extend caudally toward the cloaca and merge with the posterior wall of the urogenital sinus to form the müllerian tubercle. The ducts fuse and join with the ligamentum inguinale to differentiate into the vagina and uterus distally and the fallopian tubes proximally. The superior portion of the primitive urogenital sinus, on exstrophy of the mesonephric and ureteric ducts, forms the bladder. The anal canal forms by the fusion of the endodermal hindgut (primitive rectum) with the ectodermal proctodeum (anal pit). This dual embryologic origin of the anal canal results in a dual blood supply, drainage, and innervation (described in detail later in this chapter). Whether or not the dentate line represents the fusion site of the two embryologic derivatives remains controversial, but this anatomic landmark is generally regarded as the region in which the shift in blood supply and drainage and innervation occur. This developmental segmentation of the anal canal is clinically relevant when evaluating congenital malformations and patterns of metastases of malignant neoplasms.
Defects of anorectal development are rare; they range from 1 in 3000 births for anorectal anomalies to 1 in 50,000 for cloacal malformations. Overall, these developmental disturbances constitute approximately 10% of all anogenital malformations in females. The spectrum of anorectal malformations ranges from the mildly stenotic anus to an imperforate anus, with a fistula between the urinary and intestinal tracts, to the most severe form, persistent cloaca. Cloacal defects may affect the gastrointestinal, urinary, and reproductive tracts; cases of associated anomalies also have involved the respiratory tract, neural tube, abdominal wall, cardiac anomalies, hemivertebrae, and digits. These anomalies often present in the newborn period, but they may not be detected until adulthood. A common urogenital sinus is often present in these patients, which may be the common site of exit for the rectum, urethra, and vagina. In approximately 30% of cases, the anal opening is present in the perineum but is displaced anteriorly. The urethra may exit normally or drain into the urogenital sinus or vagina or is entirely absent. Variably, a neurogenic bladder may be present. The external genitalia in chromosomally female patients often consist of an empty skin sac or phallus-like structure, occasionally with a draining urethra. Vaginal structures may be bifid or duplexed, and they lead into the urogenital sinus or into the urethra or bladder. Abnormal sacral development is also frequently present.
Anorectal anomalies are less rare and may occur in 1 in 3000 to 5000 births. They are divided into three types, defined by the location of the defect—high (supralevator), low (translevator), and intermediate. A miscellaneous group also exists. High defects are characterized as anorectal agenesis, with fistulization of the rectum to the bladder, urethra, or vagina. Intermediate defects include anal agenesis, anorectal stenosis, and anorectal membrane. Low defects predominate in female children and include ectopic anus, anal stenosis, and membrane-covered anus.
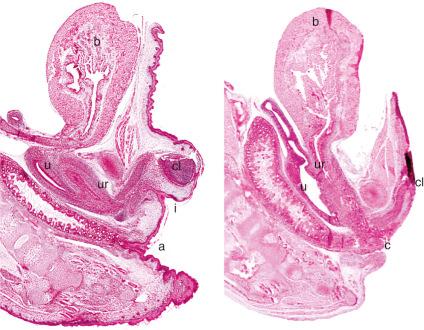
The pathogenesis of many of these anomalies lies in defects in the completion of the urorectal septum, which in turn may reflect abnormalities in signaling, documented in many cases by genetic mutation. One proposed mechanism includes defects in the expression of sonic hedgehog, of which experimental mutations lead to a dose-dependent range of anorectal malformations, including the cloaca. Mice null for p63, a p53 homologue expressed in basal epithelial cells and critical to epithelial development, fail to complete the urorectal septum, leading to a cloaca ( Fig. 10.2 ). Humans with gain of function mutations in this gene also exhibit milder urogenital anomalies (see Chapter 1 ).
