Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter covers diffuse aggressive B-cell lymphomas in immunocompetent patients. In the 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms concepts regarding diffuse and aggressive B-cell lymphomas have been further revised to reflect the current understanding of these diseases. Box 8.1 shows the current entities considered under this topic, most of which will be covered in this chapter. The immunodeficiency-related diffuse aggressive lymphomas (plasmablastic lymphoma in human immunodeficiency virus [HIV], Epstein-Barr virus–positive [EBV+] mucocutaneous ulcer) will also be briefly discussed in Chapter 10 . De novo diffuse large B-cell lymphoma (DLBCL), which represents the most common type of non-Hodgkin lymphoma, is a clinically and biologically heterogeneous disease. For the purposes of this discussion, DLBCL can be divided into morphologic variants, cell of origin variants, and clinically and biologically distinct subtypes ( Box 8.2 ). DLBCL can also result from transformation of a preexisting low-grade lymphoma, such as chronic lymphocytic leukemia–small lymphocytic lymphoma (i.e., CLL/SLL), follicular lymphoma, or marginal zone lymphoma. Unless the history is known or a low-grade component is represented in the biopsy (so-called composite histology), it might not be possible to distinguish these transformed lymphomas from de novo DLBCL.
Diffuse large B-cell lymphoma, not otherwise specified
Germinal center B-cell type
Activated B-cell type
T-cell/histiocyte-rich large B-cell lymphoma
Primary DLBCL of the central nervous system (CNS)
Primary cutaneous DLBCL, leg type
EBV+ DLBCL, NOS
EBV + mucocutaneous ulcer
DLBCL associated with chronic inflammation
Lymphomatoid granulomatosis
Primary mediastinal (thymic) large B-cell lymphoma
Intravascular large B-cell lymphoma
ALK+ large B-cell lymphoma
Plasmablastic lymphoma
Primary effusion lymphoma
HHV8 + DLBCL, NOS
Burkitt lymphoma
Burkitt-like lymphoma with 11q aberration
High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements
High-grade B-cell lymphoma, NOS
B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classic Hodgkin lymphoma
Provisional entities in italics.
Centroblastic
Immunoblastic
Anaplastic
Germinal center B-cell type
Activated B-cell type
T-cell/histiocyte-rich large B-cell lymphoma
Primary DLBCL of the CNS
Primary cutaneous DLBCL, leg type
EBV+ DLBCL of the elderly
Other types of large B-cell lymphomas (LBCLs) are recognized ( Box 8.3 ) and include EBV+ DLBCL, EBV+ mucocutaneous ulcer, DLBCL associated with chronic inflammation, primary mediastinal LBCL, intravascular LBCL, lymphomatoid granulomatosis, ALK -positive LBCL, plasmablastic lymphoma, primary effusion lymphoma, and herpesvirus 8–positive (HHV8+) DLBCL, not otherwise specified (NOS). The latter two entities are discussed with the immunodeficiency-related lymphomas (see Chapter 10 ) and will not be reviewed here. This chapter also discusses Burkitt lymphoma (BL) and the newly created diagnostic entities Burkitt-like lymphoma with 11q aberration, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, and high-grade B-cell lymphoma, NOS.
EBV+ DLBCL, NOS
EBV+ mucocutaneous ulcer
DLBCL associated with chronic inflammation
Primary mediastinal large B-cell lymphoma
Intravascular large B-cell lymphoma
Lymphomatoid granulomatosis
ALK-positive large B-cell lymphoma
Plasmablastic lymphoma
Primary effusion lymphoma
HHV8+ DLBCL, NOS
De novo DLBCL, NOS is the most common type of non-Hodgkin lymphoma, representing 30% to 40% of all non-Hodgkin lymphomas. Although it can occur in children, this disease occurs primarily in adults, with a median age of onset in the seventh decade. As with most lymphomas, there is a male predominance. Patients may have a rapidly enlarging mass. This usually represents a lymph node; however, occurrence at an extranodal site is common (40%). The most common extranodal site is the gastrointestinal tract, followed by the skin, although essentially any extranodal site can be involved. B symptoms (weight loss, fever, night sweats) are seen in approximately 30% of patients.
Most common type of non-Hodgkin lymphoma
Median age of onset in 7th decade, males > females
Rapidly enlarging mass
Diffuse infiltrate of large cells
Morphologic variants
Centroblasts
Immunoblasts
Anaplastic
CD19+, CD20+, sIg+
CD10+ (subset), BCL2+ (subset), BCL6+ (subset), MUM1+ (subset)
Germinal center B-cell type:
Hans: CD10+ or CD10−/BCL6+/MUM1−
Choi: GCET1+/MUM1−, GCET1−/CD10+, or
GCET1−/CD10−/BCL6+/FOXP1−
Non-germinal center B cell:
Hans: CD10−/BCL6− or CD10−/BCL6+/MUM1+
Choi: GCET1+/MUM1+, GCET1−/CD10−/BCL6− or
GCET1−/CD10−/BCL6+/FOXP1+
Rearranged immunoglobulin genes
20% IGH/BCL2
30% 3q27 abnormalities
Gene expression profiling suggests germinal center, activated B cell and “type 3” types
Anthracycline-containing multi-agent chemotherapy + rituximab
55% 5-year survival, outcome can be stratified based on the International Prognostic Index
Peripheral T-cell lymphoma, Burkitt lymphoma, lymphoblastic lymphoma, blastoid or pleomorphic mantle cell lymphoma, myeloid sarcoma, carcinoma, melanoma
By definition, the malignant cells proliferate and infiltrate in a diffuse pattern. Usually there is complete effacement of the lymph node architecture, although occasionally there is partial involvement. In the lymph node, partial involvement can manifest as interfollicular or sinusoidal infiltration. Sclerosis can be seen in some cases and may even impart a pseudonodular pattern. Lack of true follicle formation can be confirmed with immunostaining showing lack of a follicular dendritic cell meshwork that is typical of follicular lymphomas. In extranodal sites, obliteration of the normal epithelial components is seen. In a mucosal site, such as the stomach, epithelial invasion by large cells with formation of lymphoepithelial lesions is sometimes seen. Although this is a feature of mucosa-associated lymphoid tissue (MALT)-type lymphomas, such LBCLs are currently considered and classified as extranodal DLBCL, NOS. In such cases an effort should be made to determine whether a conventional low-grade lymphoma (MALT lymphoma) is also present in the biopsy, indicating transformation rather than a de novo DLBCL, NOS.
