Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Visceral artery aneurysms are a rare but clinically important vascular condition and have been recognized for more than 200 years. As of 2002, there were about 3000 cases reported in the literature, and the incidence of visceral artery aneurysms in the general population has been estimated at 0.1% to 2%. The first successful surgical repair of a visceral aneurysm, the repair of a mycotic aneurysm of the superior mesenteric artery, was reported by DeBakey and Cooley in 1953, and in 1954 Williams and Harris reported the first successful resection of a splenic artery aneurysm. The natural history of visceral artery aneurysms and their potential for rupture are poorly defined because of their overall scarcity. Visceral artery aneurysms encompass intraabdominal aneurysms, which are not part of the aortoiliac system, and lesions of the celiac artery, the superior and inferior mesenteric arteries, and their branches. The etiologies associated with these lesions are diverse, and there is a spectrum of anatomic locations within the visceral vasculature. One third of visceral artery aneurysms may be associated with other aneurysmal diseases that occur, in order of decreasing frequency, in the thoracic aorta, abdominal aorta, renal arteries, iliac arteries, lower extremity arteries, and intracranial arteries.
Visceral artery aneurysms include true aneurysms and false aneurysms, or pseudoaneurysms. True visceral artery aneurysms are typically degenerative or atherosclerotic, with histology demonstrating reduced smooth muscle, disruption of elastic fibers, and deficiency of the arterial media. Other conditions associated with true visceral artery aneurysms include fibromuscular dysplasia, collagen vascular diseases, inflammatory conditions, and rare inherited illnesses such as Ehlers-Danlos syndrome. In contrast to true aneurysms of the visceral vessels, splanchnic artery pseudoaneurysms are most commonly related to trauma, iatrogenic injury, local inflammatory processes, or infection.
Visceral artery aneurysms are managed with either serial observation or repair, depending on their size, the underlying clinical scenario, and the anatomic location of the lesion. Asymptomatic splenic artery aneurysms larger than 2 to 2.5 cm should be considered for repair, particularly in patient subgroups that appear to have a propensity for rupture, such as patients undergoing liver transplantation and women of childbearing age. A similar size criterion is used for hepatic and celiac artery aneurysms. All symptomatic aneurysms should be repaired, and all visceral artery pseudoaneurysms should be considered for repair regardless of size because of their increased risk of rupture. Small asymptomatic visceral artery aneurysms have a slow growth rate (0.2 mm/yr) and can be observed. Open surgical options are increasingly reserved for patients who have failed or are not candidates for endovascular approaches. Surgical options include aneurysm exclusion or ligation, aneurysmectomy, and aneurysmorrhaphy, with or without concomitant revascularization. The necessity of revascularization depends on the location of the lesion and the collateral vascular anatomy. In areas of the splanchnic circulation with an abundance of collateral flow, proximal and distal ligation of the aneurysm segment is a viable surgical option, and revascularization is often not necessary.
For a ruptured visceral artery aneurysm discovered at laparotomy, ligation of the aneurysm without vascular reconstruction is the preferred treatment. Patients with ruptured splenic artery aneurysms are usually treated with concomitant splenectomy. Ligation without revascularization can generally be performed for common hepatic artery aneurysms proximal to a patent gastroduodenal artery (GDA). Emergency surgical therapy of mesenteric branch artery aneurysms may require simultaneous intestinal resection for bowel ischemia or infarction.
The most critical aspect of appropriate preoperative planning is the exact delineation of the anatomy of the lesion. This can be done with formal arteriography, magnetic resonance arteriography, or computed tomography (CT) arteriography. Magnetic resonance arteriography and CT arteriography postprocessing using volume rendering techniques allows excellent three-dimensional reconstruction of the aneurysm in relation to its afferent and efferent branches, whereas axial images enable the surgeon to visualize mural thrombus that may not be apparent on conventional angiography. If CT arteriography or magnetic resonance arteriography cannot provide adequate details, formal arteriography should be performed and may demonstrate the feasibility of an endovascular approach.
Many patients with an idiopathic visceral artery aneurysm are younger than the typical patient who undergoes an abdominal vascular operation, and they do not have associated atherosclerotic precursors. In these patients no particular preoperative medical or cardiac workup other than routine laboratory testing, a chest radiographic examination, and an electrocardiogram is warranted. However, an older patient with medical comorbidities that may require extensive abdominal vascular reconstruction, with possible aortic clamping during the procedure, deserves a more complete preoperative medical workup, with cardiac stress testing and echocardiography as dictated by the clinical situation.
