Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the anatomy, embryology and function of the hypothalamus, pituitary, thyroid, parathyroid, pancreas and adrenals
Understand the pathophysiological basis of endocrine diseases such as diabetes (including the aetiology and identification of different forms of diabetes) and disorders of the pituitary, thyroid, parathyroid and adrenal glands
Understand the major influences on calcium and bone physiology
Understand the pathophysiological basis of endocrine emergencies, including disorders of blood glucose control (hyperglycaemia, diabetic ketoacidosis and hypoglycaemia) and adrenal failure
Understand the investigation of endocrine disease
Understand the pharmacological basis of treatment of endocrine disorders
Know the possible impact on endocrine organs of other system disorders and vice versa
Hormones are secreted by endocrine organs and, following circulation in the blood, bind to specific receptors which are broadly subdivided into two groups. Cell surface receptors on the plasma membranes bind to hydrophilic hormones such as insulin, catecholamines and those produced by the pituitary. Steroids and thyroid hormones, which are lipid soluble, enter cells to bind with cytosolic and nuclear receptors. Cell receptor sensitivity can be increased by increasing the number of binding sites through increased receptor synthesis or decreased degradation. Desensitization occurs when receptors are reduced in number, internalized from surface locations or molecules are recruited which deactivate intracellular signalling pathways.
Cell surface receptors are subdivided into two groups. In one group, the signalling is initiated by tyrosine kinase. They are known as growth factor (insulin, insulin-like growth factor-1, epidermal growth factor, fibroblast and platelet-derived growth factor) receptors. The other group are G-protein coupled receptors (GPCR). Defects in GPCRs can lead to both hormone resistance (mutations of the thyroid stimulating hormone (TSH) receptor can cause resistance to TSH) and also to upregulated activity (mutations of the luteinizing hormone (LH) receptor lead to testotoxicosis, while mutations of the TSH receptor can lead to neonatal hyperthyroidism). Mutations adversely affecting Gα s function cause pseudohypoparathyroidism and when maternally inherited lead to the downregulation of a range of GPCRs. By contrast, upregulation of Gα s function is associated with McCune–Albright syndrome, which is associated with upregulation of several GPCRs leading to the clinical features which include gonadotropin-independent precocious puberty (LH receptor), thyrotoxicosis (TSH receptor), Cushing's syndrome (ACTH receptor), hyperpigmented skin lesions and fibrous dysplasia.
Sex steroids, glucocorticoids, aldosterone and thyroxine are hydrophobic hormones which diffuse across the target cell membrane and bind to intracellular receptors located in the cytoplasm (glucocorticoids) or nucleus (androgens and thyroxine). The response to these hormone receptors therefore takes longer than those associated with cell surface receptors. Some hormones, such as testosterone and T4, may be converted once intracellular to more potent forms such as dihydrotestosterone and T3 by 5α-reducatase and 5'-deiodinase, respectively. By contrast, 11β-hydroxysteroid dehydrogenase converts cortisol to a weaker metabolite, cortisone, in aldosterone-responsive cells in the kidney to avoid overstimulation of the mineralocorticoid receptor. Defects in the genes that encode these intracellular receptors lead to hormone resistance such as androgen insensitivity syndrome, glucocorticoid resistance and resistance to thyroid hormone.
The pancreas plays a major role in the regulation of blood glucose concentrations. The pancreas is endodermal in origin, arising from the embryonic foregut. Islet cell clusters differentiate from pancreatic bud endoderm, on the edge of which pancreatic islets form. Under the influence of Pax-6 β- and δ-cells, insulin and somatostatin are produced, respectively, whereas Pax-0 facilitates development of α- and γ-cells responsible for glucagon and pancreatic polypeptide production, respectively. Endocrine function is evident from 10–15 weeks' gestation, though what contribution this makes to fetal development is unknown.
