Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
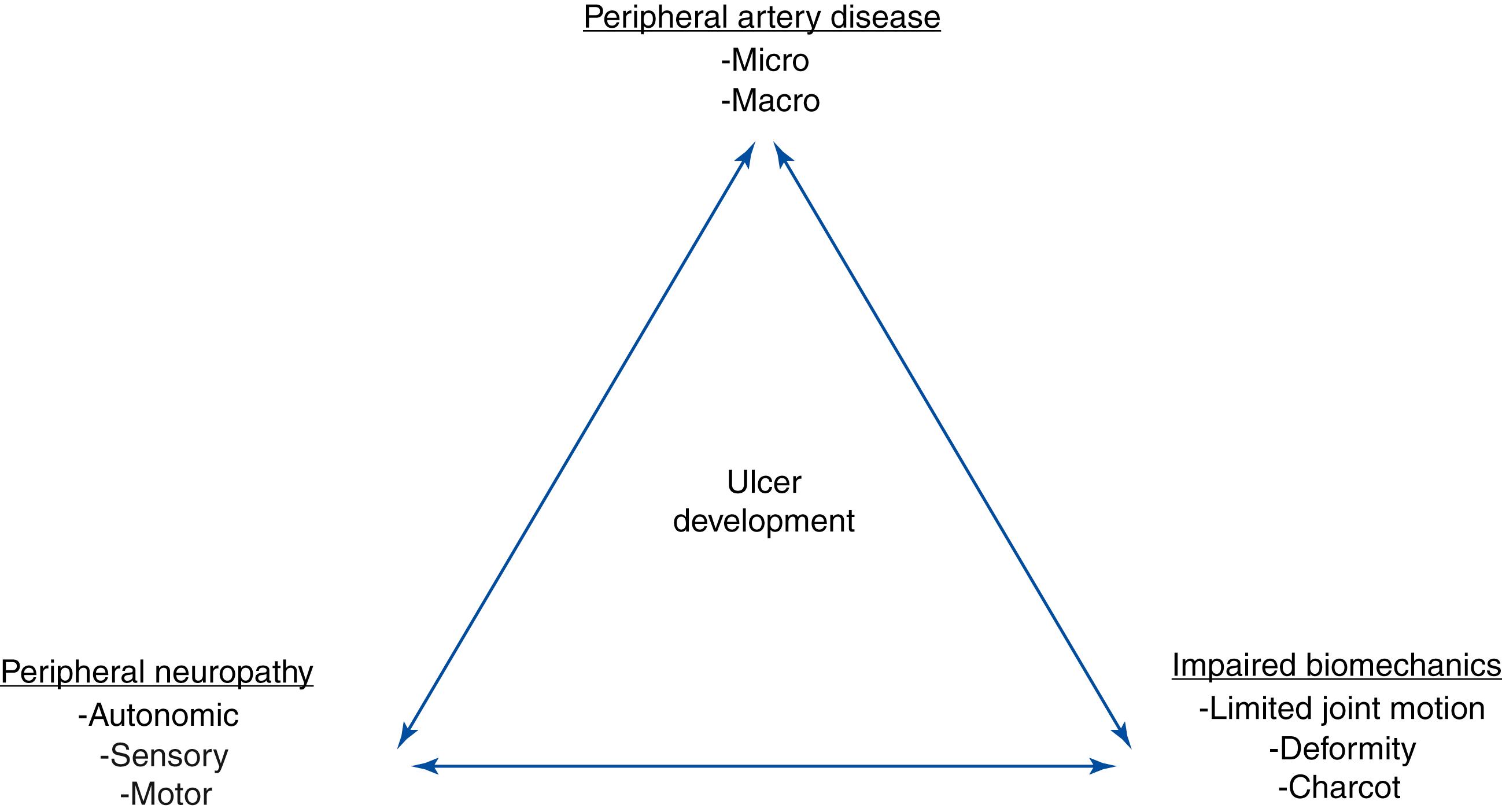
Diabetic foot ulcers (DFUs) are often erroneously thought to be a minor complication of diabetes. Diabetic foot ulcers usually start as an innocuous blister from rubbing on a shoe that is too tight, or at the site of an old callus on the sole of the foot. In reality, diabetic foot ulcers are the start of a cascade of events that contribute to repeated infections, hospitalizations, surgeries and amputations. In fact, 10%–50% of DFUs end in some type of amputation. Diabetes detrimentally affects every system in the body. Manifestations in the foot exemplify the systemic disease process with the development of peripheral sensory neuropathy, motor neuropathy, autonomic neuropathy, limited joint mobility, structural foot deformity (e.g., hammertoes and hallux valgus), Charcot arthropathy, and macro and micro-angiopathy ( Fig. 117.1 ). DFUs usually develop at the site of pressure areas on the sole of the foot that are exposed to repetitive injury (walking activity) or at the site of constant pressure at bony prominences (e.g., hammertoes or bunion deformities) from tight shoes. Patients with diabetes are often severely impaired hosts. Neuropathy eliminates the perception of pain and unrecognized injuries until infection damages enough tissue to cause pain. Immunopathy increases the risk of infection and a poor inflammatory response. , Macro- and micro-angiopathy impede the response to both infection and normal reparative processes. The purpose of this chapter is to discuss the components related to the diabetic foot.