DLBCL, NOS is cytologically heterogeneous, with morphologic variants recognized. Common to all is a nuclear chromatin pattern that is open and vesicular compared to the condensed chromatin pattern of small B-cell lymphomas. Nucleoli may be single or multiple and variably sized. Cells are usually large (compared to the nuclear size of a benign histiocyte) but occasionally can be intermediate in size (approximately the same size as a benign histiocyte). Three morphologic variants are recognized in the WHO classification, but it is not required to report them. They are the centroblastic, immunoblastic, and anaplastic variants ( Figs. 8.1 and 8.2 ). Centroblastic cells are characterized by round to oval large nuclei with vesicular chromatin and multiple (two to four) small nucleoli that are closely applied to the nuclear membrane. Cytoplasm is scant, but visible, particularly on Giemsa staining, in which it has a basophilic quality. In some cases, polylobated nuclei predominate as opposed to the typical round contours. The occurrence of polylobated nuclei typically imparts a smaller cell size and can make distinction from small B-cell lymphomas difficult. Multilobated cells are common in some extranodal lymphomas, such as primary lymphoma of bone. Immunoblasts are large cells with a single, prominent central nucleolus and more abundant basophilic cytoplasm. Plasmacytoid differentiation can be seen. Diagnosis of the immunoblastic variant is appropriate when more than 90% of cells are of this type. Some studies have suggested a worse outcome for patients with this variant. The anaplastic variant is composed of sheets of anaplastic, atypical cells with bizarre multilobated–multinucleated cells that may resemble Reed-Sternberg cells. This term reflects both cytologic features and pattern of growth. For example, anaplastic DLBCL, NOS may grow within lymph node sinuses, reminiscent of an epithelial tumor. Pleomorphic may be a better term than anaplastic, because the latter can be confused with anaplastic large-cell lymphoma of T-cell lineage.
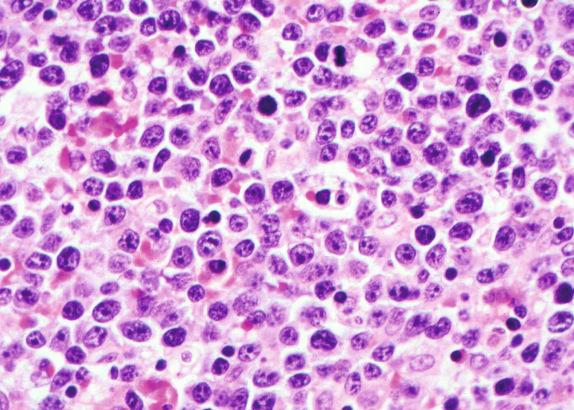
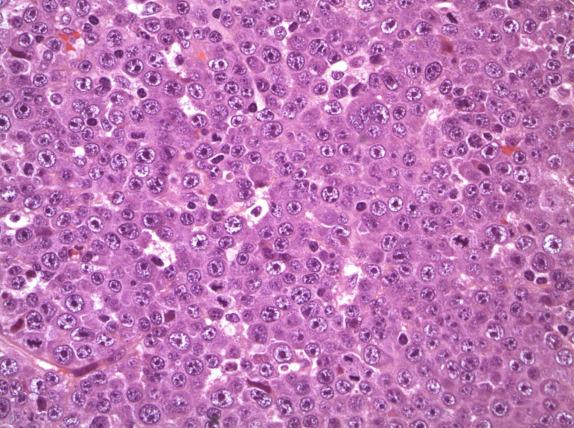
Other unusual morphologic variants have been described, and it is useful to be aware of them primarily because they can initially be mistaken for other non-hematolymphoid tumors until immunophenotyping is performed. These variants include signet ring cell, microvillus, myxoid, and spindle cell variants ( Fig. 8.3 ).
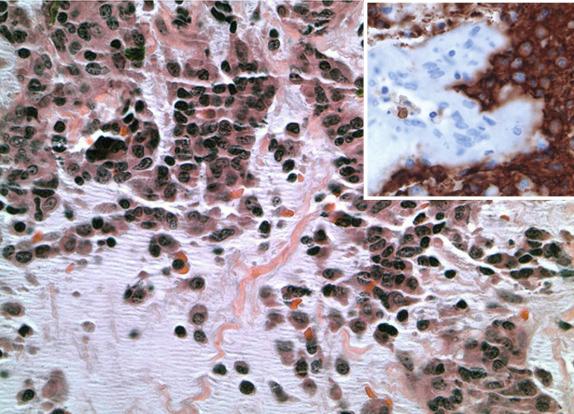
Approximately 5% to 15% of patients with DLBCL, NOS have bone marrow involvement, and this is associated with an adverse outcome. The histology, however, is highly variable. The pattern can be paratrabecular, interstitial, or diffuse. The diffuse pattern is uncommon and is usually seen when the lymphomatous infiltrate is composed of large cells. The cytology is often discordant (approximately half of the cases), meaning the marrow infiltrate is composed of small lymphocytes or mixed small and large lymphocytes. Studies have shown that patients with concordant large cells in the bone marrow have a poor response to therapy and poor overall survival. This finding is independent of the International Prognostic Index (IPI) score. The presence of discordant marrow involvement at the time of staging (small B cells in the marrow) has been shown to be a predictor of inferior progression-free survival, but it is not independent of the clinical IPI variables. Discordant disease travels with older age, elevated serum lactate dehydrogenase (LDH), advanced stage, and increased number of extranodal sites.