Pancreatic endocrine function is regulated in the human by approximately one million clusters of cells known as the islets of Langerhans. Insulin-secreting β-cells occupy the central part of the islets of Langerhans and are surrounded by a ‘rind’ of glucagon-secreting α- and somatostatin-secreting δ-cells ( Fig. 26.1 ). Islets are well vascularized to facilitate rapid hormone release and are also innervated by sympathetic and parasympathetic neurons, implying that there is a neurological contribution to pancreatic endocrine regulation.
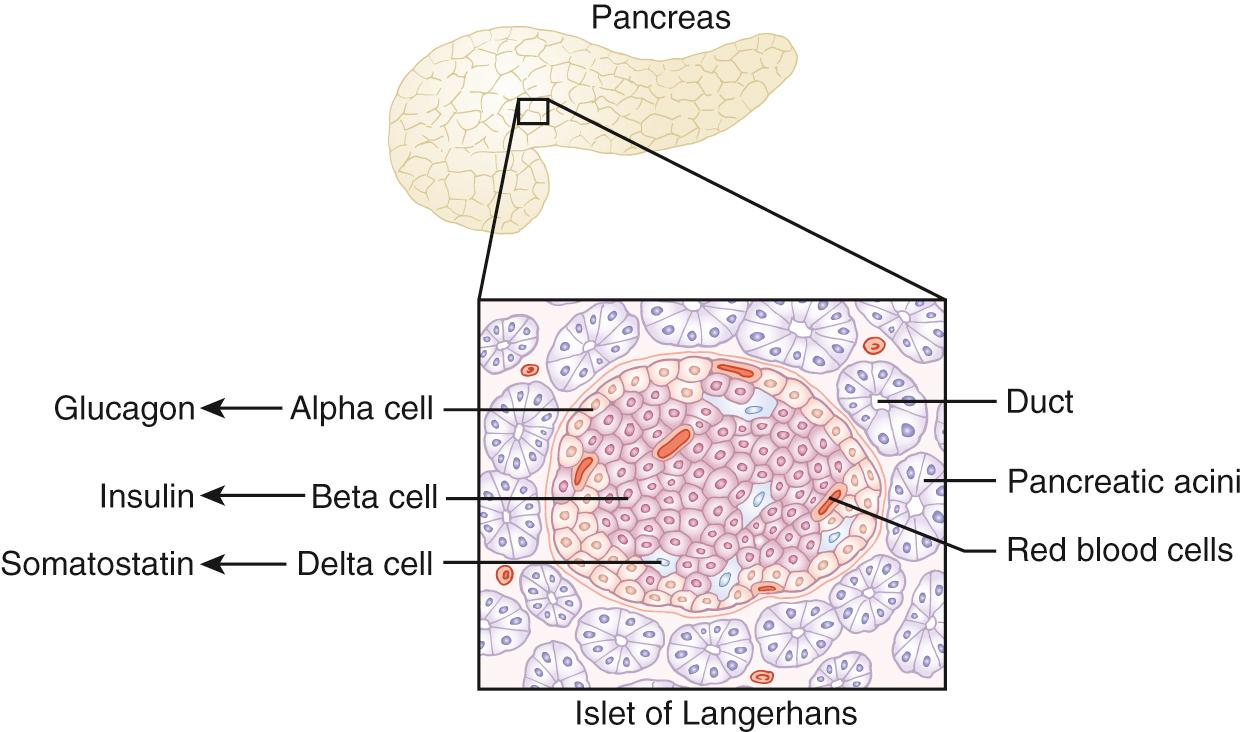
When blood glucose concentrations rise after feeding, insulin is secreted to convert glucose into glycogen ( Fig. 26.2 ) and facilitate cellular uptake, where the glucose is converted to glucose-6-phosphate. By contrast, when glucose levels fall during fasting, concentrations are maintained through secretion of glucagon, which facilitates glucose production from glycogenolysis. Other counter-regulatory hormones such as cortisol, growth hormone and epinephrine also contribute to gluconeogenesis through protein degradation and lipolysis. The latter is particularly facilitated by the switching off of insulin release as blood glucose concentrations fall.