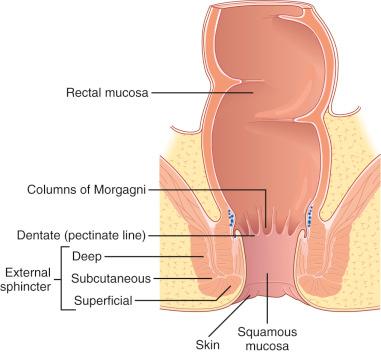
The anal canal is a grossly and histologically defined anatomic area joining the distal intestinal tract with the external perianal skin. Conceptually, the anal canal is the transition from an absorptive epithelium, which samples materials from the external environment, to a protective epithelium, which provides a barrier between the body and external environment ( Fig. 10.3 ). Grossly, the surgical anal canal is defined as the distal 3- to 4-cm segment of the alimentary tract that begins at the apex of the anal sphincter complex (palpable anorectal ring) and ends at the mucocutaneous junction of the squamous mucosa and perianal skin, which approximately coincides with the outermost boundary of the internal sphincter muscle (palpable intersphincteric groove). The proximal anal canal is composed of anal columns, which are vertical mucosal folds separated by anal sinuses. The distal edges of the anal columns are demarcated by horizontal mucosal folds, termed anal valves. The line formed by the anal valves and sinuses is the dentate line and, as mentioned earlier, marks the division of the vascular, lymphatic, and neural supply and divides the anal canal into cranial and caudal segments.
The histologic zones of the anal canal are divided into four components, defined loosely by the types of mucosal epithelium present. These include the colorectal zone, anal transitional zone, squamous zone, and perianal skin.
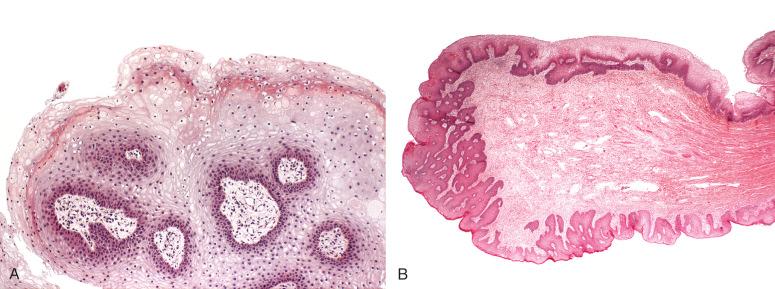
The colorectal zone ( Fig. 10.4A ) is the most cephalad segment of the anal canal and the most caudad portion of the colonic mucosa. It is a continuation of the rectal mucosa, lined by an uninterrupted absorptive columnar epithelium that extends to the proximal extent of the anal columns. There is no clear histologic demarcation between the rectal mucosa and colorectal zone of the anal canal, although the crypts tend to be shorter and more irregular in the anal canal. The epithelial cells are CK20-positive, CDX-2 positive, and CK-7 negative, an expected immunophenotype of colorectal-type mucosa. No anal transitional zone (ATZ) mucosa, anal glands, or squamous mucosa is present in this histologic zone.
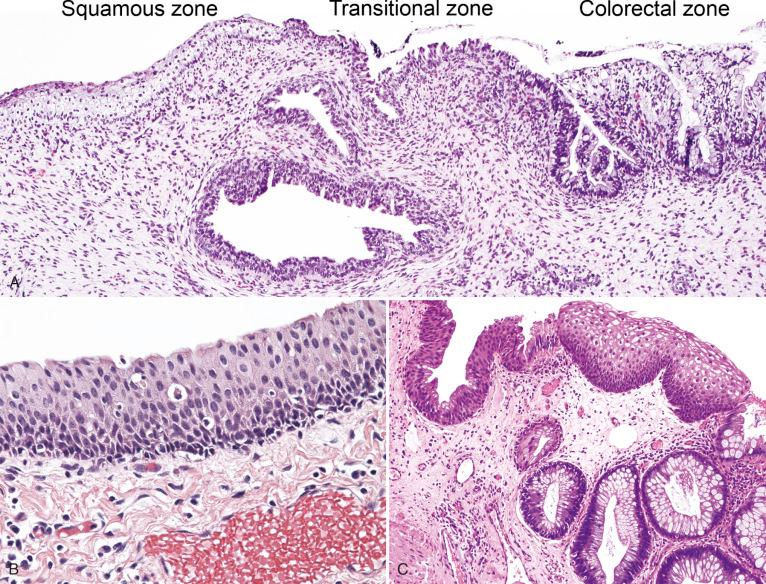
The ATZ ( Fig. 10.5 ; see Fig. 10.4 ) is a region of squamous metaplasia that extends from the dentate line (old squamocolumnar junction [SCJ]) to the new SCJ. On high-resolution anoscopy (HRA), the new SCJ is the most important and readily identifiable landmark, typically visible as a clear line of tissue that is paler than the adjacent proximal rectal epithelium (see Fig. 10.5 ). The dentate line, in contrast, is not as easily visualized on HRA. Histologically, the ATZ is flanked by colonic mucosa above and squamous mucosa below. The ATZ is defined by its relative location to the adjacent histologic zones because the epithelial types present here are variable. The prototypic epithelial type of this zone is the ATZ epithelium (see Fig. 10.4B ), which consists of multilayered columnar to cuboidal, and sometimes flattened, cells, ranging from one to nine cell layers, with variable mucin production—scant intracytoplasmic mucin to rare goblet cell metaplasia. This cell population is positive for CK7 and negative for CK20, CDX2, and p63. Scattered CK7-positive ATZ cells extend into the proximal adjacent colorectal epithelium and over the surface of the distal stratified squamous epithelium; colorectal and squamous epithelial cells are negative for CK7. There is an underlying basal cell layer that is usually one or two cells thick, with cells staining positive for p63 and negative for CK7. The cells are present as a continuation of the basal cell layer of the perianal skin and squamous zone and terminate at the ATZ and colorectal junction. The adult and fetal ATZ are remarkably similar, although the adult ATZ demonstrates occasional islands of mature squamous epithelium, as well as scattered crypts of colonic-type epithelium (see Fig. 10.4C ). These epithelial heterogeneities are relatively rare in the fetal ATZ (see Fig. 10.4A ).The overall microanatomy of the adult anal canal is established at least by 9 weeks' gestational age. The length of the ATZ is highly variable, ranging from abrupt transition (0 mm; direct squamous to colorectal epithelial transition, without intervening ATZ epithelium) to 16 mm on a longitudinal histologic section (average, 9.5 mm).
