Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The popularity of gastrointestinal (GI) cytology for the diagnosis of infection and malignancy has waxed and waned during the past few decades. The ability to distinguish between high-grade dysplasia or carcinoma in situ and invasive carcinoma in biopsy specimens and the more prevalent expertise of surgical pathology cause some to consider cytology an unnecessary duplication of GI mucosal biopsies. However, the combined use of endoscopy, ultrasound guidance, and fine-needle aspiration (FNA) has expanded the horizons of GI cytology. The refined nonendoscopic sampling methods coupled with cellular markers have rekindled interest in screening high-risk populations with cytology for esophageal carcinoma.
Types of GI tract specimens commonly received in the cytology laboratory include those obtained by endoscopic brushings and ultrasound-guided endoscopic FNA. Endoscopic FNA has enabled endoscopists to reach farther than they can with biopsy forceps to sample mural and extramural lesions, including lesions adjacent to the GI tract. The nonendoscopic specimens obtained with balloon- or mesh-type samplers have been investigated to ascertain their usefulness in the surveillance of populations at high risk for esophageal carcinoma. ,
Direct smears can be made from materials collected on the endoscopic brush, in the needle, or on the balloon and mesh samplers; these can then be either fixed immediately in 95% ethanol and stained with the Papanicolaou method or left to air-dry and stained with Diff-Quik (Dade-Behring, Inc., Deerfield, IL) or Wright-Giemsa stain. Alternatively, the material can be rinsed into a medium such as CytoLyt, CytoRich, or 50% ethanol for liquid-based preparations. The specimen can then be processed by a concentration method, such as ThinPrep Processor (Hologic, Marlborough, MA) or Cytospin (ThermoFisher Scientific, Waltham, MA), , to make slides that are then stained with the Papanicolaou method. According to a College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology, ThinPrep preparations performed better than non-ThinPrep preparations. However, liquid-based preparations, including ThinPrep, involve altered morphology and artifacts that require adjustment by cytopathologists, such as cleaner background with altered or reduced background and extracellular elements, architectural changes (smaller cell clusters and sheets and more three-dimensional clusters), altered cell distribution (more dyshesion–dissociation of cells at the periphery of cell clusters or as single cells), and changes in cytological morphology (enhanced nuclear features and smaller cell size). Residual material from liquid-based preparations lends itself to cell block making for histological examination and ancillary studies.
Cytology specimens have some advantages over specimens obtained by endoscopic biopsy. The brush can sample a wider area, and the fine needle can reach deeper lesions than can be reached by biopsy forceps. Also, both the brush and the fine needle are less invasive than biopsy forceps and less likely to cause bleeding. In addition, cytology has a shorter turnaround time than histology. Direct smears can be ready for review within minutes with no compromise of the quality of the preparation (unlike frozen sections of biopsy specimens, which compromise the quality of the final or permanent preparation). However, as mentioned, cytology is limited in its ability to distinguish between high-grade dysplasia or carcinoma in situ and invasive carcinoma.
Despite the potential duplication of cytology and biopsy, the literature has consistently shown that the highest diagnostic yield is obtained with the combined use of these specimens. The yield of cytology is significantly higher when the brushing is performed before rather than after the biopsy.
Intermediate-type squamous cells with abundant cytoplasm and vesicular nuclei are seen in the normal esophagus ( Fig. 3.1 ). Superficial-type squamous cells with abundant cytoplasm and small pyknotic nuclei can also be seen in small numbers. Single cells and clusters of ciliated columnar cells from the respiratory tract with no clinical significance may be seen rarely.
