Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are innumerable causes of chronic diffuse lung disease. This chapter reviews the common causes of diffuse lung disease, including the idiopathic interstitial pneumonias (IIPs), connective tissue disease (CTD), hypersensitivity pneumonitis (HP), sarcoidosis, cystic lung disease, eosinophilic lung disease, collagen vascular diseases, and drug reaction.
The IIPs are a group of diffuse lung diseases that present similarly. The classification system of IIPs is grounded on underlying histology-specific idiopathic conditions. However, many known nonidiopathic conditions may lead to histologic patterns identical to those in the IIPs. Other common conditions to consider include collagen vascular diseases, HP, and drug-related lung disease. The IIPs are subcategorized into the chronic fibrotic conditions, the subacute and acute conditions, the smoking-related conditions, and the rare conditions.
Idiopathic pulmonary fibrosis (IPF) is the most common type of pulmonary fibrosis and is also the most common of the IIPs. As implied by its name, the cause of IPF is unknown. The imaging and histopathologic pattern of IPF is usual interstitial pneumonia (UIP). All cases of IPF are UIP, and most cases of UIP are IPF; a minority of UIP cases are caused by collagen vascular disease (usually rheumatoid arthritis [RA]), chronic HP, drug-related pulmonary fibrosis, or occupational lung disease. IPF usually affects older male patients in the sixth and seventh decades of life; two thirds are current or former smokers.
Timely diagnosis requires a high level of clinical suspicion in all adult patients presenting with chronic exertional dyspnea or dry cough with inspiratory crackles. Inspiratory crackles and digital clubbing are often present on physical examination. Hiatal hernias are common; gastroesophageal reflux disease (GERD) may worsen pulmonary fibrosis and is associated with worse patient survival. Restrictive physiology and reduced diffusion capacity are typical on pulmonary function testing. IPF has a poor prognosis with a median survival time of approximately 3 years.
The histopathologic findings of UIP are characterized by spatially and temporally heterogeneous fibrosis with adjacent spared areas of normal lung. The subpleural lung is most severely affected. Fibroblast foci are a key finding in UIP and are associated with worse survival.
The low-contrast resolution of chest radiography limits its utility in the assessment of pulmonary fibrosis. UIP most commonly presents with reduced lung volumes and reticular and linear opacities in the basilar portions of the lungs ( Fig. 18.1, A ). In severe cases, traction bronchiectasis and honeycombing may be evident even on radiography. Full characterization of lung disease is best performed on thin-section chest computed tomography (CT).
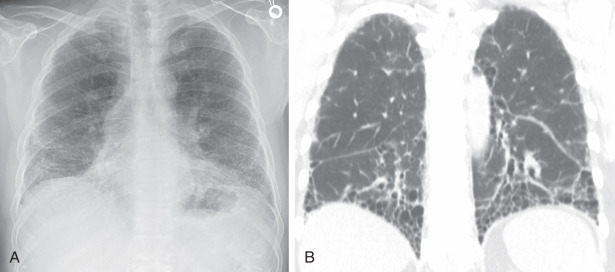
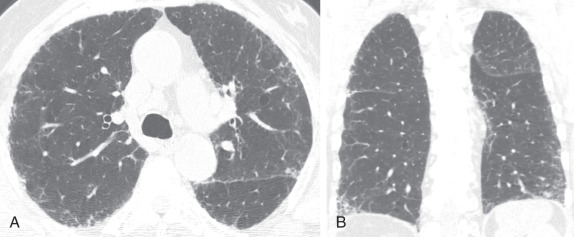
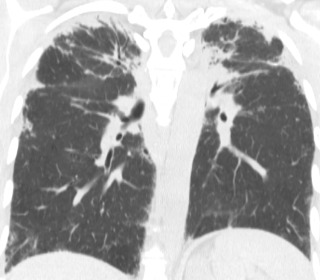
Chest CT plays a central role in the clinical workup of patients with suspected pulmonary fibrosis. A UIP pattern on chest CT is highly accurate for UIP on pathology, obviating lung biopsy. However, only half of cases of IPF have a UIP pattern on chest CT. A UIP pattern on chest CT is defined as reticulation in the peripheral and basilar aspects of the lungs with associated subpleural honeycombing without other features that are “inconsistent with UIP” ( Fig. 18.1, B ). Traction bronchiectasis or bronchiolectasis often coexists with other findings of pulmonary fibrosis. Fibrosis in UIP may be asymmetric as opposed to the distribution in nonspecific interstitial pneumonia (NSIP), which is almost always symmetric. A “possible UIP” pattern on chest CT has all the characteristics of a UIP pattern except there is no honeycombing ( Fig. 18.2 ). An inconsistent-with-UIP pattern on chest CT should be considered if there are any of the specific CT findings listed in Box 18.1 suggestive of an alternative diagnosis. In cases of possible UIP and inconsistent with UIP, biopsy should be considered for diagnosis ( Fig. 18.3 ).