Anal glands and ducts lined by ATZ-type epithelium are present in the submucosa (see Fig. 10.4A ) and internal sphincter muscle. Scattered melanocytes and endocrine cells are present. Lymphoid follicles may also be present in the lamina propria and submucosa, but in smaller quantities than in the colorectal zone.
The squamous zone (see Fig. 10.4A ) is composed of uninterrupted, nonkeratinizing squamous epithelium. The transition from the ATZ to the squamous zone generally takes place at the level of the dentate line. Melanocytes may be present in this zone, but are present in greater number toward the perianal skin. Basal and parabasal cells demonstrate strong nuclear positivity for p63, but staining gradually diminishes with epithelial maturation toward the surface. By definition, ATZ epithelium, anal glands, and skin appendages are not present.
The perianal skin is composed of keratinizing squamous epithelium with hair, sebaceous glands, sweat glands, and apocrine glands. In addition, there are anogenital mammary-like glands characterized by simple columnar epithelium with cytoplasmic snouts and an outer layer of myoepithelial cells. These glands may give rise to epithelial and fibroepithelial neoplasms, analogous to those arising in the breast. Melanocytes may be present at a greater density than in the squamous zone. Scattered Langerhans cells and Merkel cells may be present. The transition from the squamous zone to perianal skin is gradual, without any abrupt histologic landmarks.
In addition to the major epithelial types listed above, components of the anal wall include smooth (internal sphincter) and striated muscle (external sphincter), nerves, blood vessels, and lymphatics. The supply and drainage of these components differ significantly above and below the dentate line, as discussed below.
Understanding the gross and histologic anatomy of the anal canal is important for understanding the pattern of neoplastic development in this complex site. Squamous carcinoma commonly arises in the canal and is linked to human papillomavirus (HPV) infection. Adenocarcinoma of the anal canal may arise from the distal rectal mucosa and, more rarely, from anal ducts. Melanomas, lymphomas, leiomyomas, leiomyosarcomas, and neural tumors such as schwannomas have also been reported.
The anatomy of the anal canal is also key to understanding the spread of neoplastic disease based on its origin in the upper or lower anal canal. The upper two-thirds of the anal canal is supplied with blood mainly from the superior rectal artery (a branch of the inferior mesenteric artery) and drains into the superior rectal veins. The venous drainage of the superior rectal vein empties into the inferior mesenteric vein, but branches of the superior mesenteric and middle veins may also anastomose in that area. Drainage from the upper two-thirds of the canal leads to the inferior mesenteric lymph nodes. The autonomic nervous system innervates the anal canal above the dentate line, whereas the somatic nervous system via the pudendal nerve and sacral plexus innervates the canal below the dentate line. The lower third is supplied by the inferior rectal arteries, branches of the internal pudendal arteries. Venous drainage is provided by the inferior rectal veins leading to the internal iliac veins via the internal pudendal veins. Lymphatics for the lower third of the canal lead to the superficial inguinal lymph nodes. The somatic nervous system supplies that area via the inferior rectal nerve. Understanding the histologic zones and gross anatomy of the anal canal can provide important clues for clinical disease. For example, iliac and periaortic lymph node metastases from tumors originating above the dentate line are frequently seen in adenocarcinomas, whereas lymphadenopathy of the inguinal and femoral nodes more likely reflects spread from tumors below the dentate line and are more likely to be squamous cell carcinoma.
