Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pediatric diffuse lung disorders, also known as childhood interstitial lung diseases (chILDs), comprise a rare and heterogeneous spectrum of progressive and often lethal pulmonary pathology. They are characterized by otherwise unexplained respiratory signs and symptoms, hypoxemia, and widespread pulmonary opacities on imaging. The diseases are not limited to the interstitium and may affect the alveoli, airways, blood vessels, lymphatic channels, and pleural spaces. Although much remains unknown, the revised chILD classification scheme, recognizing disorders distinctly occurring in infants under 2 years of age ( Box 56.1 ), along with advances in imaging and lung biopsy techniques, has greatly improved understanding. In this chapter, the diseases of infancy are discussed, followed by an overview of diffuse lung disorders more prevalent in children over 2 years of age. The connective tissue, collagen vascular, and storage diseases are detailed in Chapter 57 .
Acinar dysplasia
Congenital alveolar dysplasia
Alveolar capillary dysplasia with misalignment of pulmonary veins
Prenatal: secondary pulmonary hypoplasia
Postnatal: chronic lung disease
Prematurity-related chronic lung disease (bronchopulmonary dysplasia)
Chronic lung disease in term infant
Associated chromosomal or genetic abnormalities
Trisomy 21
Other (e.g., filamin A mutation)
Associated congenital heart disease
Surfactant dysfunction disorders
SpB genetic mutations (pulmonary alveolar proteinosis and variants)
SpC genetic mutations (chronic pneumonitis of infancy; also pulmonary alveolar proteinosis, desquamative interstitial pneumonitis, and nonspecific interstitial pneumonia)
Adenosine triphosphate–binding cassette transporter protein A3 genetic mutation (pulmonary alveolar proteinosis; also CPI, diffuse interstitial pneumonitis, and nonspecific interstitial pneumonia)
Congenital GM-CSF receptor deficiency (pulmonary alveolar proteinosis histologic pattern)
Thyroid transcription factor-1 genetic mutations
Others: histology consistent with surfactant dysfunction, unrecognized genetic disorder
Lysinuric protein intolerance (pulmonary alveolar proteinosis histologic pattern)
Neuroendocrine cell hyperplasia of infancy
Pulmonary interstitial glycogenosis
Primary
Associated with other pulmonary conditions, especially alveolar growth disorders
Modified with permission from Lee EY, Cleveland RH, Langston C: Interstitial lung disease in infants and children: new classification system with emphasis on clinical, imaging, and pathologic correlation. In: Cleveland RH, ed. Imaging in Pediatric Pulmonology , New York: Springer; 2011.
Diffuse developmental disorders are characterized by marked alveolar gas exchange impairment and are thought to arise early in prenatal lung development. The three main entities within this category are acinar dysplasia, congenital alveolar dysplasia, and alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV). Acinar dysplasia and congenital alveolar dysplasia are caused by arrest of lung development in the pseudoglandular/early canalicular and late canalicular/early saccular phases, respectively. ACD/MPV results from an abnormal location of pulmonary vein branches next to the pulmonary arteries along with medial hypertrophy of the pulmonary arterioles, reduced alveolar capillary density, and maldevelopment of pulmonary lobules; FOXF1 gene mutations and 16q24.1 microdeletions are implicated in some cases.
Chest radiographs, often the only imaging available, may initially appear normal but progress to hazy bilateral pulmonary opacities, resembling surfactant deficiency. Lung volumes are typically normal to decreased but increase with ventilator support. Barotrauma-related air leaks such as pneumothorax and pneumomediastinum develop in about half of patients ( Fig. 56.1 ). With concurrent pulmonary hypertension (PHT), the main pulmonary artery may enlarge. Ultimately, imaging findings are nonspecific; however, the diagnosis should be considered in the term neonate with persistent unexplained respiratory distress.
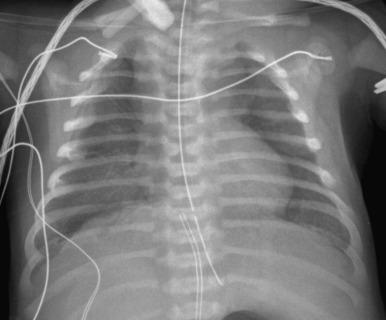
Patients develop rapidly progressive and nearly always lethal respiratory failure in the first 2 months of life despite treatment for PHT, intensive ventilation, and extracorporeal membrane oxygenation (ECMO). Serial chest radiographs can monitor the progression of the disease and help identify acute complications that may be seen with prolonged ventilatory support. Patients generally do not survive long enough for lung transplantation, the only viable treatment. More than 80% of patients with ACD/MPV have associated extrapulmonary anomalies (e.g., cardiac, gastrointestinal, or genitourinary), for which screening may be performed. Genetic counseling may be offered to relatives of patients with ACD/MPV, which appears heritable in 10% of cases.