Clinical features
40–60 years old
Dyspnea, dry cough
Histologic feature: temporal heterogeneity
Reticulation
Lower zones
Honeycombing
Small lungs
Reticulation
Honeycombing
Traction bronchiolectasis
Traction bronchiectasis
Peripheral and subpleural distribution
Upper, mid, or peribronchovascular distribution
Extensive ground-glass opacity
Consolidation
Discrete cysts
Diffuse mosaic attenuation or air trapping
Profuse micronodules
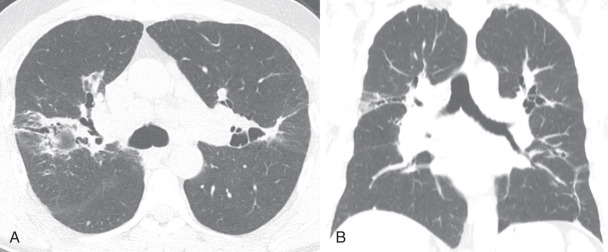
Honeycombing is a finding of localized end-stage pulmonary fibrosis. Honeycombing on CT is an important finding because it is the single most specific finding of UIP and has poor prognostic ramifications. In certain cases, differentiating paraseptal emphysema from honeycombing on CT can be challenging because smoking is associated with both entities. Honeycombing usually manifests as rows or stacks of thin-walled subcentimeter lung cysts and adjacent reticulation, often in the mid and lower lung zones; paraseptal emphysema presents as longer and often larger cystic regions often in the upper lung zone and often contain subtle internal septations, which would be highly unusual for honeycombing ( Fig. 18.4 ). Unfortunately, differentiation of these two entities may be impossible in a minority of cases.
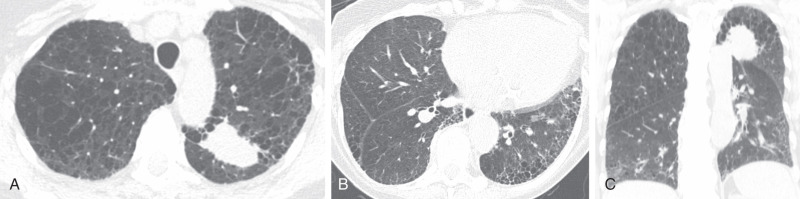
Combined pulmonary fibrosis and emphysema (CPFE) is a distinct clinical entity and is separate from the IIP classification system. Approximately one third of IPF patients have concomitant emphysema. On pulmonary function tests, lung volumes are typically normal or near-normal because of the offsetting effects of fibrosis (restriction) and emphysema (obstruction). However, diffusion capacity is typically markedly reduced. Emphysema is usually upper lung preponderant, and fibrosis is usually basilar in distribution and often has a UIP-like configuration, although other fibrotic patterns may also predominate (see Fig. 18.4 ). The majority of patients have some degree of diffuse ground-glass opacity likely reflecting superimposed smoking-related respiratory bronchiolitis (RB) or desquamative interstitial pneumonitis (DIP). Patients with CPFE are at increased risk of pulmonary hypertension and lung cancer.
Nonspecific interstitial pneumonia is most often secondary to an underlying condition, most often collagen vascular diseases (particularly scleroderma, myositis, and mixed connective tissue disease [MCTD]), drug-induced pneumonitis, and HP. Idiopathic NSIP is a distinct clinical entity with a good prognosis and typically responds well to steroid treatment.
The histologic features consist of various amounts of interstitial inflammation and fibrosis with a uniform appearance. There are two distinct types: cellular NSIP, with mild to moderate inflammation and little fibrosis, and fibrosing NSIP, with interstitial thickening by uniform fibrosis and preservation of alveolar architecture. The fibrosis is of the same age, unlike the temporal heterogeneity seen in UIP.