Peripheral neuropathy is one of the most important components in diabetic foot ulcers and infections. Peripheral neuropathy is a progressive pathologic process that develops in the environment of diabetes, prolonged poor glucose control and microvascular disease. , Glycation-induced metabolic and vascular changes of nerve fibers result in sensory, autonomic, and motor neuropathy in the lower extremity. Neuropathy plays a devastating role in the cascade of events that lead to ulceration, infection, and amputation. Patients often present with a combination of small and large fiber neuropathy. Small fiber neuropathy is usually diagnosed based on symptoms of burning, tingling, and radiating electrical pain. , Clinical assessment is based primarily on the patient’s inability to perceive temperature differences, so it is not often evaluated. Large fiber neuropathy generally presents with symptoms of numbness, tingling, and formication. Patients will often complain that their feet feel cold when they feel warm to their spouse or in the clinic. Large fiber neuropathy is easily assessed in the clinic with a 128-Hz tuning fork, 10-g monofilament, or Achilles deep tendon reflexes.
Large fiber sensory neuropathy is often so severe that patients lack sufficient peripheral sensation to protect their feet from injury. This is referred to as “loss of protective sensation” (LOPS). Initially, diabetic peripheral sensory neuropathy with the “loss of protective sensation” begins distally in the toes and progresses to the ankle and legs. Large fiber neuropathy is one of the sentinel disease processes that leads to diabetic foot ulceration. LOPS provides an environment for foot injury without being recognized by the patient. For example, in people with normal sensation, a sharp object such as a tack is reflexively identified and the foot is spontaneously withdrawn, avoiding further injury. In an environment of sensory neuropathy, the patient will not withdraw the foot. The tack is unrecognized, resulting in repetitive damage to deep structures. Foreign materials such as dirt and fabric are pushed into deep tissue that creates abscess and necrosis. Because of neuropathy, there is a longer time after a puncture event before the patient seeks medical care and the risk of surgery and amputation are much higher among people with diabetes and neuropathy. Patients with diabetes are 5 times more likely to require multiple surgeries and 46 times more likely to have an amputation as a result of an infected puncture wound. , Patients with large fiber neuropathy have postural instability and an increased risk of falls and associated trauma.
Autonomic peripheral neuropathy is related to the increase in sympathetic tone in the diabetic lower extremity. Dysregulation can lead to arterial-venous shunting, small vessel ischemia, and changes in the soft tissue turgor. , Xerosis, with its loss of moisture balance and elasticity, can lead to developing fissure or tears in the skin. Further, chronic edema applies tension on the compromised soft tissue envelope, increasing the susceptibility of minor tissue injury, causing breaks in the skin leading to an ulceration.
There is no established treatment for large fiber neuropathy. The symptoms of small fiber peripheral neuropathy (paresthesias, burning, pins and needles, tingling, radiating pain) are often treated off-label with oral medications including antidepressants, antiarrhythmics, and anticonvulsants such as gabapentin. In addition, vitamin supplements with L-methylfolate, methylcobalamin, and pyridoxal 5′-phosphate and topical medications including topical clonidine and capsaicin formulations have shown efficacy to reduce symptoms. , There are two FDA-approved drugs for painful neuropathy (pregabalin and duloxetine). Both have demonstrated reduction in pain and improved sleep compared to placebo.