A variety of developmental anomalies may lead to perianal or anal cysts. It is believed that cysts in these areas may arise from remnants of the neuroenteric canal, tailgut, or hindgut, but in practice these mucus-producing cysts may be difficult to distinguish from anal duct cysts or anal gland cysts. The cyst lining typically is composed of mucus-producing cuboidal to columnar epithelium. Clinically, these cysts come to attention because of infection and perianal abscess formation.
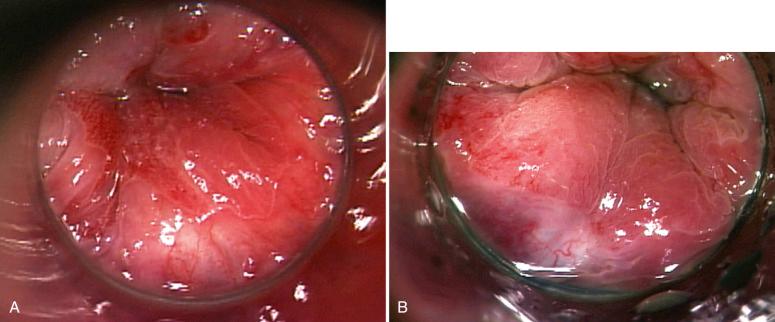
Hemorrhoids are characterized by dilations of superficial mucosal vessels accompanied by enlargement or prolapse of connective tissue. Traditionally, it was believed that hemorrhoids were caused by the pathologic development of superficial varicosities of the submucosal plexus of venules. However, hemorrhoids are now presumed to result from enlarged or prolapsed fibrovascular and connective cushions in the submucosa, which perform a physiologic protective role during defecation. Portal hypertension does not cause hemorrhoids but may precipitate bleeding ( Fig. 10.6 ).
Risk factors for hemorrhoids include a low-fiber diet, constipation, and prolonged intra-abdominal pressure (straining). Other factors implicated in their development include diarrhea, obesity, hypertension, and hereditary tendency. Increased age also predisposes to symptomatic hemorrhoids because loss of elastic tissue and replacement of muscle with collagen fibers cause instability and venous stasis. Patients with concomitant portal hypertension or coagulopathy are then susceptible to bleeding and prolapse.
Hemorrhoids can occur caudal (external) or cephalad (internal) to the sphincter (external), and the location will determine the symptoms. Internal hemorrhoids primarily cause painless bleeding, whereas external hemorrhoids are associated with pain, particularly when thrombosed, strangulated, or inflamed. Erosion of the surface of a prolapsed hemorrhoid may also cause pain or infection.
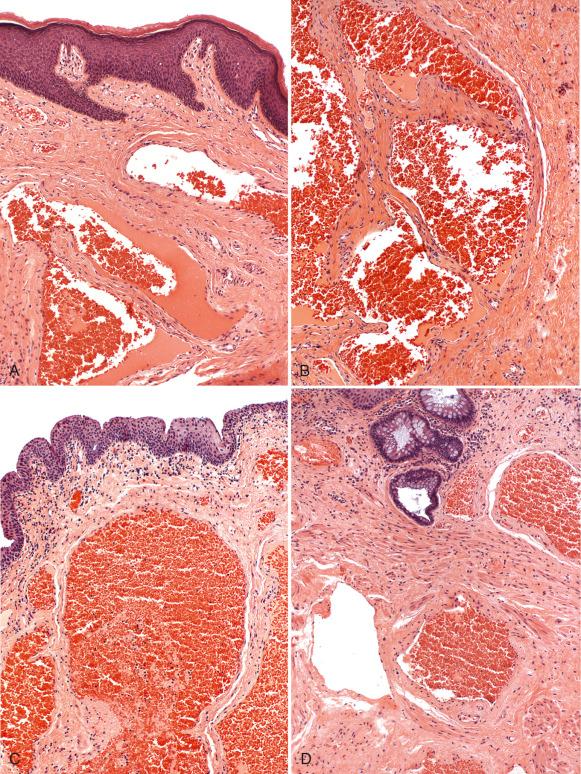
The hemorrhoidal tissue is composed of thick-walled, medium-sized vessels in the submucosa and background stroma containing connective tissue and smooth muscle fibers ( Fig. 10.7 ). Additional features include thromboses, neuronal hyperplasia, and erosion, with inflammatory changes of the mucosal surface. Incidental anal intraepithelial neoplasia (AIN) has been reported in 2% of excised hemorrhoids.