Nonspecific interstitial pneumonia is more common in women and is diagnosed at a younger age (average age of 50 years at diagnosis) than UIP and IPF. There is no strong association with smoking. The clinical presentation is nonspecific and includes dyspnea, cough, and weight loss.
The plain radiographic features consist of reticular opacities in the lower lungs without honeycombing ( Box 18.2 ). Chest CT findings are more specific. Typical findings include basilar-predominant ground-glass opacity, reticulation, and traction bronchiectasis—at times quite exuberant and above expected for the severity of adjacent lung disease ( Fig. 18.5 ). The distribution of disease is invariably symmetric in contrast to the often asymmetric distribution of UIP. The axial distribution may be central or peripheral but with subpleural sparing (see Fig. 18.5 ). Subpleural sparing is highly suggestive of NSIP and essentially excludes UIP as the predominant pattern of lung injury. Honeycombing is uncommon in NSIP and, if present, should be mild.
Clinical features
Average age, 50 years
More women than men affected
Dyspnea, dry cough
Histologic feature: temporal homogeneity
Reticulation
Lower zones
Absent or rare honeycombing
Small lungs
Reticulation
Traction bronchiectasis
Ground-glass opacity
Almost always basilar
Axial central or subpleural sparing highly suggestive; often peripheral
Cryptogenic organizing pneumonia (COP), formerly known as bronchiolitis obliterans with organizing pneumonia (OP), is the idiopathic form of OP. Slightly more than half of cases of OP are idiopathic. The more common secondary causes of OP include collagen vascular disease, radiation therapy, medication, aspiration, pneumonia, and solid organ as well as stem cell transplantation. OP typically has a good prognosis and usually responds very well to corticosteroid therapy, although relapses are common. Histologically, OP is characterized by organizing cellular infiltrate and collections of young myxoid colagen with fibroblasts in the distal alveoli, which extend into the distal airways.
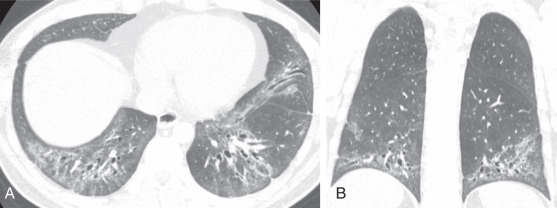
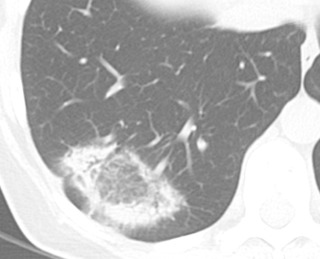
The imaging findings of OP are somewhat heterogeneous and diverse. Consolidation with a variable degree of ground-glass opacity is the most common finding. The changes are usually in a peripheral or peribronchovascular distribution and may be migratory. Airways within areas of lung opacity may transiently dilate. The borders of lung opacity are often “wispy” on CT, which is suggestive of the diagnosis ( Fig. 18.6 ). A reasonably specific finding for OP on imaging is a “perilobular pattern” consisting of irregular opacity with or without septal thickening that surrounds the secondary pulmonary lobule, often in the lower lung zones ( Fig. 18.7 ). Another frequent finding of OP is the atoll or reversed halo sign: a central region of ground-glass opacity with a circumferential border of consolidation ( Fig. 18.8 ). The presence of a substantial degree of reticulation in OP suggests a worse prognosis.
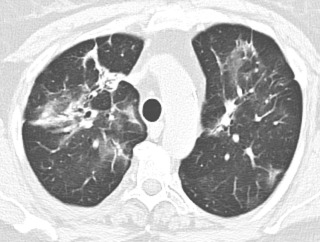
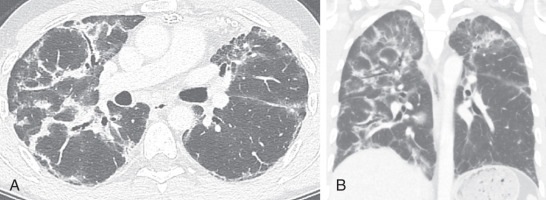
Acute interstitial pneumonia (AIP) is essentially idiopathic acute respiratory distress syndrome (ARDS) and is most often characterized by a diffuse alveolar damage pattern. Affected patients often present with a viral-like prodrome followed by rapid respiratory decompensation. The diagnosis of AIP is that of exclusion, and secondary causes of ARDS such as pneumonia, trauma, transplant rejection, and blood transfusion must be excluded.