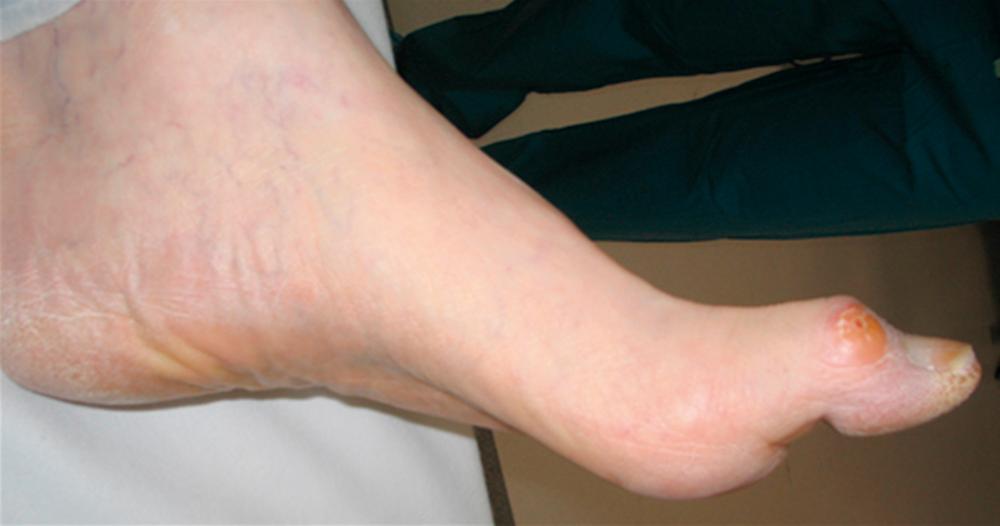
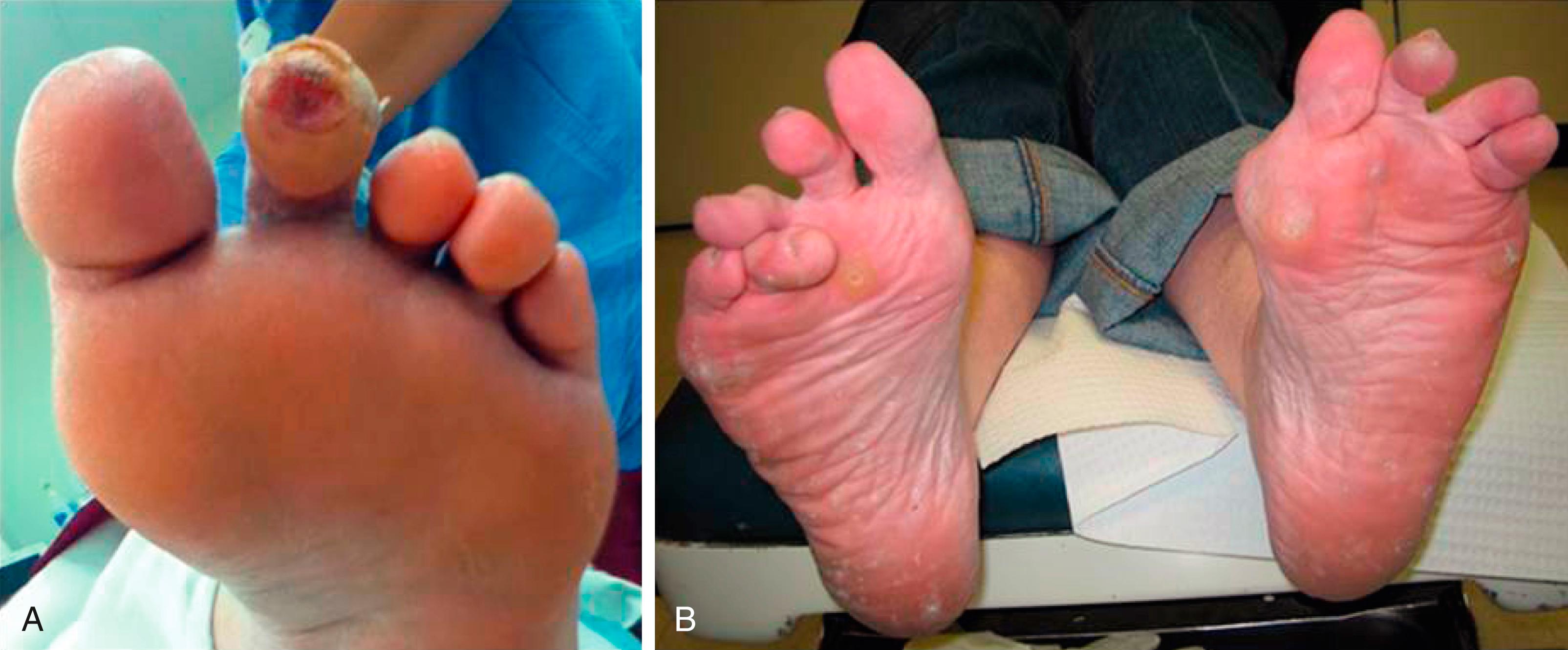
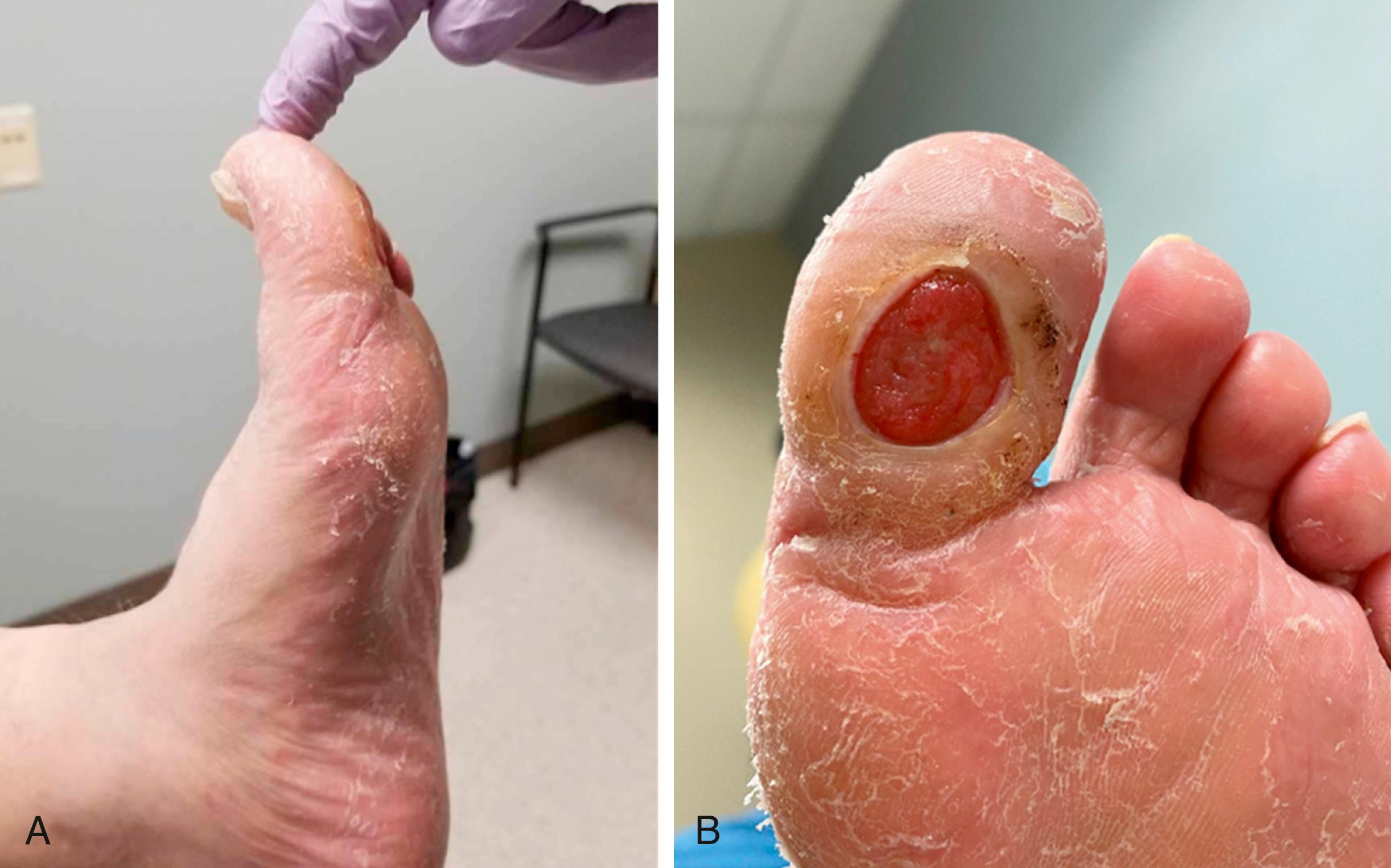
Motor neuropathy in the lower extremity leads to muscle atrophy and imbalance of opposing muscle groups. Motor neuropathy is especially apparent in the smaller intrinsic muscles of the hands and feet. Even in people without diabetes, an imbalance of the extensors and flexors; evertors and invertors; and the abductors and adductors can cause capsular laxity and bony mal-alignment, joint subluxation, and structural foot deformity. With severe motor neuropathy, patients’ hands can develop a “monkey paw” or an “intrinsic minus” foot, the atrophy of intrinsic muscles ( Fig. 117.2 ). In the foot, motor neuropathy contributes to the development of forefoot deformities such as hammertoe and hallux abductovalgus, which are reported to be major contributors of ulceration (92% related to neuropathy, and 77% related to deformities) ( Figs. 117.3 and 117.4 ). For example, the intrinsic flexors, interossei, and lumbricals in the foot normally stabilize the toes against the metatarsal heads in the stance and propulsive phases of the gait cycle. When the intrinsic muscles atrophy, the balance of the stronger long flexors and extensors causes the toes to contract and eventually to dislocate dorsally at the metatarsophalangeal joints. This imbalance and deformity can literally push the head of the metatarsals through the bottom of the foot.