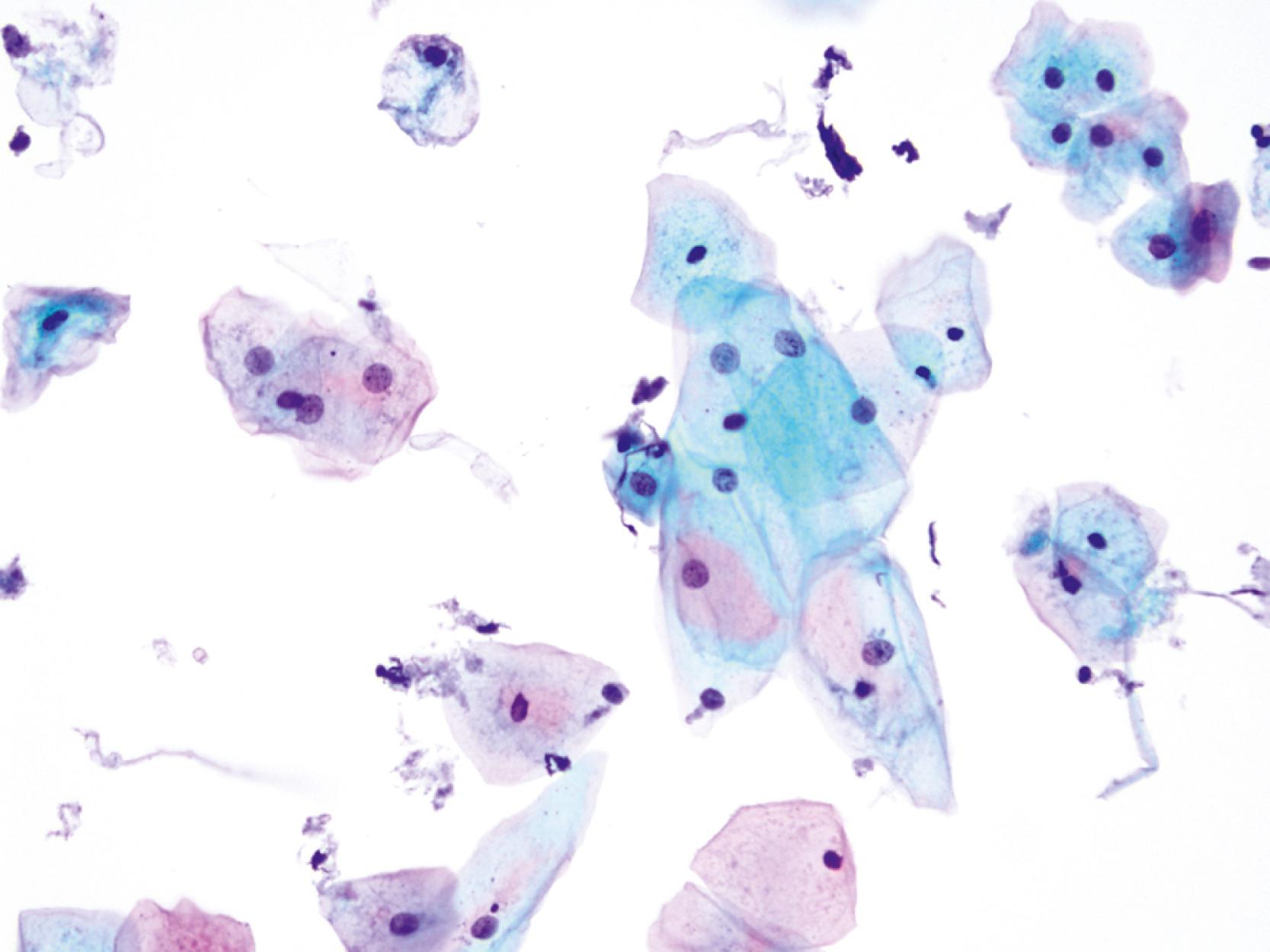
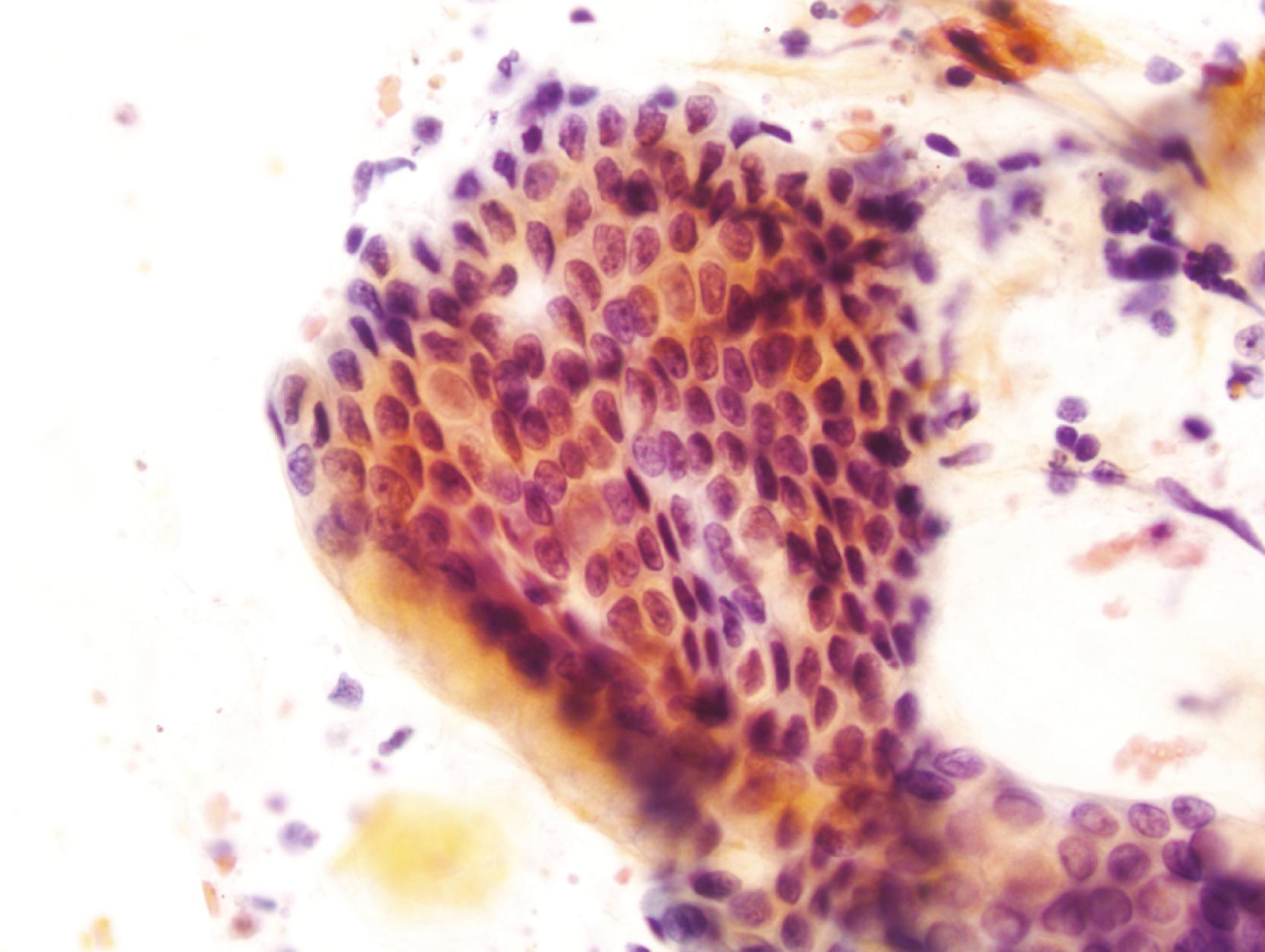
Gastric surface foveolar cells can shed as single cells or in sheets. When in sheets, the columnar cells exhibit abundant cytoplasm, regularly spaced nuclei, and open chromatin arranged in a honeycomb or palisaded pattern ( Fig. 3.2 ), depending on the orientation. When they are shed as single cells, they often lose their cytoplasm to become naked nuclei. In endoscopic FNA specimens, the sheets of foveolar cells can mimic cells from a mucinous neoplasm, and the single naked nuclei, because of their small, monomorphic appearance, can mimic cells from a pancreatic endocrine tumor.