As in ARDS, there are typical stages of AIP. First, during the exudative phase, histology will demonstrate edema, hyaline membranes, acute lung inflammation, and alveolar hemorrhage. The organizing phase can develop within a few days with type II pneumocyte hyperplasia and organizing fibrosis. If patients survive their acute illness, some patients may progress to a chronic phase of pulmonary fibrosis.
Imaging findings mirror the histologic stages. On radiography, patchy bilateral consolidation is present during the early exudative phase. On CT, there are diffuse or dependent ground-glass opacities and consolidation ( Fig. 18.9 ). Usually the zonal distribution of disease is diffuse, although there may be a slight basilar preponderance. In the organizing stage, consolidation usually improves both on radiography and CT. A peribronchovascular distribution and bronchial dilation may be evident on CT. In patients who survive and develop fibrosis, the anterior and upper aspects of the lungs are usually more severely affected.
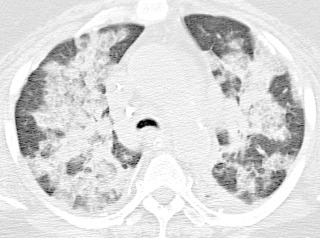
Interstitial pneumonias associated with smoking represent a spectrum of lung disease ranging from the asymptomatic RB to symptomatic respiratory bronchiolitis–interstitial lung disease (RB-ILD) to DIP. RB is very common in smokers and is found on pathology in the vast majority of patients with significant smoking history. Histologically, RB is characterized by collections of pigmented macrophages primarily within a bronchiolar distribution. In patients with RB, RB-ILD is diagnosed if patients have associated symptoms or functional impairment. Radiologic and histologic findings in RB and RB-ILD are essentially identical.
Respiratory bronchiolitis is usually not apparent on radiography, although more severe cases of may manifest as diffuse increased interstitial opacities sometimes referred to as “dirty lungs” or “dirty chest.” CT findings of RB typically include upper lung or diffuse centrilobular ground-glass nodules ( Fig. 18.10 ), similar to findings in subacute HP. A history of smoking is highly suggestive of RB as opposed to HP. The mild immunosuppressant effects of cigarette smoke protect from HP, but smoking is the causative agent in RB. Although most patients improve clinically and on imaging with smoking cessation, a substantial minority of patients will not respond or even worsen over time.
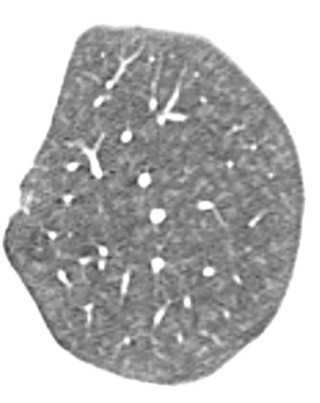
Desquamative interstitial pneumonitis is much less common than RB, and up to 40% of patients with DIP have no history of smoking. Other less common causes of DIP include autoimmune disease, hepatitis C infection, and drug toxicity. The histologic finding in DIP is characterized by a diffuse alveolar infiltration of pigmented macrophages.
A chest radiograph may appear normal or demonstrate a subtle diffuse or basilar-predominant increased opacity in the lungs. On CT, DIP usually manifests as confluent areas of ground-glass opacity primarily in the lung periphery and at the lung bases ( Fig. 18.11, A ). In most cases, small clustered cystic lesions are superimposed on ground-glass opacity; these cysts may represent mild emphysema or very early traction bronchiolectasis caused by pulmonary fibrosis ( Fig. 18.11, B ). Indeed, a minority of cases of DIP progress to frank pulmonary fibrosis, although progression to a classic UIP pattern is not common. Given the relatively nonspecific imaging appearance of DIP, surgical lung biopsy may be necessary to achieve an accurate diagnosis.