Surgical decompression of the peroneal and tibial nerves may have a role if the disease is not advanced. , Wieman and colleagues decompressed the posterior tibial nerve by excising the flexor retinaculum at the level of the medial malleolus in 26 patients. Within 1 month, 92% of patients reported their pain had subsided. Macare van Maurik’s group performed decompression prospectively on 42 patients whose contralateral limb served as the control. Randomization was performed to determine which leg received surgical intervention. At 3 months there was a significant improvement in pain in the postoperative leg that was maintained for 12 months. There are currently few prospective studies and long-term effectiveness of the procedure is yet to be measured.
Perfusion is arguably the most critical element in diabetic foot ulcer healing, amputation level selection, and limb salvage. Tools to measure functional perfusion to determine wound healing are an unmet need in the diabetic foot. Traditional arterial Dopplers are unreliable because of Monckeberg medial calcific sclerosis. Often, arteries in the foot and ankle are not compressible. Toe pressures and waveforms may be more helpful, however many patients have had previous amputations and do not have toes to evaluate. There have been a number of new technologies such as hyperspectral imaging (HSI) and the SPY fluorescence imaging system that have been met with enthusiasm but are still being evaluated. Early proof of concept studies with hyperspectral imaging indicated the technology could be used to predict DFU healing. However, subsequent studies have reported contradictory results. Nouvong and colleagues reported the results of a prospective cohort study of 54 patients with 73 diabetic foot ulcers over a 24-week period. She reported the sensitivity, specificity, positive predictive value was 80%, 74% and 90%. Likewise, Khaodhiar evaluated 23 patients with 34 DFUs and reported the sensitivity, specificity, and positive and negative predictive values of the HT index for predicting healing were 93%, 86%, 93%, and 86%, respectively. In contrast, Jeffcoate and colleagues evaluated 43 patients with DFUs. They found a negative association with HSI and healing at 12 weeks and a positive association at 24 weeks. Indocyanine green angiography in the SPY system may help to evaluate the effectiveness of vascular interventions but the technology does not provide quantitative measurements, so it is difficult to compare patients and quantify results. There is no evidence that indocyanine green angiography is useful to determine wound healing or amputation level selection.