In patients with multiple or unusual visceral aneurysms, the surgeon must consider the possibility of an underlying condition such as an infectious or inflammatory process, periarteritis nodosa, or Ehlers-Danlos syndrome. Although rare, the proper diagnosis of these conditions may have significant implications before undertaking operation. For example, in patients with periarteritis nodosa, regression of these aneurysms after pharmacologic management with immunosuppressive or cytotoxic agents is well documented. Visceral aneurysms in patients with Ehlers-Danlos syndrome appear to be equally distributed among the hepatic, splenic, renal, and celiac arteries. Surgical repair in these patients is exceedingly difficult, and ligation is preferred to vascular reconstruction when possible.
A ruptured visceral artery aneurysm is typically not diagnosed until laparotomy is performed for an abdominal catastrophe. These patients are often in hypovolemic shock, and massive hemoperitoneum can be encountered. Aggressive resuscitation with blood products must be instituted, and temporary clamping of the supraceliac aorta at the level of the diaphragm may be necessary. Packing and exploration of the abdomen to localize the area of pathology are then performed. These patients typically have not had detailed preoperative imaging studies, and localization of the area of pathology can be challenging. The surgeon must use careful judgment in deciding whether ligation or arterial reconstruction is appropriate. This is based on both the location of the aneurysm and the underlying condition of the patient.
In the specific case of ruptured splenic artery aneurysms, a vascular surgeon is occasionally called by an obstetric team when a pregnant patient has been explored for a presumed obstetric calamity. This is an extremely difficult situation, because the obstetrician typically has explored the patient through a Pfannenstiel incision or lower abdominal situation. This must be rapidly converted to a midline laparotomy for adequate visualization of the upper abdominal cavity.
Careful review of detailed preoperative imaging studies is necessary to delineate the anatomy and location of the aneurysm and the adequacy of collateral circulation to avoid end-organ ischemia if ligation without revascularization is planned.
Careful review of detailed preoperative imaging studies is required to identify associated variations in the mesenteric circulation, such as stenoses or vascular anomalies, that may dictate the need for revascularization as opposed to ligation.
Awareness is necessary of the possibility of associated medical and surgical conditions, such as pancreatitis, infection, collagen vascular disease, or collagen production disorders, that may complicate open surgical repair.
The surgeon should be aware of multiple options for aneurysm treatment if revascularization is required, including aneurysm plication, aneurysmorrhaphy, and interposition bypass grafting.
Awareness of alternative inflow sources for revascularization, such as the supraceliac aorta, is important should technical issues arise.
The surgeon should avoid kinking of autologous bypass grafts.
Injury to associated venous and visceral structures should be avoided.
Careful monitoring is necessary in the postoperative period for hemorrhage or signs of end-organ ischemia that could indicate bypass thrombosis.
The celiac artery is typically a short, thick trunk that arises from the anterior surface of the abdominal aorta, just below the diaphragmatic hiatus. It typically divides into three large branches: the left gastric artery, the hepatic artery, and the splenic artery ( Fig. 42-1 ). The hepatic artery is directed toward the right and forms the lower boundary of the foramen of Winslow. A large branch, the GDA, descends from the hepatic artery and subsequently divides into the right gastroepiploic and superior pancreaticoduodenal arteries. The GDA functions as an important collateral between the celiac artery and the superior mesenteric artery. The portion of the hepatic artery proximal to the GDA is termed the common hepatic artery, and the portion distal to the GDA is called the proper hepatic artery. The proper hepatic artery lies in relation to the common bile duct and portal vein and subsequently divides into the right and left hepatic arteries.
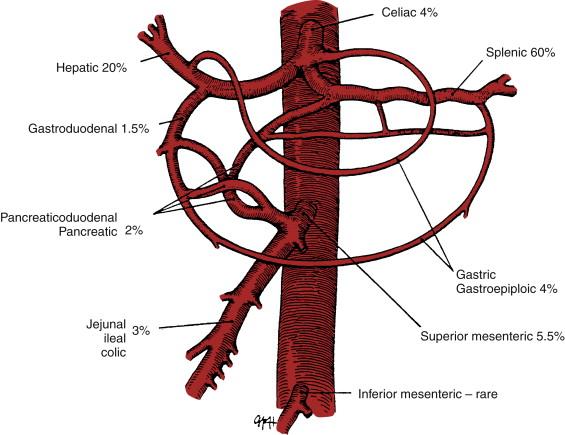
The splenic artery is usually the largest branch of the celiac artery and often is notable for marked tortuosity. It passes to the left side, behind the stomach, and along the upper border of the pancreas, where it gives rise to numerous pancreatic branches. The other branches of the splenic artery before its termination in the splenic hilum are the short gastric and left gastroepiploic arteries.