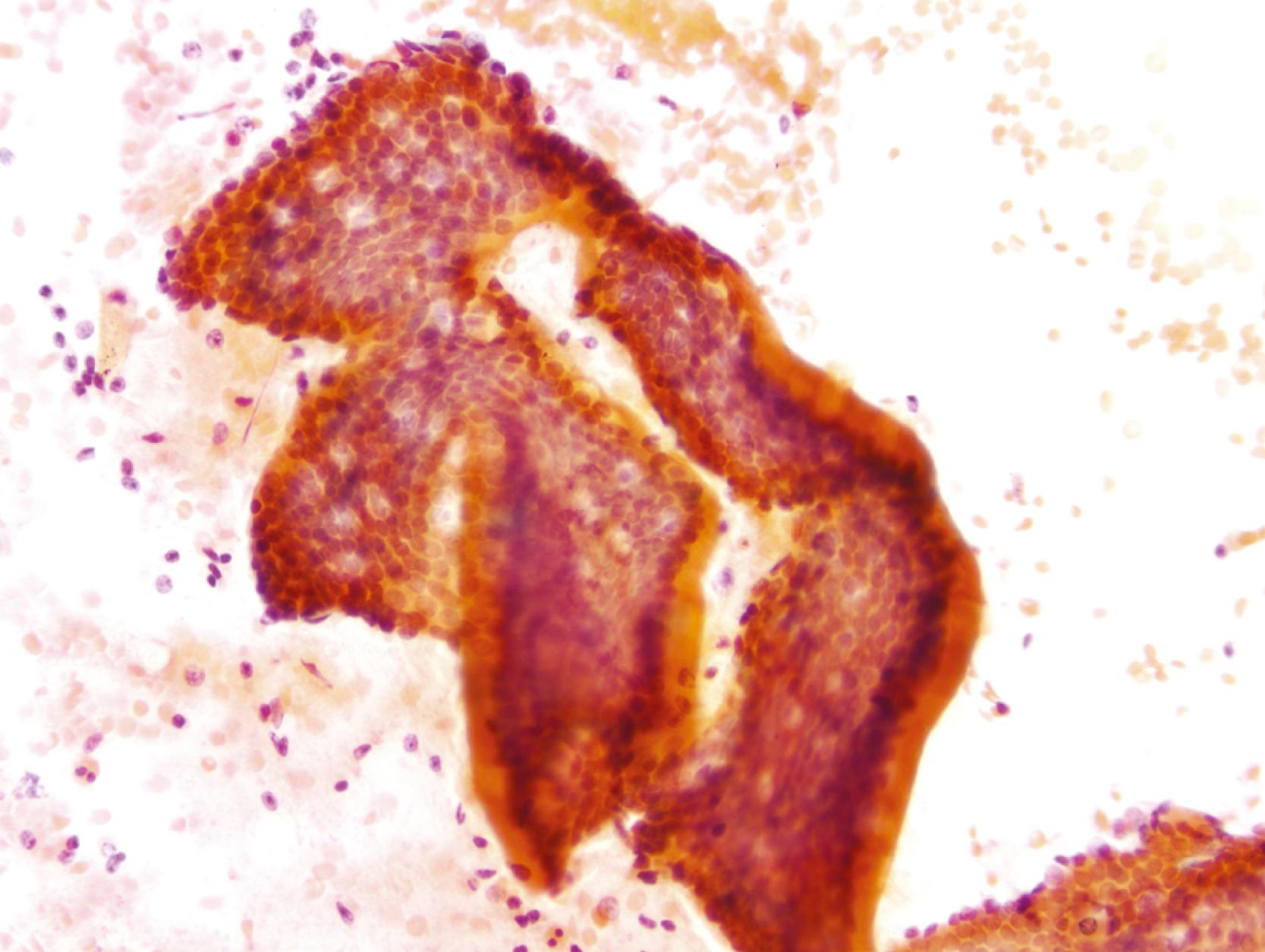
The lining cells of the small intestine can be easily distinguished from gastric foveolar cells by the presence of goblet cells. On low magnification, the specimen typically has a Swiss cheese appearance, with the “holes” representing either goblet cells or gland openings of the crypts ( Fig. 3.3 ). On high magnification, the absorptive cells have either finely granular or vacuolated cytoplasm, and the goblet cells have single large mucin vacuoles and crescent-shaped nuclei with rounded contours. The striated border of the absorptive cells may be seen at the periphery of the sheets.