Immunophenotypically, the malignant B cells of DLBCL, NOS express the pan B-cell markers CD19, CD20, CD22, CD79a, and PAX5 in the vast majority of cases. Although lack of CD20 expression is rarely encountered in pretreatment cases, and CD20 may be lost in cases occurring following rituximab therapy. Beyond this DLBCL, NOS is an immunophenotypically heterogeneous disease. CD10 is expressed in 25% to 50% of cases, BCL6 is expressed 50% to 90% of cases, and MUM1 is expressed in 35% to 65% of cases. Surface immunoglobulin (Ig) is expressed in most cases, but in approximately 10% of cases it is undetectable by flow cytometry. Other variably expressed antigens include CD30 (10% to 40% of cases), CD5 (<10% of cases), and cyclin D1 (~2% of cases with weak and focal staining). Expression of Ki-67 is highly variable and can range from 30% to more than 95%.
Gene expression profiling (GEP) has shown that DLBCL, NOS can be classified into prognostically significant subtypes based on the cell of origin (COO)—namely, germinal center B-cell type (GCB) and activated B-cell (ABC) type—with the ABC-type DLBCL, NOS exhibiting a different biology and worse overall response to cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab (R-CHOP) therapy compared to the GCB type. For this reason the current WHO classification requires the identification of the COO subtype. Until recently GEP has required the use of fresh frozen tissue and has not been widely used in clinical laboratories. However, algorithms based on immunohistochemical (IHC) staining have been devised that can be routinely performed and can differentiate the COO subtypes of DLBCL, NOS with close concordance to the GEP classification scheme. The Hans algorithm uses the expression pattern of CD10, BCL6, and MUM1 and has approximately 80% to 86% concordance with the GEP classification. The Choi algorithm uses the expression pattern of CD10, BCL6, MUM1, GCET1, and FOXP1 and has approximately 93% concordance with the GEP classification ( Fig. 8.4 ). Another approach referred to as the tally algorithm uses a combination of IHC antibodies but without regard to order and shows the highest concordance with GEP. Germinal center markers (CD10 and GCET1) and activated B-cell markers (MUM1 and FOXP1) are scored (≥30% of cells), and cases are classified based on whether more germinal center or ABC markers are present. In case of a tie, LMO2 is used to determine subtype (≥30% + Cells = GCB; <30% + Cells = non-GCB).

MYC and BCL2 are two other proteins that are also variably expressed in DLBCL, NOS and approximately 25% to 30% of cases express both when using cut-offs of ≥40% and ≥50% for MYC and BCL2, respectively. These so-called dual expressers (also sometimes referred to as “double expressers”) are enriched in the ABC type and are associated with worse response to R-CHOP therapy, independent of the IPI, COO type, and underlying MYC and BCL2 gene rearrangements. Dual expression of MYC and BCL2 also defines a group of patients at high risk of central nervous system relapse. Importantly, dual expression of MYC and BCL2 is not a surrogate marker for dual rearrangement of the MYC and BCL2 genes (so-called double-hit lymphoma; see discussion below). Molecular genetic testing by fluorescence in situ hybridization (FISH) analysis is required to exclude underlying gene rearrangements of the MYC and BCL2 genes.
Because DLBCLs are monoclonal B-cell neoplasms, Ig genes are usually clonally rearranged whereas T-cell receptor genes are typically in the germline configuration.
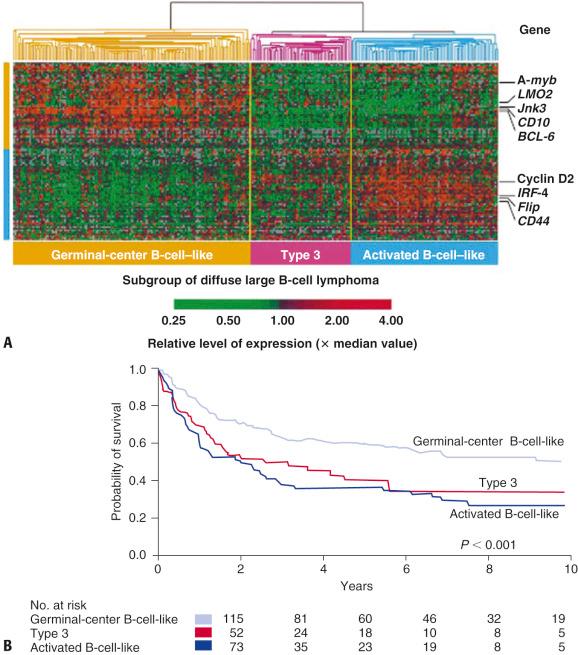
Our understanding of the molecular heterogeneity of DLBCL, NOS has been advanced by gene expression profiling. DLBCL, NOS can be segregated into at least three subgroups based on similarities to normal cell types: GCB (45% to 55%), ABC (35% to 45%), and the unclassifiable “Type 3” group (10%), which shows a probability of less than 90% based on gene expression of belonging to either GCB or ABC subtypes ( Fig. 8.5 ). The IHC classifiers use binary GCB versus ABC/non-GCB categories only, and thus the unclassifiable cases may be variably distributed within these two groups, making these algorithms less biologically meaningful. However, GEP has recently become possible in formalin-fixed paraffin-embedded (FFPE) tissue specimens, and assays, such as Lymph2Cx, have been developed to determine COO in DLBLC, NOS using FFPE with near equivalent concordance to the fresh frozen tissue–based assays. These FFPE-based assays are likely to find their way into routine clinical practice in the near future, particularly if the results of ongoing clinical trials with targeted drugs indicate that therapeutic decisions should be made based on COO. Mini-gene predictors, using two to six genes, have also been described that appear to be independent of the clinical IPI variables.