Another pertinent concept relates to angiosomes, the anatomic areas that are vascularized by a source artery. The three major vessels (posterior tibial, peroneal, anterior tibial arteries) feed specific areas of the lower extremity. Several reports have stated that there is a correlation between the ulcer site and the angiosome that is compromised. However, there are redundancies inherent in the foot with arterial-to-arterial connections that may compensate for occlusive disease in one or more vessels. Successful healing of ulcers through indirect revascularization implies that in many cases, these redundancies exist and may be adequate. , Current consensus is that angiosome-targeted revascularization should be performed when possible (direct revascularization), because it enhances wound-healing.
When patients present with infection, surgical excision of the infection needs to be achieved before revascularization to reduce the risk of graft or stent infection. Ischemic ulcers on the other hand should not be debrided until revascularization has been performed, unless there is concern for underlying infection. While necrotic areas or frankly gangrenous digits will ultimately require some sort of amputation, this must be performed at a level where perfusion is adequate for healing. As long as there is no significant infection, debridement and amputation for dry necrosis should also be delayed until after revascularization. However, because this is an impaired host, local signs of infection are often blunted and it may be difficult to determine if there is underlying soft tissue or bone infection. The Wagner classification system is the oldest in use specifically for diabetic foot ulcers but does not take ischemia into account until the ulcer has progressed to gangrene. The University of Texas classification system builds on this and encompasses depth, infection, and ischemia with some overlap between the two systems ( Table 117.1 ).
| Stage | Grade | |||
|---|---|---|---|---|
| 0 | I | II | III | |
| A | Pre- or post-ulcerative lesion. Completely epithelialized (Wagner 0) |
Superficial wound not involving tendon, capsule, or bone (Wagner 1) |
Wound penetrates to tendon or capsule (Wagner 2) |
Wound penetrates to bone or joint |
| B | Infection | Infection | Infection | Infection (Wagner 3) |
| C | Ischemia | Ischemia | Ischemia | Ischemia (Wagner 4/5) |
| D | Infection and ischemia | Infection and ischemia | Infection and ischemia | Infection and ischemia |
The foot is a complex structure with 26 bones and multiple articulations; intrinsic and extrinsic tendon insertions; fascia and ligaments that are both flexible and rigid. There is a redundant network of lymphatics, veins, arteries, and nerves, encased in a resilient soft tissue envelope. The complications associated with diabetes impart a devastating toll on foot function. Amputation often causes biomechanical changes in the foot that contribute to adaptive, progressive structural deformities, tendon imbalances, and limited joint mobility. After amputation, the risk of recurrent ulcers, infections, and amputations increases dramatically. ,
Structural changes occur in the diabetic foot that alter the underlying biomechanics. Stiffening of soft tissue structures, including tendons, ligaments, and joint capsules, leads to a limitation of range of motion across joints, joint subluxation, and dislocation. , Deformity and limited joint motion is thought to be caused by atrophy of intrinsic muscles of the foot from peripheral motor neuropathy and advanced glycosylation end products that induce changes in the collagen of tendons. This process can lead to shortening of the tendon and loss of elasticity. Patients subsequently develop deformities such as hammertoes or equinus deformity, the limitation of dorsiflexion at the ankle joint. Limited joint mobility often contributes to compensatory gait changes. For instance, to compensate for equinus, patients abduct and pronate their foot in gait. This causes increased pressure and shear on the ball of the foot, leading to plantar forefoot ulcerations under the metatarsal heads.
Callus distribution is a reliable method of identifying areas of pressure and shear forces that are often associated with foot ulceration. , Alterations in gait patterns (i.e., shuffling, antalgic, wide base, unsteady) imply an underlying abnormality. , Limited joint range of motion and structural deformities like bony prominences or joint contractures are signs of mal-alignment. Reducibility of any mal-aligned joint articulation is a key feature when examining the deformity. As the term implies, a reducible deformity is one that can be placed back into an anatomical position. A semi-reducible deformity cannot be completely realigned into an anatomical position, and a non-reducible deformity is a fixed deformity that cannot be reduced at all. Typically, there is a progression from a reducible to a rigid deformity, and the options become more limited as the deformity progresses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here