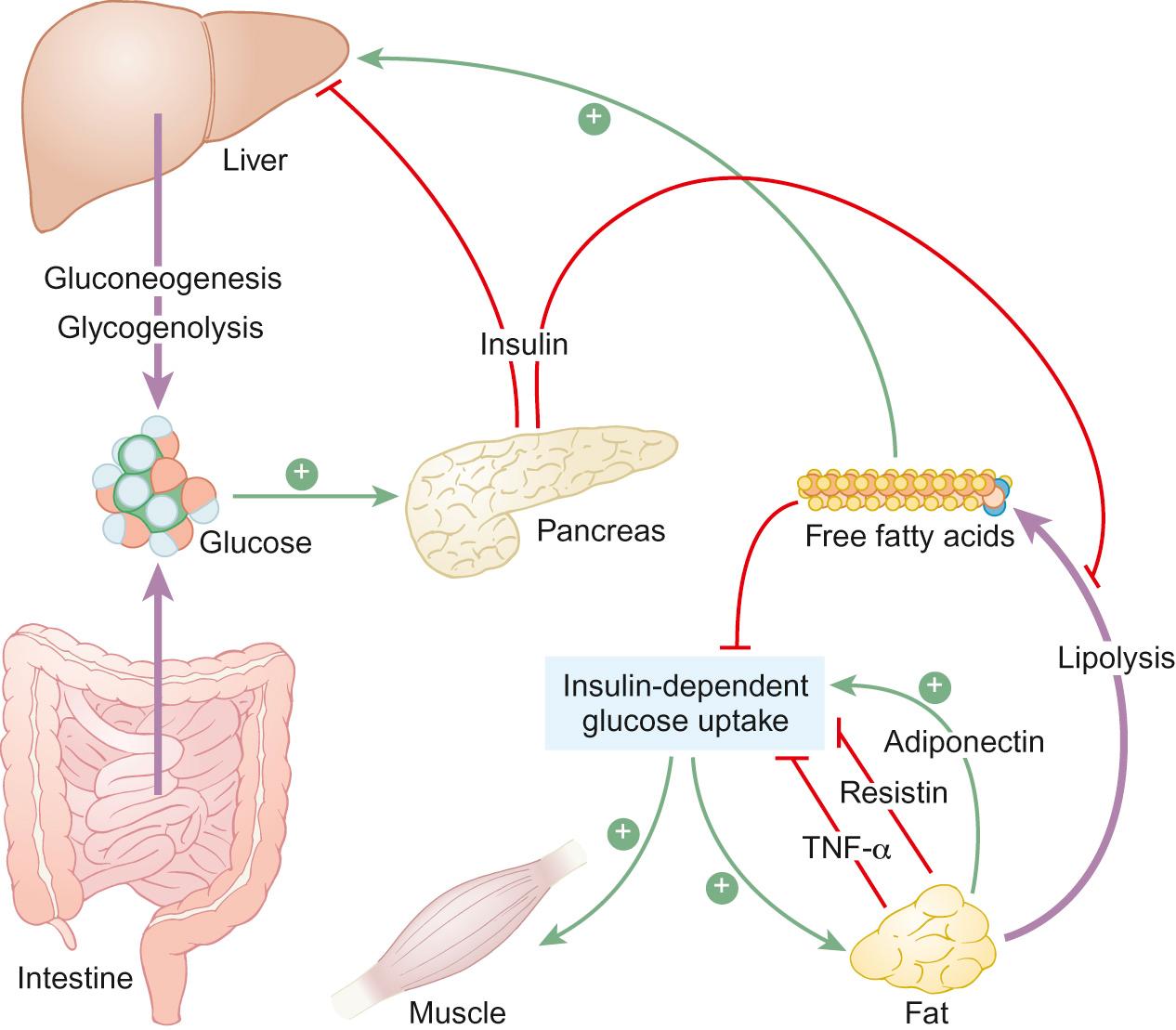
Triglycerides are transported in blood by very-low-density lipoproteins to target tissues where lipases promote hydrolysis of triglycerides into glycerol and free fatty acids. Glycerol is then metabolized to rejoin the glycogenolysis and gluconeogenesis pathways. Free fatty acids are transported across the mitochondrial membrane to undergo a process of beta oxidation, which generates two-carbon molecules of acetyl co-A, which can enter the citric acid cycle to ultimately generate ATP.