The cell-of-origin distinctions also have underpinnings in biology, with molecular genetics and next-generation sequencing having been helpful in furthering our understanding of the molecular alterations underlying these disease subtypes. GCB-type DLBCL harbors rearrangements of BCL2 exclusively, usually in the form of a t(14;18)(q32q21) ( IGH-BCL2 ), which is seen in approximately 35% to 45% of cases. MYC rearrangements are also largely restricted to the GCB-type occurring in approximately 10% of all DLBCL, NOS cases. Co-occurrence of BCL2 and MYC rearrangements is seen in approximately 5% of all DLBCL cases, called double-hit lymphoma, and is associated with aggressive behavior. DLBCL cases that harbor a double-hit abnormality are re-classified as high-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements (discussed later); thus laboratories must have a strategy to find these cases. Some have elected to preform FISH testing on all cases of diffuse aggressive B-cell lymphomas, whereas others have elected screening strategies that involve MYC IHC. GCB-type DLBCL is also enriched with recurrent mutations or deletions in genes associated with epigenetic regulation ( EZH2 , CREBBP , EP300 , MLL2 , MLL3 , MEF2B , and TET2 ). Recurrent mutations in genes associated with B-cell receptor, Toll-like receptor, and NF-κB signaling pathways ( CARD11, CD79A , CD79B , MYD88, TNFPAI3 , and NFKBIE ) are also seen in a minority of cases. In addition, recurrent mutations in DNA repair/genomic integrity pathways are also identified ( TP53 ) in 10% to 20% of cases. The EZH2 mutation is exclusive to GCB-type DLBCL, NOS and is found in approximately 20% of cases. It is a gain-of-function mutation that leads to increased expression of the EZH2 protein, which is responsible for methylation of histone H3 at lysine 27 leading to transcriptional repression and gene silencing. ABC-type DLBCL is enriched with 3q27/ BCL6 gene rearrangements as well as gains/amplifications of BCL2 and MYC ; double-hit lymphoma is rare in this type of DLBCL. ABC-type DLBCL, NOS is enriched with recurrent mutations or deletions in genes associated with Toll-like receptor signaling, NF-κB signaling, and B-cell receptor signaling pathways ( MYD88 , TNFPAI3 , CD79A , CD79B , CARD11, and NFKBIE ), although recurrent mutations and deletions in genes related to epigenetic modification ( CREBBP , TET2 , EP300 , MLL2 , and MLL3 ) are occasionally observed. Recurrent mutations in DNA repair/genomic integrity pathways are also identified ( TP53 ) in 10% to 25% of cases. PRDM1/BLIMP1 mutations are seen exclusively in ABC-type DLBCL, NOS and are found in approximately 25% of cases. It is a loss-of-function mutation that prevents terminal differentiation of the malignant B cells.
In aggregate, the survival data and molecular genetic findings underscore the rationale behind distinguishing DLBCL, NOS cases based on cell of origin. Constitutive alterations in specific pathways in these subtypes, such as epigenetic regulation and NF-κB signaling in GCB and ABC-type lymphomas, respectively, support the potential effects of specific biological therapies for patients with GCB versus ABC-type DLBCLs.
The differential diagnosis of DLBCL, NOS includes other hematopoietic neoplasms of intermediate to large cells, including T-cell lymphomas, Burkitt lymphoma (BL), blastoid and pleomorphic variants of mantle cell lymphoma, lymphoblastic lymphoma, and myeloid sarcoma. Usually these neoplasms can be easily distinguished from DLBCL, NOS by routine immunophenotyping. T-cell lymphomas can be identified because of a lack of B-cell markers and the expression of T-cell–associated antigens such as CD2, CD3, CD5, and CD7 by the malignant cells. BL generally has a high mitotic index with a starry-sky pattern and is composed of medium-sized cells with multiple small nucleoli. MYC translocations are virtually always present in BL; however, this finding is not specific because MYC translocation can be seen as a primary or secondary event in DLBCL, NOS. Importantly, BL cells have a characteristic immunophenotype with expression of CD20, CD10, and BCL6, an absence of BCL2 expression, and a high proliferative rate in nearly all cases. Therefore consideration of morphologic, immunophenotypic, and molecular genetic features is required for accurate diagnosis. Blastoid and pleomorphic mantle cell lymphoma can resemble DLBCL, NOS but will typically express CD5 and cyclin D1, with the latter in a strong and uniform pattern not seen in DLBCL, NOS. Lymphoblastic lymphoma cells have a fine, blastic chromatin pattern unlike the more vesicular chromatin of DLBCL, NOS, and the neoplastic cells will usually be positive for terminal deoxynucleotidyl transferase (TdT) or CD34, or both. Myeloid sarcoma will lack B-cell markers and express myeloid markers such as CD13, CD33, lysozyme, myeloperoxidase, or CD68. Pleomorphic examples of DLBCL may mimic other anaplastic neoplasms (carcinomas, including small-cell carcinoma, melanomas, or sarcomas). Expression of B-cell markers and lack of markers expected in these other nonlymphoid malignancies, such as keratins or melanoma-associated antigens, will aid in correct diagnosis.
The current standard-of-care treatment for DLBCL, NOS combines the multiple-agent chemotherapy regimen CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) with humanized anti-CD20 monoclonal antibody, rituximab (R-CHOP). With this type of therapy, patients can be cured in approximately 60% to 70% of cases, representing a significant improvement over CHOP alone. Prognosis of patients can also be stratified by the five clinical factors of the IPI score ( Table 8.1 ). Segregation of DLBCL, NOS into GCB and ABC types provides further prognostic information, as does identifying the presence of dual MYC and BCL2 protein expression. Identification of these additional prognostic factors may help determine alternative therapies in the future.
| Prognostic Factors | Risk Score a | 5-Year OS (mo.) | |
|---|---|---|---|
| Age (>60) | Low | 0, 1 | 73 |
| LDH (nl) | Low Int | 2 | 51 |
| Performance status | High Int | 3 | 43 |
| Stage (III, IV) | High | 4, 5 | 26 |
| Extranodal (>1 site) | |||
a The risk score is the sum of the number of prognostic factors (0-5).