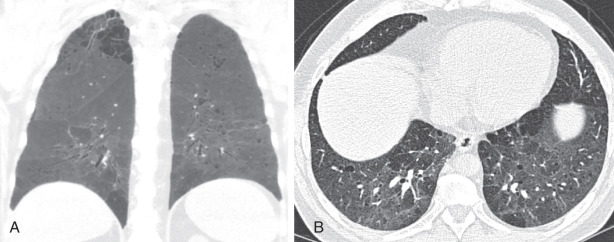
Lymphocytic interstitial pneumonitis (LIP) is one of the rare interstitial pneumonias and is almost always secondary to an underlying condition. Most cases of LIP in adults occur in association with Sjögren syndrome. Other causes include human immunodeficiency virus (usually in children) and hematopoietic stem cell transplantation. The histologic pattern is marked by polymorphic lymphocyte infiltration in the pulmonary interstitium and lymphatics. Imaging findings on radiography are nonspecific, with most cases being normal. On chest CT, a variable degree of patchy ground-glass opacity and nodules may be present, often in the lung periphery and along the bronchovascular tree. In chronic cases, LIP manifests most commonly as a diffuse cystic lung disease, which is basilar and peribronchovascular predominant ( Fig. 18.12 ).
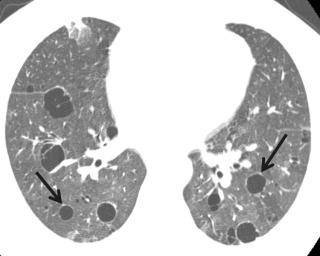
Idiopathic pleuroparenchymal fibroelastosis (IPPFE) is a recent addition to the IIP classification but is poorly understood. Although it is an idiopathic condition, an association with chronic infection and stem cell transplantation has been described. On histology, there is exuberant biapical subpleural fibrosis. CT mirrors the histology; dense pleural and subpleural lung fibrosis affects the lung apices symmetrically and often extends along the superior margins of the upper lobes ( Fig. 18.13 ). A similar imaging appearance occurs in the setting of chronic lung transplant rejection, known as restrictive allograft syndrome (RAS).
Thoracic involvement in CTD is quite common. Each CTD is associated with a particular autoantibody or collection of antibodies; indeed, often serology is the main means by which diagnosis is achieved ( Table 18.1 ). Thoracic involvement from CTD may be pulmonary, airway, pleural, gastrointestinal, or vascular. The pulmonary manifestations of CTD usually mirror the patterns described in the IIPs ( Fig. 18.14 and Table 18.2 ). In approximately 15% of cases, lung disease may be the initial manifestation of CTD and can precede overt clinical presentation by 5 years. Other common manifestations of CTD in the thorax that aid in achieving accurate diagnosis are the presence of pleural or pericardial effusion, esophageal dilation, pulmonary arterial enlargement (from pulmonary hypertension), soft tissue calcification (more common in scleroderma or dermatomyositis), and shoulder or acromioclavicular joint erosive arthritis in RA.
| Disease | Autoantibodies |
|---|---|
| RA | RF, anti-CCP |
| Scleroderma | Anti-centromere antibody, anti–SCL-70 |
| MCTD | Anti-RBNP |
| DM, PM, AS | Anti–Jo-1, anti–aminoacyl-tRNA synthetases |
| SLE | Anti-ds DNA, anti-Smith, antiphospholipid |

| Imaging Pattern | RA | SLE | MCTD | Scleroderma | PM, DM, AS | Sjögren |
|---|---|---|---|---|---|---|
| UIP | ++ | + | + | ++ | + | + |
| NSIP | + | + | ++ | ++++ | ++ | + |
| OP | ++ | + | + | + | ++ | − |
| LIP | − | − | − | − | − | ++ |
| PH | + | + | + | ++ | − | + |
| Bronchiectasis | ++ | − | − | − | − | ++ |
| OB | ++ | + | − | − | − | − |
The most common ILD pattern to affect patients with RA is a UIP pattern. Often, the imaging manifestation of UIP in RA is indistinguishable from that in IPF. However, there are some CT findings that are more suggestive of RA than IPF: substantial fibrosis in the anterior upper lobes (in addition to basilar fibrosis), exuberant honeycombing that affects the vast majority of fibrotic lung, and isolation of fibrosis to the lung bases without substantial extension along the lateral margins of the lungs on coronal images. NSIP and OP may also occur in RA but are less common patterns.