Fibroepithelial stromal polyps (FSPs) are polypoid projections of the anal mucosa with stromal hyperplasia that occur in response to mucosal trauma. FSPs are composed of two components, including squamous mucosa with minimal acanthosis and underlying loose to compact stromal tissue, often with distinctive stellate or multinucleate cells showing fibroblastic and myofibroblastic differentiation. Various degrees of inflammation are present, depending on the degree of trauma to the polyp. Fibroepithelial polyps lack the dilated submucosal vessels of a hemorrhoid and are thought to be the equivalent of acrochordons found in other sites of the body (see Fig. 10.7 ).
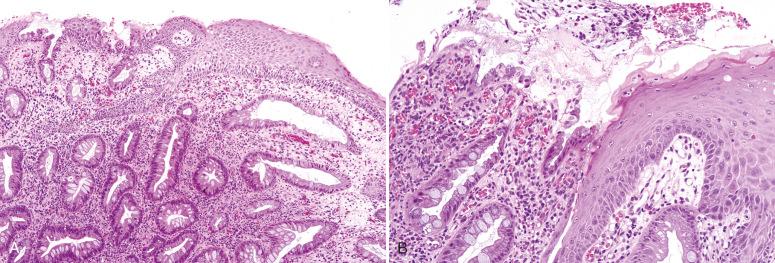
Inflammatory polyps, or inflammatory cloacogenic polyps, are prolapsed mucosal projections with histologic features suggesting an ischemic or reactive origin. Inflammatory polyps are believed to occur as a component of the solitary rectal ulcer syndrome or mucosal prolapse syndrome. These polyps occur most often in middle-aged patients, although they also occur in children and often present with rectal bleeding. A few case reports of dysplasia found in these polyps are present in the literature.
Grossly, the lesions are pink or tan polypoid excrescences of the anal canal mucosa, which are varied in size and are typically sessile, with a tubulovillous architecture.
The lesions are lined by a mixture of squamous and colorectal epithelium, often with surface ulceration or erosion. In addition, fibrosis of the lamina propria and thickening of the muscularis propria are present, with some extension of smooth muscle into the lamina propria. Mucosal gland hypertrophy and surface telangiectasia may also be seen ( Fig. 10.8 ).
Treatment of the lesions is simple excision, usually with curative results.
Anal fissures are traumatic erosions of the mucosal surface, primarily caused by the passage of hard stool. These so-called stercoral fissures usually occur in the posterior midline of the anal canal and may develop into chronic ulcers with repeated trauma. A reactive stromal hyperplasia, histologically similar to a fibroepithelial polyp, can form in chronic cases at the proximal end of the lesion and is termed a sentinel tag . Secondary causes of anal fissures include inflammatory bowel disease, particularly Crohn disease, neoplasms, or infectious processes. Trauma and anal intercourse are also implicated in the formation of fissures.
The fissures or ulcers show mucosal erosion, with fibrinoid ulcer formation and underlying granulation tissue. Foreign body giant cells may also be present and should not be mistaken for the granulomas of Crohn disease.
Most anal fistulous disease is believed to arise from infection of the anal ducts and subsequent tracking into the anal glands. Because of the position of the anal glands deep to the wall of the internal anal sphincter muscle, deep abscesses may result acutely. Fistulous tracts may form in the chronic inflammatory phase. Hidradenitis suppurativa is an associated condition, which is a chronic infection of the apocrine glands and surrounding connective tissue, occurring most often in males ( Fig. 10.9 ). Patients present with complaints of pain, purulent discharge, and pruritus. Inflammation may involve the distal anus as well as the anal canal and rectum, with the formation of fistulas ( Fig. 10.10 ). Associated conditions include oily skin, acne, obesity, smoking, and diabetes mellitus. Crohn disease may also result in perianal and anal fistulous disease.