Important differences exist between young children and adults with respect to blood glucose regulation. The young child is at particular risk from hypoglycaemia due to the relatively large brain size and limited glycogen stores. To compensate, infants in particular are able to generate ketones as an alternative cerebral fuel source more easily than adults.
In a child presenting with hyperglycaemia, the following history is important:
Duration of symptoms such as polyuria, polydipsia, weight loss, lethargy, constipation or blurred vision
Infection, particularly candidiasis
Vomiting or abdominal pain might suggest ketoacidosis
Family history of diabetes of any type or other autoimmune disease.
Many children with newly diagnosed diabetes will have no abnormal findings on clinical examination. In children with hyperglycaemia and presumed diabetes, the critical need is to distinguish between type 1 diabetes (T1D) and type 2 diabetes (T2D), as the approach to treatment will be very different for each form:
Weight loss, dehydration, signs of acidosis such as Kussmaul breathing (deep and tachypnoeic, reflecting the respiratory effort to correct the metabolic acidosis) with sweet (acetone-smelling) breath, depressed consciousness and signs of cerebral oedema suggest type 1 diabetes
Overweight, hypertension and acanthosis nigricans suggest developing insulin resistance and type 2 diabetes.
In hyperglycaemic children, the following investigations need to be considered:
If there is uncertainty about a diagnosis of diabetes, a random or two hour glucose tolerance test blood glucose of >11.1 mmol/L or fasting blood glucose >7 mmol/L indicates the presence of diabetes; however, in most children with type 1 diabetes, the diagnosis can be determined by clinical features and random blood glucose and no further investigations are required
The degree of elevation in HbA1c levels, which may indicate the length of prodrome
The presence of glutamic acid decarboxylase (GAD) antibodies indicates probable autoimmune-mediated T1D
If a diagnosis of T1D seems likely, screening for other autoimmune disease (hypothyroidism, hyperthyroidism and coeliac disease) is indicated
There can be uncertainty whether the child has T1D or T2D and a formal oral glucose tolerance test with measurement of high concentrations of insulin and c-peptide on baseline and two hour blood samples along with suppressed sex hormone binding globulin concentrations would indicate the presence of T2D.
This is much the most common childhood form of diabetes, caused by T cell-mediated autoimmune damage to pancreatic β-cells evidenced by raised titres of GAD (glutamate decarboxylase) antibodies. There are strong HLA associations with linkage to the major histocompatibility class II genes DQA, DQB and DRB. It presents in genetically susceptible individuals with a more rapid onset evident in pre-school aged children than adolescents. The rapidly increasing incidence of type 1 diabetes, particularly in pre-school aged children implies in addition a change in some unknown environmental precipitant such as diet, viruses, hygiene or toxins. Although a classical presentation of polyuria, polydipsia and weight loss remains common, an increasing proportion are overweight at diagnosis, reflecting population changes in weight gain. Symptoms occur when approximately 90% of β-cells have been destroyed. Individuals with T1D are at increased risk of other autoimmune-mediated diseases such as coeliac and thyroid disease.
Treatment of T1D requires insulin injections two to four or more times daily or delivered by pump in a continuous subcutaneous infusion. In recent years, bioengineered insulin analogues have become available. One group (insulin lispro and insulin aspart) is very rapidly acting due to a change in their molecular structure which prevents polymerization into inactive hexamers following subcutaneous injection. Other long-acting forms work through molecular changes, which either shift their isoelectric point to result in precipitation and slow dissolution to release bioactive molecules (insulin glargine) or alternatively promote potent binding to albumin (insulin detemir) to prolong duration of action. Current research is evaluating autoimmune modulation at diagnosis to prolong residual β-cell activity, ‘closing the loop’ between continuous glucose sensing and insulin pump and islet cell transplants.