Patients with RA may also be affected by obliterative bronchiolitis (OB). Although it may be difficult to differentiate OB from severe asthma on CT because both conditions present with severe air trapping, a history of RA and nonreversibility are suggestive of OB rather than asthma. Follicular bronchiolitis is a rare condition that manifests as centrilobular or tree-in-bud nodularity on imaging and can affect patients with RA but should only be considered after more common causes of tree-in-bud opacity (aspiration or pneumonia) have been thoroughly excluded.
Interstitial lung disease is rare in systemic lupus erythematosus (SLE), although patients with SLE are predisposed to development of pulmonary hemorrhage and pulmonary infections. SLE more often presents with pleural or pericardial effusions or thickening. Pleural thickening may result in restrictive thoracic physiology that mimics pulmonary fibrosis on pulmonary function testing and can lead to substantial patient morbidity. Some patients develop diaphragmatic weakness, which leads to chronically low lung volumes—the so-called shrinking lung syndrome that may mimic mild ILD on radiography caused by vascular crowding and atelectasis.
Mixed connective tissue disease is a distinct clinical entity defined by the presence of the antiribonucleoprotein (RNP) antibody. ILD in MCTD is common and occurs in up to 60% of patients with MCTD; NSIP is the most common ILD pattern in MCTD. It differs from interstitial pneumonia with autoimmune features (IPAF), which is a heterogeneous group of conditions that present with ILD and suspected autoimmune disease not meeting criteria for a well-defined CTD.
Patients with scleroderma are especially susceptible to the development of ILD, with some series showing that the majority of patients develop ILD. The most common CT pattern is NSIP followed by UIP. Given the high prevalence of esophageal dysmotility and pulmonary hypertension in scleroderma, findings of dysmotility (esophageal dilation, gas–fluid level, open esophageal sphincter) and pulmonary hypertension (pulmonary artery size and cardiac chamber enlargement) should be specifically addressed.
Inflammatory myositis (dermatomyositis, polymyositis, and antisynthetase syndrome) commonly cause ILD, occurring in 50% to 75% of cases. The Jo-1 antibody is associated with ILD in the setting of myositis and can predict the development of ILD in the future as well as response to therapy. Most patients with ILD present initially with NSIP and OP—CT shows basilar ground-glass opacity, mild reticulation, mild traction bronchiectasis, and superimposed bronchovascular or peripheral lung consolidation. With corticosteroid therapy, consolidation (OP) often resolves, leaving residual ground-glass opacity and reticulation (NSIP). This disease course is typical and highly suggestive of myositis-related ILD.
Sjögren syndrome is a relatively rare disease; the most common ILD to develop in this setting is LIP as described previously. Mild bronchiectasis is also common in this condition.
Hypersensitivity pneumonitis ( Box 18.3 ), also known as extrinsic allergic alveolitis, describes a spectrum of granulomatous and interstitial pulmonary disorders associated with intense and often prolonged exposure to a wide range of inhaled organic or inorganic dusts, gases, or organisms. The site of inflammatory host response in these disorders is located primarily in the alveolar air exchange portion of the lung and not in the large conducting airways that are involved in asthmatic diseases.
Causes
Inhaled organic dust
Occupational antigens
Animal proteins
Saprophytic fungi
Dairy and grain products
Water vaporizers
Stages
Acute (4–6 hours)
Subacute: after resolution of acute stage, between episodes
Chronic: fibrosis, occurring months or years after exposure
Diagnosis
History related to exposure
Precipitating antibodies
Positive inhalational challenge
Acute
Airspace consolidation (diffuse)
Rapid clearing
Subacute
Nodular or reticular nodular opacities
Upper zones
Centrilobular nodules on computed tomography
Ground-glass opacity on computed tomography
Chronic
Medium to coarse linear opacities
Honeycombing
Upper zones
An innumerable number of antigens may cause HP. However, the most common antigens to cause HP are avian and mold related. These antigens, usually disseminated as aerosol dust, can be derived from animal dander and proteins; saprophytic fungi (i.e., spores) in contaminated vegetables, wood, bark, or water reservoir vaporizers; and dairy and grain products. The exposure is often occupational. The organic antigen may be a microbial organism, and the most commonly incriminated microbe is thermophilic Actinomyces, a ubiquitous bacterium that has the morphologic features of a fungus. This is the offending antigen in one of the most common types of HP, farmer's lung.