Alveolar growth disorders are the most common form of neonatal interstitial lung disease (ILD), caused by an unprogrammed insult to lung development, resulting in defective alveolar formation, lobular simplification, lack of alveolar septation, and airspace enlargement. Prematurity-related chronic lung disease (bronchopulmonary dysplasia [BPD]) is a frequently encountered subtype. Also included within this group are structural pulmonary changes related to chromosomal abnormalities or congenital heart disease and pulmonary hypoplasia due to oligohydramnios, space-occupying masses, and neuromuscular disease, among other etiologies.
Features are variable. Chest radiographs in infants with classic BPD show coarse reticular opacities, cystic appearing lucencies, and disordered lung aeration due to alveolar septal fibrosis and hyperinflation. However, with ever-earlier delivery possible, there has been a shift in the imaging features of BPD. Findings in so-called “new” BPD range from near normal to markedly disordered lung parenchyma, variably sized pulmonary lobules, thick perilobular reticular opacities, linear and triangular subpleural opacities, ground-glass opacities, and hyperlucent areas, some of which resemble cysts or may be mistaken for emphysema ( Fig. 56.2 ). Recently, the pulmonary findings of BPD known by radiography and computed tomography (CT) have been successfully delineated by a novel ultrashort echo time (UTE) magnetic resonance imaging (MRI) technique. In infants with trisomy 21, small subpleural cysts are characteristic ( Fig. 56.3 ). In patients with X-linked filamin A gene mutations causing alveolar growth disturbances, characteristic findings include central pulmonary artery enlargement, atelectasis, progressive severe pulmonary hyperinflation, hyperlucency, and peripheral pulmonary vascular hypoattenuation resembling congenital lobar or acquired emphysema ( e-Fig. 56.4 ).
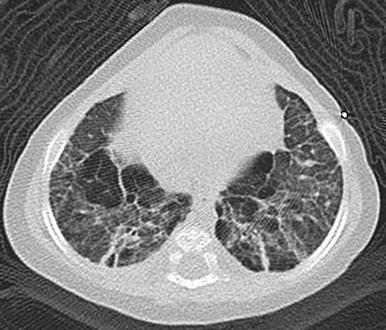
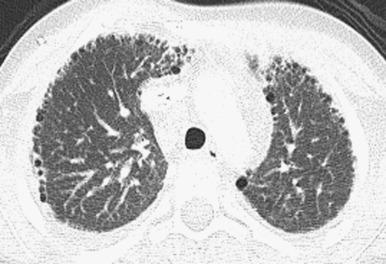
Decreasing respiratory support helps reduce BPD incidence and severity. Low-dose corticosteroids, fluid restriction, vitamin A, and patent ductus arteriosus closure are of equivocal benefit. Linear and subpleural opacities seen on CT correlate with interstitial fibroproliferation but not symptom severity. Lung biopsy may be considered to exclude the not-uncommon coexistence of pulmonary interstitial glycogenosis (PIG) (detailed later), which can be steroid-responsive. Lung transplantation is usually inevitable with filamin A mutations due to severe respiratory decline.
These disorders are caused by genetic mutations affecting surfactant metabolism, including surfactant proteins B (SpB) and C (SpC) and the adenosine triphosphate–binding cassette transporter protein A3 (ABCA3). Also included in this category are rare genetic disorders that indirectly influence surfactant production, such as thyroid transcription factor-1 (TTF-1)/NK homeobox 1 (NKX2.1) abnormalities (“brain-lung-thyroid syndrome”), lysinuric protein intolerance, and granulocyte-macrophage colony-stimulating factor–Rα mutations. Many etiologies remain uncharacterized.
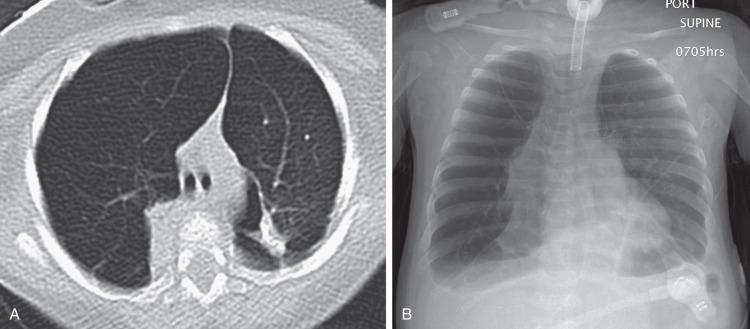
Chest radiographs show diffuse or patchy hazy granular pulmonary opacities. CT demonstrates diffuse ground-glass opacity, consolidation, interlobular septal thickening, or a crazy-paving pattern typical of pulmonary alveolar proteinosis (PAP) (detailed in a later section) ( Fig. 56.5 ). With increasing age, ground-glass opacities recede, and progressively larger and more numerous cysts develop ( e-Fig. 56.6 ). Pectus excavatum may develop due to the effects of chronic lung disease on the growing chest wall.