Affected individuals require considerable support and a detailed educational programme to help learn how to self-manage their diabetes through:
Appropriate changes in insulin dose guided by the results of self blood glucose testing
Their estimation of the carbohydrate content of food (‘carb counting’)
Their estimation of the known effects of exercise on their blood glucose measurements.
The aim of treatment is to produce optimal blood glucose control, which reduces the risks of microvascular complications to a minimum. This is judged by repeat measurement of glycosylated haemoglobin (HbA1c), a consequence of non-enzymatic glycation of haemoglobin when exposed to plasma glucose. This provides an integrated measure of circulating blood glucose concentrations over the previous 2–3 months but may be unreliable in conditions which affect circulating red cell half-life such as haemoglobinopathies, in which circumstances other measures such as continuous glucose monitoring or fructosamine may be required.
Patients and their carers need to be trained to recognize hypoglycaemia and how to treat these episodes and also to have an awareness of the longer term effects of suboptimally managed diabetes. Inadequate insulinization in the short term leads to hyperglycaemia which, when it exceeds the renal threshold for capacity to reabsorb glucose, results in glycosuria and polyuria through concomitant osmotic effects. Excessive urinary glucose losses result in a negative calorie balance and in time weight loss. A cachexic state leads in the long term to growth hormone resistance with impaired growth and suppressed gonadotropin secretion causing pubertal delay. Excessive insulin deficiency leads not only to hyperglycaemia but also to ketosis due to lipolysis. Accumulation of ketones causes a progressive acidosis with vomiting and impaired consciousness. In the longer term, persistent hyperglycaemia produces microvascular changes in which the basement membrane of epithelial cells becomes damaged through the presence of excess glycoproteins, leading to retinopathy, nephropathy and neuropathy. There is clear evidence from the Diabetes Control and Complications Trial (DCCT) study in the USA, in which teenagers and young adults were randomized to either ‘normal care’ or intensive management with multiple daily injections of insulin and blood testing, that improved blood glucose control leads to dramatic reductions in all diabetes-related complications evaluated within 10 years. Furthermore, these benefits have persisted even when blood glucose levels increased after the end of the trial, suggesting a ‘memory effect’ from this period of tight glycaemic control. These observations have driven an increased use of multiple daily injections and insulin pumps in routine clinical practice, to try and replicate the DCCT findings in routine care.
This remains a relatively unusual cause of diabetes in childhood. Symptoms at presentation are similar to those of T1D, though ketoacidosis is less common. There is a clear genetic predisposition to T2D through different genes to those responsible for T1D. The primary mechanism appears to be obesity-induced insulin resistance with a secondary relative degree of insulin insufficiency leading to excessive hepatic release of glucose.
Management requires lifestyle changes to increase physical activity and reduce calorie intake to promote weight loss and increase insulin sensitivity. Most children require additional medical therapy using metformin, which acts primarily by suppressing hepatic gluconeogenesis. Additional therapy may be necessary using sulphonylureas, which bind to the potassium channel on the pancreatic β-cell, precipitating membrane depolarization, calcium influx and insulin release. Some individuals will also require additional insulin injections when adequate blood glucose concentrations cannot be achieved. Young people with T2D require similar screening to those with T1D for the development of microvascular complications. In addition, teenage girls are at risk of polycystic ovary syndrome (PCOS), which may cause distressing hirsutism and impair the menstrual cycle and fertility. The symptoms of PCOS may respond to the treatment of associated insulin resistance with metformin.