T-cell/histiocyte-rich LBCL (TC/HRBCL) is primarily a disease of young to middle-aged adults, with a median age of onset of 30 years; it affects men more often than women. Patients usually present with B symptoms, advanced stage disease (III and IV), and a high IPI score. Extranodal disease is common, with the spleen, liver, and bone marrow being the most commonly involved extranodal sites.
Middle-aged adults, males > females
Lymph node, spleen, liver, and bone marrow involvement common
Large, atypical lymphocytes rare (<10% of cells)
Numerous small lymphocytes and histiocytes (>90% of cells)
Diffuse pattern of infiltration
Large, atypical lymphocytes: CD45+, CD20+ B cells
Background small lymphocytes: CD3+ T cells
Gains of 2p11, 4q13, 18q21, Xq, Xp21
Loss of 9p11, 17p
Anthracycline-containing multi-agent chemotherapy + rituximab
45% to 75% 5-year survival
Classic Hodgkin lymphoma, lymphocyte-predominant Hodgkin lymphoma, peripheral T-cell lymphoma
Lymph nodes involved by TC/HRBCL show architectural effacement by a diffuse or vaguely nodular cellular infiltrate consisting predominantly (>90%) of small T cells and nonepithelioid histiocytes. Malignant cells are scattered singly among the cellular background, although in some cases they may cluster. They are large, highly atypical cells that are often multilobated with variably prominent nucleoli ( Fig. 8.6 ). In some cases the cells can resemble the lymphocyte-predominant (LP) cells characteristic of nodular lymphocyte predominance Hodgkin lymphoma (NLPHL) or Hodgkin and Reed-Sternberg cells characteristic of classic Hodgkin lymphoma (HL). Some cases may have cells that have more conventional immunoblastic or centroblastic features.
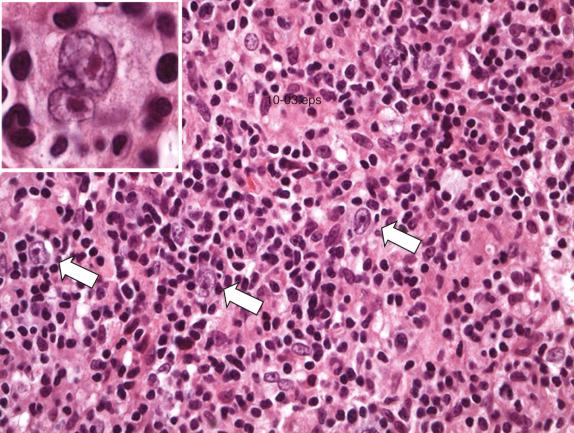
Splenic involvement occurs in a multifocal and micronodular pattern with expansion of the white pulp by an infiltrate of cells similar in composition to that described in the lymph node. The red pulp is not usually involved. Within the liver the lymphomatous infiltrate is confined principally to the portal tracts. Bone marrow involvement in TC/HRBCL occurs at a significantly higher rate than de novo DLBCL, NOS (60%). Involvement is often paratrabecular with a polymorphous infiltrate of small T cells, histiocytes, and scattered large atypical CD20+ B cells. Small B cells are typically scarce.
As noted, most cells in the infiltrate are reactive CD3+ T cells or CD68+ histiocytes. Only occasional CD20+ large B cells should be seen, and small CD20+ B cells should be absent or only rarely seen ( Fig. 8.7 ). The immunophenotype of the malignant cells is somewhat heterogeneous. The malignant cells are positive for CD45 and the pan B-cell markers CD20, CD79a, and PAX5; however, expression of CD10, BCL6, BCL2, and EMA is variable. Some have suggested, based on BCL6 expression, that a germinal center origin can be entertained, but others could not replicate these findings. It is likely that TC/HRBCL, as currently described, is heterogeneous, in part because of a lack of precise diagnostic criteria. Many believe that a subset of cases within the category of TC/HRBCL might define a specific entity, closely related to NLPHL. Evidence in support of this theory includes the occurrence of the two lymphomas in the same patient and an identical immunophenotype of large B cells in both (CD20+, CD79a+, BCL6+, EMA+, and CD75+). EBV is absent, as are follicular dendritic cell meshworks when using stains for CD21, CD23, or CD35. Background T cells are typically CD8+ and express cytotoxic markers, including TIA1, granzyme B, and perforin. The distinguishing features between TC/HRBCL and NLPHL rely primarily on the surrounding non-neoplastic microenvironment.
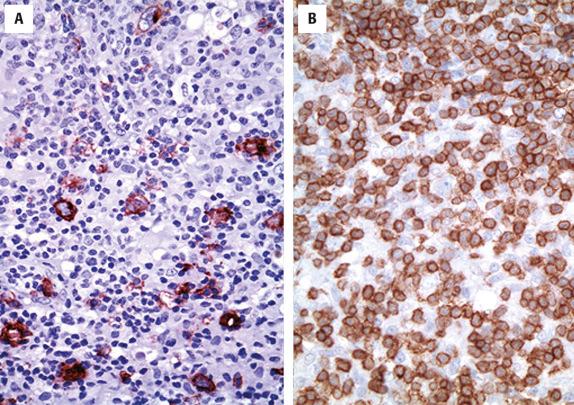
Immunoglobulin genes are clonally rearranged and somatically hypermutated in the majority of cases suggestive of a germinal center B-cell stage of differentiation. Few studies have investigated the molecular genetics landscape of TC/HRBCL, but there appears to be an average of approximately five genetic abnormalities per tumor, with reported abnormalities including gains of 2p11, 4q13, 18q21, Xq, and Xp21 and losses of 9p11 and 17p.