Hypersensitivity pneumonitis is often categorized into acute, subacute, and chronic stages. However, there is often substantial overlap in the presentation of patients. Acute HP is caused by intermittent high-intensity antigenic exposure, with symptoms occurring hours after antigen exposure and with resolution within 1 day to 2 weeks of antigen withdrawal. Subacute HP is caused by continuous low-intensity antigen exposure; repeated exposure; or, rarely, the manifestation of long-standing undiagnosed acute HP. Chronic HP may occur as progression of known acute or subacute HP but may also arise from chronic low-level exposures to an antigen without any acute or subacute events.
The first clue to the diagnosis of HP is a good clinical history that suggests the temporal relationship between the patient's symptoms and certain activities, including hobbies and occupation. The acute form of the disease is characterized by cough, dyspnea, and fever, which usually begin 4 to 6 hours after exposure to large quantities of the causative agent. There is often a leukocytosis, and pulmonary function studies reveal restrictive dysfunction. Usually characterized by dyspnea and chronic cough, subacute and chronic disease associated with low-grade exposure to the offending antigen may be confused with other forms of ILD. Laboratory studies consistent with the diagnosis include precipitating antibodies reactive to the offending dust antigen and a positive inhalational challenge that can reproduce the symptoms.
The imaging features of HP vary with the stage of the disease. A minority of patients with acute or subacute HP have a normal or near-normal CT scan.
In the acute stage , consolidation may be observed, especially in the lower lung zones. This reflects pathologic findings of alveolar filling by polymorphonuclear leukocytes, eosinophils, lymphocytes, and large mononuclear cells. Consolidation may be quite extensive, simulating pulmonary edema, but it is transitory and usually clears within hours or days. It is uncommon to image patients during the acute stage of disease.
The subacute stage is characterized by a fine nodular or reticulonodular pattern, although the radiographic appearance is largely nonspecific and usually not helpful in achieving a specific diagnosis. This nodular appearance corresponds pathologically with alveolitis, interstitial infiltration, small granulomas, and some degree of bronchiolitis. Histologic abnormalities are usually most severe in a peribronchiolar distribution. CT is very helpful in the diagnosis of the subacute HP. Characteristic findings include ground-glass opacity, often in a centrilobular distribution (see Fig. 18.10 ), with superimposed mosaic attenuation and air trapping. The “head cheese” sign manifests as three distinct densities demarcated by the margins of secondary pulmonary lobules on expiratory chest CT and is nearly pathognomonic for HP in the subacute to chronic setting ( Fig. 18.15 ).
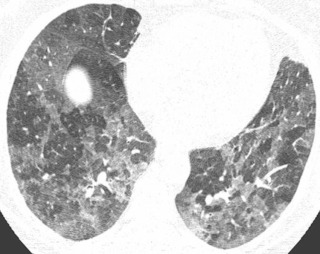
In the chronic stage of HP, continued exposure to the antigen results in changes characteristic of pulmonary fibrosis: reticulation, traction bronchiectasis, traction bronchiolectasis, volume loss, and honeycombing. Although upper lung preponderance is a helpful finding in differentiating HP from UIP and NSIP, HP is only upper lung preponderant in a minority of cases ( Fig. 18.16 ). In many cases, the distribution of chronic HP is basilar preponderant, mimicking UIP or NSIP. However, residual imaging findings of subacute HP (centrilobular ground-glass opacity or air trapping) may be present and suggest the correct diagnosis in these cases. In a minority of chronic HP cases, emphysema may develop even in the absence of smoking history, particularly in farmer's lung.
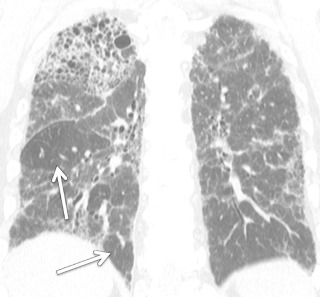
If the environmental source of the inhaled antigen is identified, simple avoidance is usually sufficient treatment; however, in up to 50% of cases, the antigen is never definitively identified. The acute form of the disease abates without specific therapy. With chronic forms of disease, a trial of corticosteroids can be given. A small proportion of cases may progress despite antigen avoidance and corticosteroids.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here