Genetic defects of genes (mostly transcription factors) expressed in the β-cell known as maturity-onset diabetes of the young (MODY) produce variable defects in insulin secretion (but not action). MODY is characterized by mild asymptomatic hyperglycaemia in non-obese children, often with a strong autosomal dominant family history of ‘diabetes’ associated with low or non-existent risks of complications. Most cases do not require treatment.
Rare causes of diabetes include those associated with mutations in mitochondrial DNA (e.g. maternally inherited diabetes and deafness syndrome and Kearns–Sayre syndrome) and those where insulin sensitivity is impaired by genetic defects in insulin receptor signalling (e.g. leprechaunism).
With increasing length of survival, teenagers with cystic fibrosis are at increasing risk of developing diabetes due to the combination of pancreatic destruction and insulin resistance, particularly during pulmonary exacerbations. This requires insulin treatment to support an unrestricted diet, whilst taking care to avoid hypoglycaemia, given the increased risks associated with coexistent α-cell damage.
Recently, neonatal diabetes has been recognized to be due to defects of genes expressed in the pancreatic β-cell. A particularly exciting outcome from understanding its pathogenetic basis has been the subsequent prediction and observation that in those with mutations of certain genes (encoding the Kir6.2 subunit of the ATP-sensitive potassium channel, KCNJ11), treatment with sulphonylureas can be even more effective than insulin, allowing patients to be weaned off daily injections.
A 14-year-old girl who was diagnosed with type 1 diabetes aged 8 years is admitted with an episode of ketoacidosis. Following rehydration and stabilization with intravenous insulin, she is found to have lost 8 kg in weight since her previous clinic visit 6 months earlier, having missed two appointments subsequently. She reports problems with recurrent hypoglycaemia and having had to reduce her daily dose of insulin (four injections daily) to less than 0.5 units/kg/day. She does not report any other symptoms of ill health. Inspection of her blood glucose diary shows largely normal blood glucose values but repeat measurement of her HbA1c shows a markedly elevated value (104 mmol/mol).
What are the likely explanations for her weight loss? Answer each with true (T) or false (F).
Addison's disease
Coeliac disease
Eating disorder
Hyperthyroidism
Inadequate insulin therapy
What additional treatment(s) is/are required? Answer each with true (T) or false (F).
Carbimazole
Gluten-free diet
Hydrocortisone
Recommended increased insulin doses
Referral to a psychologist
A. False; B. False; C. True; D. False; E. True.
A. False; B. False; C. False; D. True; E. True.
The presentation of ketoacidosis, weight loss and an elevated HbA1c value in a child with established type 1 diabetes who is a poor attender in clinic should always raise concerns about inadequate insulin therapy, most commonly due to poor adherence, regardless of self-reported blood glucose values, which may be fabricated. The fact that this girl openly reports reducing her insulin injections to an unusually small dose for a teenager should also raise the question that this represents a deliberate attempt to manipulate her body weight and the possibility of an eating disorder should be considered. Coeliac disease and hyperthyroidism are unlikely in an asymptomatic individual. Finally, the rare development of another autoimmune disorder such as Addison's disease should be considered in an individual forced to reduce their insulin dose and who has lost weight, but the increase in insulin sensitivity that drives this effect would make development of ketoacidosis and such a markedly elevated HbA1c value less likely. This girl clearly requires increased doses of insulin and, if an eating disorder is suspected, help from a clinical psychologist will be required to optimize management.
Hypoglycaemia in the immediate neonatal period is common and usually transient. It is considered in Chapter 11 , Neonatal medicine. This section considers neonates, infants and children with recurrent hypoglycaemia.
When evaluating a child for hypoglycaemia, attention should be paid to:
Autonomic symptoms of hypoglycaemia, such as pallor, sweating, tachypnoea in a neonate or anxiety, palpitations and tremor in an older child
Neuroglycopenic symptoms of jitteriness, apnoea, hypotonia, feeding problems, irritability, abnormal cry, convulsions or coma in a neonate or hunger, abdominal pain, nausea, vomiting, pins and needles, headache, weakness, dizziness, blurred vision, irritability, mental confusion, odd behaviour, fainting, convulsions or coma in an older child
In a neonate, pregnancy details (e.g. maternal symptoms of diabetes), mode of delivery (breech said to be more common in hypopituitarism), birth weight (hypoglycaemia more common in intrauterine growth restriction or large for gestational age due to maternal diabetes) and the relationship of hypoglycaemia to feeding (i.e. is this a question of inadequate fuel supply or excess fuel requirements as in hyperinsulinism?)