TC/HRBCL must be distinguished from NLPHL, classic HL, and peripheral T-cell lymphomas. In differentiating NLPHL from TC/RBCL, a diffuse pattern and lack of a follicular dendritic cell meshworks supports a diagnosis of TC/HRBCL over NLPHL. Furthermore, NLPHL contains large numbers of small non-neoplastic IgD+ B cells and increased numbers of CD57+, PD1+ T cells within the nodules; these are not features of TC/HRBCL. Strong expression of CD20 and CD45 in the atypical large cells with lack of CD15 helps to exclude classic HL and T-cell lymphoma.
T-cell/HRBCL is an aggressive lymphoma. Treatment is usually with a multiple-agent anthracycline-containing regimen (CHOP) plus rituximab. Five-year overall survival rates with CHOP alone are reported to be in the range of 45% to 58%, similar to DLBCL, NOS. The effect of adding rituximab is not well established, but one small study has identified a similar 3-year overall survival to DLBCL, NOS. Distinguishing TC/HRBCL from NLPHL and classic HL is important because therapies for HL do not adequately treat TC/HRBCL.
Primary DLBCL of the central nervous system (PCNSL) is a rare tumor accounting for 1% of non-Hodgkin lymphomas and 5% of brain tumors. The most common type of primary central nervous system (CNS) lymphoma, it encompasses all cases of DLBCL that arise in the brain, spinal cord, meninges, or eye parenchyma. DLBCL occurring in the CNS in the setting of immunodeficiency is not included in this diagnostic category. The median age of onset is 55 years, with a slight male predominance. Patients may have headache, mental status changes, or focal neurologic symptoms related to mass effect at the site of involvement. In one series, 81% of cases occurred in a supratentorial location, 7% infratentorially, and 12% in both locations. Because this neoplasm grows rapidly, the duration of signs and symptoms is on the order of weeks.
Rare tumor but most common type of primary CNS lymphoma
Supratentorial location most common
Median age at diagnosis 55 years
Presenting symptoms include mental status changes, headache, focal neurologic deficit
Sheets of large centroblastic or immunoblastic cells
Perivascular distribution
CD20+, BCL2+/−, BCL6−/+, MUM1+, MYC+/−, EBV−
Rearranged immunoglobulin genes
del6q21-22 and BCL6 rearrangement
MYD88 , TBL1XR1 , CD79B mutations
Multi-agent therapy with high-dose methotrexate and radiation
Aggressive lymphoma, median survival approximately 3 years (age >60 is poor prognostic factor)
Burkitt lymphoma, metastatic carcinoma, melanoma, inflammatory reaction (encephalitis)
The tumor resembles other DLBCLs cytologically. In a series of 62 PCNSL cases in immunocompetent patients, 87% were centroblastic and 13% were immunoblastic. The pattern of involvement is diffuse with a propensity for perivascular involvement ( Fig. 8.8 ). A diffuse pattern is more readily seen when a large mass is biopsied; perivascular involvement may be the only pattern seen in smaller stereotactic biopsy specimens. Necrosis and surrounding gliosis are commonly associated features.
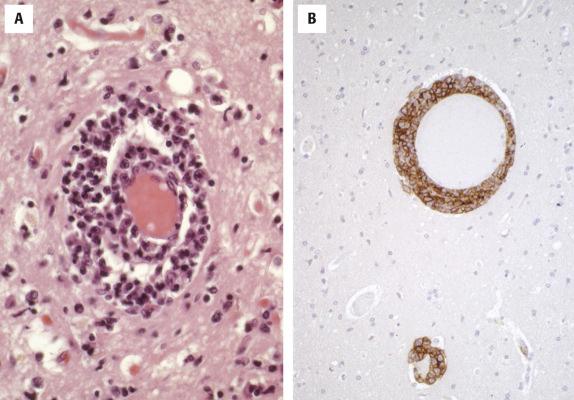
The lymphoma cells express pan B-cell markers CD19, CD20, CD79a, and PAX5. CD10 is expressed in less than 20% of cases, BCL6 in 60% to 80% of cases, and MUM1 in more than 90% of cases. The reported expression of BCL2 is variable but is seen in at least 50% of cases, with dual expression of MYC and BCL2 identified in upward of 80% of cases in some series. Using the Hans algorithm, approximately 80% to 95% of cases are classified as ABC/non-GCB type; this is due to the frequent expression of MUM1. Because many cases co-express BCL6, it has been suggested that this tumor is derived from a B cell in the late germinal center to early post-germinal center stage of development. EBV is usually absent.
Monoclonal immunoglobulin gene rearrangement is present. There are no specific diagnostic molecular genetic abnormalities identified in PCNSL, but abnormalities of 1q21, 6q, 7q, and 14q are reported. Comparative genomic hybridization studies have shown that gains outnumber losses, with chromosome 12q showing the most frequent gains (63%). The most commonly deleted locus is located at chromosome 6q (47%), with 6q21-22 being the commonly deleted region. Rearrangement of the BCL6 gene is also seen in a significant minority of cases (23% to 37%). Gene expression profiling shows that PCNSL is a tumor of late germinal center B cells, and its transcriptional signature closely resembles that of systemic DLBCL, NOS. Using an integrative analysis combining GEP and high-resolution array comparative genomic hybridization, it has been shown that 65% of PCNSLs harbor deletions of 9p21.3 involving CDKN2A as well as a number of previously unrecognized deletions associated with diminished expression of the candidate genes. Several recent studies have identified highly recurrent somatic mutations affecting the NF-κB pathway in the majority of PCNSL, including mutations in MYD88 , TBL1XR1 , and CD79B . These mutations occur with greater frequency than is seen in DLBCL, NOS, likely reflecting the paucity of antigen stimuli from the surrounding immune-privileged environment and serving as a genetic hallmark for this disease.
The differential diagnosis is similar to that of DLBCL, NOS at any site. Given the perivascular arrangement of lymphoid cells, inflammatory conditions such as encephalitis should be considered. However, the cytologic atypia and predominance of B cells help differentiate PCNSL from inflammatory conditions. EBV studies are helpful for distinguishing PCNSL from lymphomatoid granulomatosis involving the brain.