Splenic artery aneurysms comprise 60% of all visceral aneurysms ( Fig. 42-1 ). They are often saccular, usually less than 2 cm in diameter, and most are located in the mid- or distal splenic artery ( Fig. 42-2 ). Most splenic artery aneurysms are found incidentally during unrelated abdominal imaging. When rupture occurs, patients usually experience acute left-sided abdominal pain and shock. However, an initial contained rupture into the lesser sac may occur, providing a window of opportunity for treatment. This “double rupture” phenomenon may be seen in 20% to 30% of cases. Splenic artery aneurysms may occasionally rupture into adjacent structures, including the gastrointestinal tract, pancreatic ducts, or splenic vein. Splenic artery pseudoaneurysms secondary to pancreatitis may rupture into a pancreatic pseudocyst or into the pancreatic duct, a condition called hemosuccus pancreaticus.
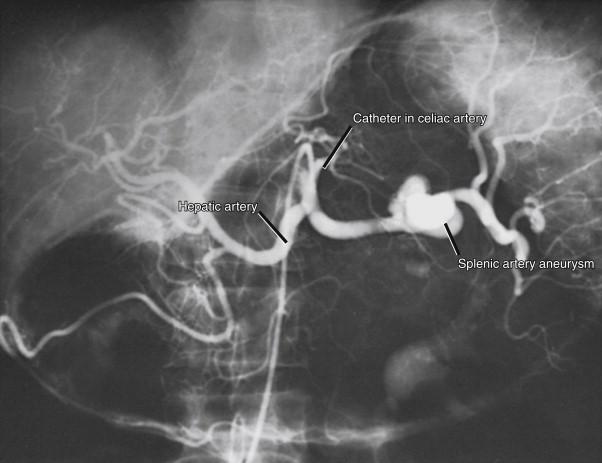
The overall mortality rate of ruptured splenic artery aneurysms is as high as 25%. Rupture of a splenic artery aneurysm during pregnancy, which usually occurs during the third trimester, has devastating maternal and fetal mortality rates of 80% and 90%, respectively. The frequent occurrence of rupture in the third trimester, and the presentation of abdominal pain and shock, often leads to misdiagnosis as an obstetric emergency.
Splenic artery aneurysms that are ruptured or symptomatic require urgent treatment, and aneurysms in pregnant women or in women of childbearing age also warrant intervention. Less stringent indications for treatment include aneurysms that are enlarging or those more than 2 cm in diameter, but these size criteria are not absolute. The traditional surgical management of splenic artery aneurysms consists of proximal and distal ligation or aneurysmectomy for lesions in the proximal or middle portion of the splenic artery. Revascularization of the distal splenic artery is generally not warranted, because collateral flow to the spleen is maintained by the short gastric arteries. For more distal lesions adjacent to the splenic hilum, splenectomy has been the most commonly performed operation.
The appropriate incision for open surgical treatment of an aneurysm in the middle portion of the splenic artery is either a midline laparotomy or a bilateral subcostal approach. An aneurysm in the distal portion of the splenic artery can be approached from either a left subcostal or a midline abdominal incision.
After initial exploration of the abdominal cavity, the greater omentum is reflected upward while downward traction is maintained on the transverse colon. The omentum is separated using sharp dissection, and the lesser sac is entered. The posterior gastric wall is swept away from the underlying pancreas, and the entire pancreas is exposed from its head to the hilum of the spleen. The splenic artery and vein are located as they run along the superior surface of the body and tail of the pancreas. An incision in the posterior peritoneum allows direct exposure of the origin and middle portion of splenic artery, where most splenic artery aneurysms are located.
If the aneurysm is located in the distal splenic artery or adjacent to the splenic hilum and concomitant splenectomy is planned, the lesser sac may be entered via the gastrosplenic ligament using medial traction on the stomach. The distal portion of the splenic artery can be palpated after its course along the upper margin of the pancreas. The peritoneum overlying the vessel is incised, proximal vascular control is gained, and the artery, along with the splenic vein, is ligated. Splenectomy with the distally located aneurysm can then be performed en bloc.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here