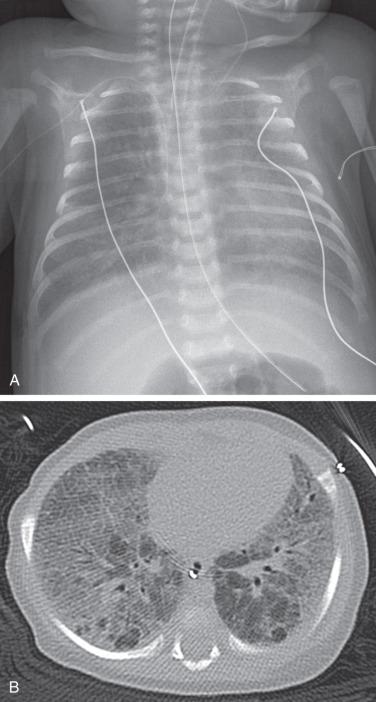
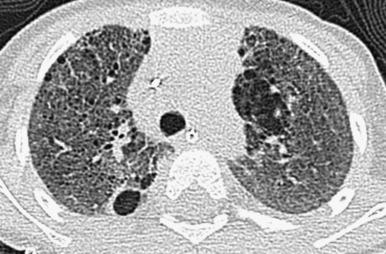
The diagnosis can be established by genetic testing, obviating lung biopsy. The mainstays of treatment are chronic ventilator support, nutritional supplementation, and potential lung transplantation, with anecdotal roles for pulse corticosteroids, hydroxychloroquine (Plaquenil), and azithromycin. Targeted gene therapy remains an area of investigation.
The etiology of neuroendocrine cell hyperplasia of infancy (NEHI) is unknown. Histopathologically, the disease (previously referred to as persistent tachypnea of infancy) is characterized by increased numbers of pulmonary neuroendocrine cells (PNECs), which function in oxygen sensing and fetal lung development and normally rapidly decline after the neonatal period. Some cases have an identifiable genetic component, including links to NKX2.1 mutations.
Chest radiographs demonstrate hyperinflation and variable increased perihilar opacity resembling bronchiolitis or reactive airways disease ( Fig. 56.7 ). CT characteristically shows a mosaic attenuation pattern indicating air trapping that affects at least four lobes, with geographic ground-glass opacities most prominent in the right middle lobe, lingula, and paramediastinal lung regions ( Fig. 56.8 ). In experienced hands, the sensitivity and specificity of CT for NEHI diagnosis are reported to be 78% to 83% and 100%, respectively; thus CT may obviate the need for lung biopsy.
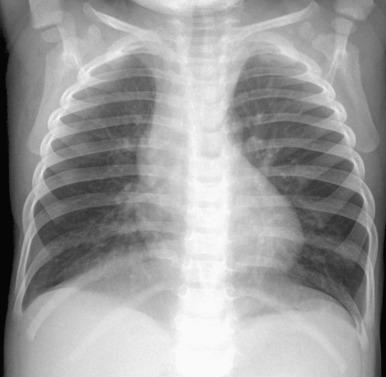
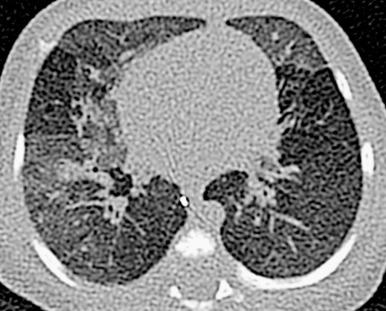
Treatment of NEHI at present is supportive, geared toward preventing hypoxemia and infection and maintaining nutritional support. Although patients may be persistently symptomatic or require long-term oxygen therapy, the prognosis is usually favorable, without progressive respiratory failure or directly attributable death. In later life, acute exacerbations may occur due to intermittent air trapping or infection.
The etiology of PIG, previously known as infantile cellular interstitial pneumonitis and histiocytoid pneumonia, remains unknown. PIG is characterized histopathologically by infiltration of the interstitium with immature mesenchymal cells containing copious cytoplasmic glycogen and staining positively for vimentin. The lack of PIG in lung biopsies from children older than 10 months suggests that the disorder may be related to lung growth and development. Patchy PIG and alveolar growth abnormalities commonly coexist.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here