Therapy is generally multiple-agent therapy with high-dose methotrexate combined with radiotherapy. The latter is generally withheld in older patients because of unacceptable toxicity. This lymphoma is aggressive, with a median overall survival of 37 months. An important prognostic factor is age. Patients older than 60 years fare substantially worse than those younger than 60 years. Deletion of chromosome 6q21-22 and rearrangement of BCL6 have also been shown to correlate with a decreased overall survival.
Primary cutaneous DLBCL, leg type, is primarily a disease of elderly adults (median age, 77 years) with a female predominance. It usually manifests with localized disease of the skin with a single or multiple, localized, rapidly growing erythematous or violaceous plaques or nodules, often with ulceration. The lower leg is the most frequent site of involvement, but other sites can be involved as well. Dissemination to nodal or extranodal sites is common with disease progression.
Presents most commonly in elderly, females > males
Rapidly enlarging mass on the lower extremities
Diffuse sheets of large centroblasts and immunoblasts
Absent or minimal stromal response
CD20+, CD10−, BCL6+, MUM1+, FOXP1+, BCL2+, cIgM, p63
Rearranged immunoglobulin genes
Amplification of 18q21 ( BCL2 and MALT1 )
Deletion of 9p21 ( CDKN2A )
Translocation of BCL6, MYC, and/or IGH present in many cases
MYD88 mutation
Aggressive lymphoma with 50% 5-year survival
Treatment with anthracycline-containing multi-agent chemotherapy ± radiation
Secondary involvement in skin of systemic DLBCL, NOS
Cutaneous lymphoblastic lymphoma
The tumor infiltrates the dermis and hypodermis and is composed of diffuse sheets of medium to large centroblasts and immunoblasts without admixed small centrocytes ( Fig. 8.9 ). The cells have a characteristic round nuclear shape. There is little stromal reaction, and few infiltrating, small non-neoplastic lymphocytes are present. Mitoses are easily seen. The epidermis is uninvolved in most cases and separated from the tumor by a Grenz zone.
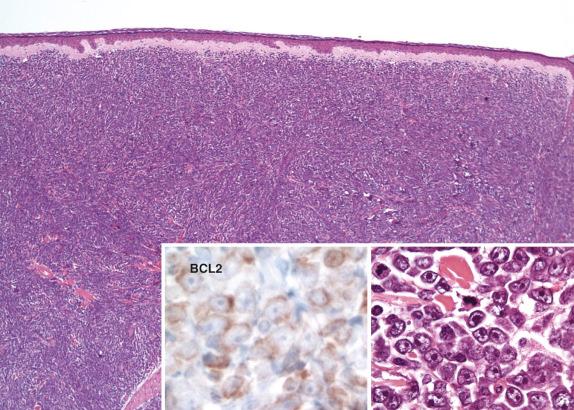
The tumor is positive for the pan B-cell markers CD20, CD79a, and PAX5. Most cases (>85%) express BCL2, MUM1, and FOXP1 and lack expression of CD10. BCL6 expression is seen in a variable number of cases. p63 is also variably expressed but seen in most cases. Cytoplasmic IgM with or without coexpression of IgD is reported in virtually all cases. EBV is negative. Follicular dendritic cell meshworks are absent.
Monoclonal Ig rearrangement is present. The tumor lacks t(14;18); however, translocations involving BCL6 , MYC, and IGH are common. Recurring genetic abnormalities include amplification of chromosome 18q21, where the BCL2 and MALT1 genes are located; deletion of chromosome 9p21.3, where the CDKN2A gene is located; deletion of 6q, where the BLIMP1 gene is located; and recurrent somatic mutation of MYD88 , which is seen in approximately 60% to 70% of cases. Amplification of BCL2 is believed to be the mechanism responsible for the frequent expression of BCL2 protein seen in this tumor. Gene expression profiling shows that the tumor is similar to activated B-cell–like DLBCL, NOS.
The main differential diagnosis of DLBCL, leg type, is cutaneous involvement by systemic DLBCL, NOS. This can be excluded only by careful staging procedures and knowledge of the clinical history. Another B-cell lymphoma that must be excluded is B-cell lineage lymphoblastic lymphoma. Cutaneous lymphoblastic lymphoma is rare. Unlike primary cutaneous DLBCL, leg type, lymphoblastic lymphomas usually express CD10 and TdT. Primary cutaneous follicle center lymphoma (described in Chapter 7 ) may be composed of large cells, but they contain centrocytic cells, often lack BCL2, express germinal center markers such as CD10, and uncommonly express cytoplasmic IgM and p63.
Systemic anthracycline-containing multiple-agent immunochemotherapy with the selective addition of radiotherapy is the preferred type of treatment for this lymphoma. Primary cutaneous DLBCL, leg type, has an intermediate clinical course with a 50% 5-year survival. Adverse prognostic factors include advanced age, multiple skin lesions, and disease localized to the leg. Some authors have suggested that it is the expression of BCL2 protein, a known poor prognostic indicator in other lymphomas, along with other clinical factors that contribute to the poor outcome of this lymphoma, rather than location on the leg. Other studies have shown that deletion of 9p21.3, methylation of the promoter region of CDKN2A , and MYD88 mutation are associated with an unfavorable prognosis.
Epstein-Barr virus (EBV)–positive diffuse large B-cell lymphoma, not otherwise specified (EBV+ DLBCL, NOS) is a monoclonal counterpart of a related group of diseases collectively known as EBV-positive B-cell lymphoproliferative disorders that does not meet the criteria for a diagnosis of a more specific EBV+ disease entity. It is seen most commonly in Southeast Asia, particularly Japan, and Latin America, and it is less commonly seen in Western countries. The disease is thought to result from age-associated immune senescence and affects patients without known immunodeficiency or previous history of an EBV-associated lymphoproliferative disorder (i.e., DLBCL associated with chronic inflammation, lymphomatoid granulomatosis, primary effusion lymphoma, or plasmablastic lymphoma). EBV positivity in DLBCL is proportionately more common with increasing age, reaching a peak after the age of 90 years. Although peak incidence occurs in the eighth decade, cases occurring in patients younger than 50 years of age have been increasingly recognized. Hence, the prior nomenclature of “EBV + diffuse large B-cell lymphoma of the elderly” has been dropped in favor of the current name. It is important to exclude other causes of underlying immunodeficiency before making this diagnosis.