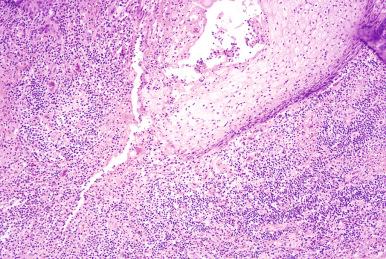
The characteristic features include ulceration and granulation tissue in the tracts, with foreign body giant cells and fibrosis.
Inflammatory bowel disease can manifest in the anal canal in several ways.
Ulcerative colitis uncommonly involves the anal region (ulcerative proctitis) and produces nonspecific inflammatory changes in the anal canal, restricted to the mucosa of the colorectal zone. Fissures and fistulas are more rare. Of note, however, one study has found that the columnar epithelium affected by ulcerative colitis often extends in irregular tongues into the transitional epithelium, occasionally reaching the dentate line. Thus, surgical intervention for ulcerative colitis is a balance between removing all the mucosa of the colorectal zone and preserving sphincter function.
Female fecundity decreases following surgical procedures for ulcerative colitis, specifically following an ileal pouch–anal anastomosis (IPAA). Studies have shown that fecundity before surgery in patients with ulcerative colitis is equivalent to a control population. However, following bowel excision and revision, women had more difficulty getting pregnant. A small study of hysterosalpingography in 21 women after IPAA found abnormalities in two-thirds of patients. Abnormalities included fibrous bands, fallopian tubes adherent to the pelvic side wall, hydrosalpinx, and tubal occlusion.
Crohn disease affects the anus more frequently and with a wider variety of symptoms and pathology. Approximately 25% of patients with small bowel Crohn disease have anal canal involvement, compared with involvement in 50% to 75% of patients with small and large bowel disease. Anal tags, fissures, ulcers, fistulas, and abscesses may form. Granulomatous inflammation affecting the anal canal mucosa and wall are seen histologically.
Intraepithelial neoplasia of the ATZ may also occur following IPAA for ulcerative colitis or Crohn disease. A study from the Cleveland Clinic has shown that although no patients in their series (total of 210 patients) developed carcinoma of the transitional zone, LSILs and HSILs developed in six patients and one patient, respectively. All patients who developed a lesion had a prior history of intraepithelial neoplasia or carcinoma, either in the colon or rectum.
Endometriosis rarely involves the anus and perianal area. The patients typically report cyclic pain that correlated with menses, occasionally with perianal nodule formation. Many cases are associated with an episiotomy scar and occasionally may involve the sphincter muscles. The histologic correlate is an endometrioma, composed of a blood-filled cyst containing endometrial-type glands and stroma and hemosiderin deposition.
The anus may be involved in a wide range of infections, including tuberculosis, amebiasis, and fungal and yeast infections. Other infections typically associated with the vulva may also involve the anus, including herpes, syphilis, and, as discussed later, papillomaviruses (see Chapter 4 ).
Various iatrogenic insults to the anal region have been reported in the literature. Irradiation of the anal region following abdominoperineal resection for anal cancer is one of the most common causes of iatrogenic injury. Histologically, the anal sphincter muscle shows increased fibrosis and nerve density. Ergotamine-induced strictures from ergotamine suppositories for migraine treatment have also been reported. In addition to trauma from repeated suppository use, ergotamine toxicity results from the drug's vasoconstrictive properties, producing local ischemia. Rectal ulcerations are the most common early manifestation of this disorder, leading to progressive fibrosis and stricture formation.
The most recent World Health Organization (WHO) classification (2010) divides tumors of the anal canal into epithelial tumors, mesenchymal tumors, and secondary tumors. Epithelial tumors are subdivided into premalignant lesions of the anal canal mucosa and perianal skin and carcinoma. The carcinoma group consists of squamous cell carcinoma, adenocarcinoma, and neuroendocrine neoplasms.
For practical purposes, neoplastic lesions can be divided into three categories—benign adnexal lesions, squamous intraepithelial lesions (SILs) and carcinoma, and other malignancies. Squamous lesions are the most commonly encountered neoplasms and will occupy the bulk of the discussion that follows.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here