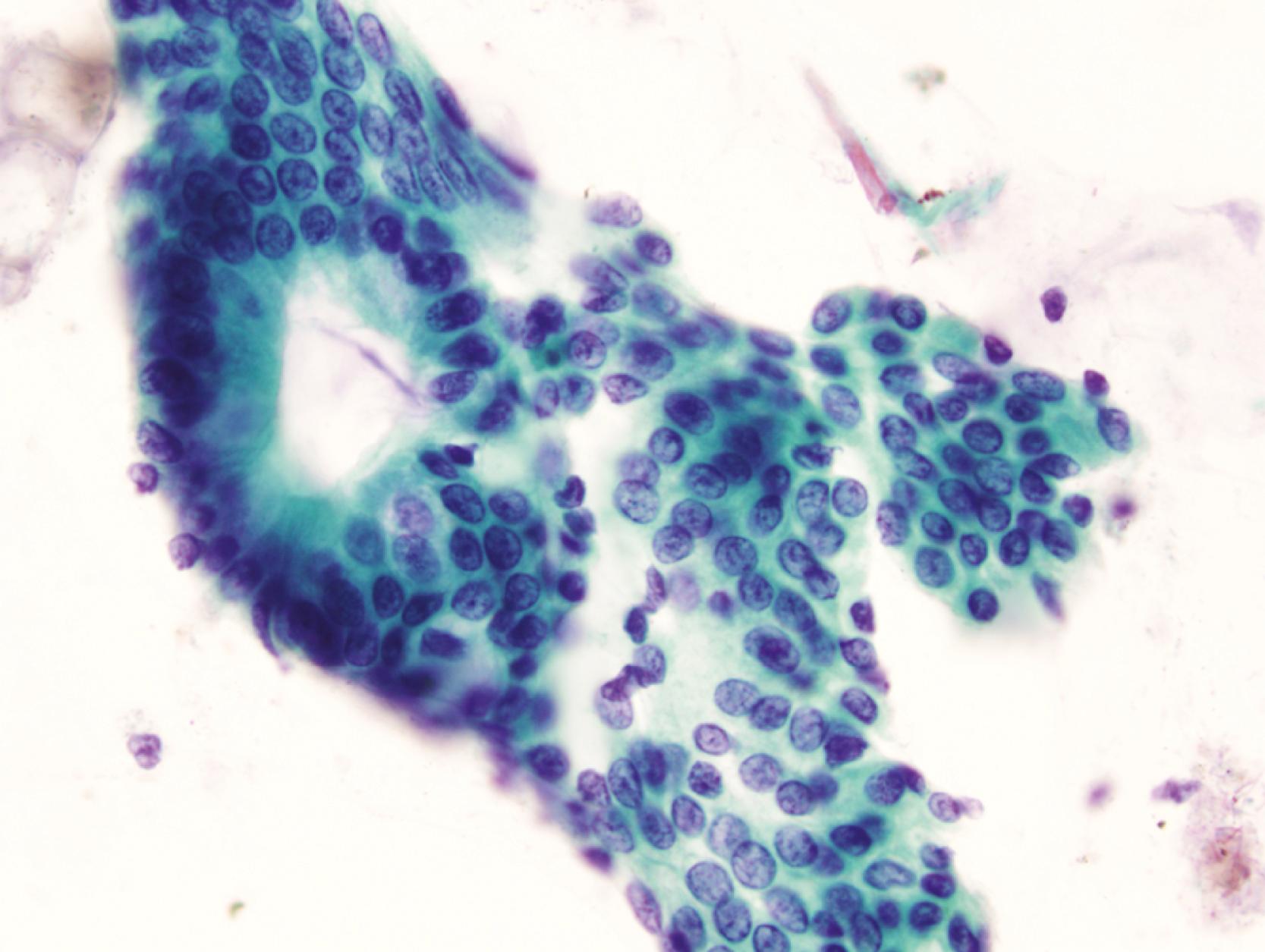
Normal epithelium is characterized on cytology by sheets or strips of tall columnar cells with abundant cytoplasm and basal nuclei. Partial or complete openings of the colonic crypts may be seen ( Fig. 3.4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here