EBV+ DLBCL, NOS affects men slightly more frequently than women and appears in lymph nodes and at extranodal sites, including the skin, gastrointestinal tract, lung, and tonsil. Greater than 50% of patients exhibit B symptoms, elevated low-density lipoprotein (LDH), and advanced-stage disease, although solid organ and bone marrow involvement is relatively infrequent.
Typically affects patients >50 years of age with no history of immunosuppression but can be seen in younger patients
Usually presents with B symptoms and advanced stage
Nodal and extranodal disease common; solid organs and bone marrow not usually involved
Rare in Western countries; more common in Southeast Asian and Latin American populations
Polymorphic or monomorphic infiltrate of immunoblast-like transformed cells
Hodgkin and Reed-Sternberg–like cells
Mixed inflammatory cells in polymorphic infiltrate
Can resemble TC/HRLBCL in younger patients
CD20+, CD79a+, CD10−, BCL6−, MUM1+, CD30±, CD15−, EBV+
Clonally rearranged IGH gene
Clonal EBV genome
NF-κB and JAK/STAT pathway activation
Gains of 9p24 (PD-L2)
Combination immunochemotherapy (CHOP-R) ± radiation
2-year median survival in older patients; younger patients, better prognosis
Infectious mononucleosis
Classic Hodgkin lymphoma
EBV+ mucocutaneous ulcer
Epstein-Barr–positive DLBCL, NOS is morphologically heterogeneous. It can be divided into polymorphous and large-cell lymphoma subtypes; however, these are of no clinical significance, and usually both morphologies can be seen in the same specimen when examined thoroughly. In all cases, the tumor consists of a polymorphic or monomorphic infiltrate of immunoblast-like transformed cells and Hodgkin and Reed-Sternberg (HRS)-like cells that efface normal architecture ( Fig. 8.10 ). Inflammatory cells, including small reactive lymphocytes, plasma cells, histiocytes, and epithelioid cells, are admixed in polymorphic areas. Foci of geographic necrosis are also common and reminiscent of posttransplant lymphoproliferative disorder. In younger patients, cases often resemble T-cell/histiocyte-rich large B-cell lymphoma.
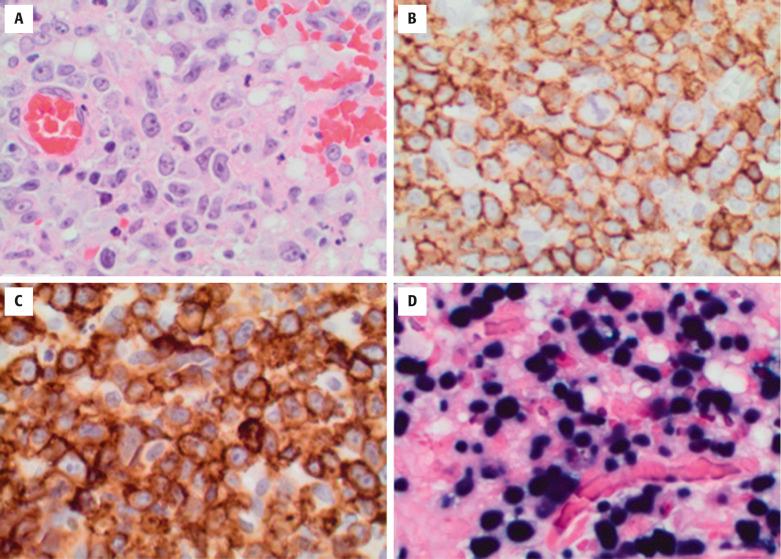
The malignant cells in EBV+ DLBCL, NOS express the pan B-cell antigens CD20, CD79a, and PAX5 and exhibit a nongerminal center immunophenotype, being negative for CD10 and BCL6 and positive for MUM1. Approximately three fourths of cases express CD30, and this is more commonly seen in Southeast Asian and Latin cases. CD15 is universally absent. PD-L1 is frequently expressed. EBV is present in all cases, typically seen in more than 50% of malignant cells, and best detected by in situ hybridization for EBV-encoded RNA (EBER).
Epstein-Barr virus–positive (EBV+) DLBCL, NOS is a monoclonal disorder, and a clonal rearrangement of the IGH gene is usually detectable. A clonal EBV genome is also usually identified. Rearrangements of BCL2 , BCL6 , and MYC are absent. GEP studies have identified enhanced activity of the NF-κB and JAK/STAT pathways within this disease; however, mutations affecting these pathways ( CD79B , CARD11 , MYD88 ) are only rarely detected, which is suggestive of EBV-mediated activation of these pathways. In addition, gains of 9p24 ( PD-L2 ) have been identified, which promote immune evasion.
The main differential diagnosis includes infectious mononucleosis and EBV+ classic Hodgkin lymphoma (classic HL). Unlike EBV+ DLBCL, NOS, infectious mononucleosis shows preservation of lymphoid architecture with preferential expansion of the paracortex by a polymorphous cellular infiltrate. Zonal necrosis is uncommon, and a monoclonal B-cell population is not detected. The HRS cells of classic HL usually differ from the HRS-like cells of EBV+ DLBCL, NOS in that they do not express CD79a, usually lack CD20, andare positive for CD15 in most cases. In addition, reactive T cells with an activated immunophenotype (TIA1+/perforin+) are common in EBV+ DLBCL, NOS but infrequent inclassic HL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here