Access to oral hypoglycaemic medication, which may have been accidentally ingested
Family history of sudden infant death or consanguinity might suggest an inborn error of metabolism
Development of symptoms in response to foods containing lactose, fructose or sucrose might suggest galactosaemia or disorders of fructose metabolism.
Most children with a history of hypoglycaemia will not have any abnormal clinical signs on examination unless actually hypoglycaemic at the time. However, the presence of the following signs may indicate an associated diagnosis:
Optic atrophy – septo-optic dysplasia
Cranial midline defects, short stature, microgenitalia – hypopituitarism
Increased skin or buccal pigmentation, hypotension – Addison's disease
Underweight or signs of malnutrition – accelerated starvation
Tall stature, excess weight – hyperinsulinism
Abnormal ear-lobe creases, macroglossia, umbilical hernia, hemihypertrophy – Beckwith–Wiedemann syndrome
Hepatosplenomegaly – glycogen storage disorder.
The aim of investigations is to establish the severity of hypoglycaemia and to evaluate the counter-regulatory responses and intermediary metabolite pathways by obtaining a blood sample before instituting treatment of the hypoglycaemic episode for measurement of:
Glucose to confirm severity of hypoglycaemia
Urea and electrolytes for evidence of adrenal insufficiency
Bicarbonate or pH, as acidosis might imply adrenal failure or an inborn error of metabolism
Liver function tests, as may be abnormal in primary liver disease, sepsis, glycogen storage disease, galactosaemia, fatty acid oxidation defects and hereditary fructose intolerance
Ammonia, may be elevated in a number of inborn errors of metabolism and some forms of hyperinsulinism
Insulin and c-peptide, should normally be suppressed
Cortisol, ACTH and growth hormone should normally be elevated, implying an appropriate stress response
Free fatty acids and β-hydroxybutyrate, if elevated in proportion imply appropriate lipolysis
Acylcarnitine will be abnormal in some fatty acid oxidation defects
Lactate will be elevated in metabolic liver disease, glycogen storage disorder and sepsis
Alanine, if low suggests ‘accelerated starvation’ (see below)
After glucose has been administered to correct the hypoglycaemia, the next urine sample should be collected to measure the presence of ketones, reducing sugars, dicarboxylic acids, glycine conjugates, carnitine derivatives, and amino and organic acids to screen for inborn errors of metabolism and a toxicology screen.
Treatment depends on aetiology. Hypoglycaemia may be broadly subdivided into two groups:
Causes associated with reduced glucose availability arise due to defects in the counter-regulatory response including limited supplies of glucose precursors. These include:
Being born with intrauterine growth restriction
Prematurity
Hypopituitarism
Adrenal insufficiency
Growth hormone deficiency
Hypothyroidism
Glucagon deficiency
Accelerated starvation (ketotic hypoglycaemia)
Inborn errors of metabolism
Drugs such as alcohol, aspirin and beta blockers
Liver dysfunction
Congenital heart disease.
Investigation of hypoglycaemia frequently fails to demonstrate any underlying abnormality. Accelerated starvation is an example of this. It can only be diagnosed once other endocrine and metabolic causes are excluded, and is of unknown aetiology. It is characterized by demonstration of a normal endocrine counter-regulatory response with raised fatty acid and ketone responses. It is more common in boys than girls and in those who were born small for gestational age or who have a thin physique; it usually resolves by puberty. Treatment of this group of causes involves avoidance of prolonged fasting, particularly during intercurrent illness, administration of complex carbohydrates such as cornstarch at bedtime to provide a prolonged glucose response to feeding and intravenous dextrose as required to reverse acute hypoglycaemia.
Alternatively, hypoglycaemia may occur in the context of increased glucose consumption due to:
Congenital hyperinsulinism
Transient neonatal hyperinsulinism
Being an infant of a diabetic mother
Insulinoma
Beckwith–Wiedemann syndrome
Rhesus haemolytic disease
Perinatal asphyxia
Malaria.
Markedly excessive glucose requirements (e.g. in excess of 12 mg/kg body weight/hour) to avoid hypoglycaemia suggest underlying hyperinsulinism. Congenital hyperinsulinism may occur in relation to several genetic defects affecting the regulation of insulin release. The most commonly identified are those of genes encoding the sulphonylurea receptor (ABCC8) and associated potassium inward rectifying channel (KCNJ11). Affected children are at increased risk of hypoglycaemia-induced brain damage as the hyperinsulinism suppresses not only glucose but also ketone body production, which is an alternative cerebral fuel source. Some respond to diazoxide and chlorothiazide treatment, diazoxide exerting its effect through actions on the potassium channel by inducing hyperpolarization, decreased calcium influx and reduced insulin secretion. If these fail, then a somatostatin analogue may be used to exert a direct receptor-mediated inhibition of insulin release. Scientific advances in our understanding of the genetics and new imaging techniques such as [ 18 F]DOPA positron emission tomography scanning allow, in persistent cases, distinguishing focal from diffuse pancreatic disease, the former being curable by surgical excision with minimal morbidity.
A 4-day-old baby boy presented with a seizure. His blood glucose was extremely low at 0.8 mmol/L. There was no relevant family history and the pregnancy and birth had been unremarkable. His birthweight was 3.94 kg and there were no abnormalities on examination. He experienced recurrent hypoglycaemia despite full volume feeds and an intravenous dextrose infusion providing an additional 14 mg glucose/kg/min.
What is the most likely diagnosis? Select ONE answer only.
Accelerated starvation
Congenital adrenal hyperplasia (CAH)
Congenital hyperinsulinism
Hypopituitarism
Medium chain acyl coA dehydrogenase (MCAD) deficiency
What additional treatment is required? Select ONE answer only.
Chlorothiazide and diazoxide
Increased feed volume
Growth hormone
Hydrocortisone
Low-fat milk
C. Congenital hyperinsulinism.
A. Chlorthiazide and diazoxide.
When faced with a neonate with severe, recurrent hypoglycaemia, it is critical, in order to make a diagnosis, to establish whether the problem is associated with restricted calorie intake, implying a defect in counter-regulation (e.g. CAH, hypopituitarism, MCAD deficiency or accelerated starvation), or whether it is associated with excess glucose requirements. Such dramatically elevated glucose requirements to avoid hypoglycaemia are seen with hyperinsulinism, for which specific treatment with diazoxide, which decreases insulin secretion by direct action on potassium inward rectifying channel, is indicated. Chlorothiazide is also given as it has a synergistic effect on insulin secretion as well as assisting in the tendency for diazoxide to cause fluid retention. Increased feed volume will not overcome such high glucose requirements. Although low cortisol levels may occur in congenital hyperinsulinism, there is no evidence of any benefit from hydrocortisone or growth hormone therapy. Finally, this child requires increased calories to prevent hypoglycaemia-induced brain damage, not a low-fat milk.
The thyroid develops from four weeks' gestation from an outpouching of the floor of the pharynx (the precursor of T4-producing follicular cells) and bilateral protrusions of the fourth pharyngeal pouches (which give rise to calcitonin-secreting cells). The thyroid descends along the thyroglossal tract, which then regresses, though remnants may come to form thyroglossal cysts. Failure of descent results in an ectopic thyroid gland, a common form of congenital hypothyroidism. The process of thyroid development is regulated by a number of transcription factors (including PAX-8, FOXE-1 and NKX2.1).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here