Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Megakaryocytes (MKs), among the rarest and most unusual hematopoietic cells in the human bone marrow, comprise 0.02% to 0.1% of the total nucleated marrow cells. , Over the past decades, the study of these cells lagged behind that of other hematopoietic lineages, partly because of the rarity and fragility of MKs in the bone marrow, and partly because of the lack of a potent thrombopoietic factor to stimulate the growth of these cells in culture. The cloning of thrombopoietin (Tpo) in 1994 broke many of these barriers and led to major advances in our understanding of megakaryopoiesis. The first part of this chapter is dedicated to reviewing the biology of MK progenitors, the differentiation and maturation of MKs, the effects of thrombopoietic cytokines (particularly Tpo), and how these processes differ between fetal, neonatal, and adult megakaryopoiesis. The second part of the chapter summarizes our current understanding of neonatal thrombocytopenia, with an emphasis on the factors underlying the predisposition of ill neonates to develop severe and prolonged thrombocytopenia. Although many questions in this field remain unanswered, it is now clear that fetal and neonatal MKs have cellular and molecular features clearly different from those of their adult counterparts. These developmental differences have implications for the pathogenesis of common and less common platelet disorders, and expand beyond neonatal hematology into the field of cord blood (CB) transplantation, a therapy frequently complicated by delayed platelet engraftment.
In a very schematic fashion, the complex process of platelet production can be represented as consisting of four main steps ( Fig. 110.1 ): (1) the production of thrombopoietic factors (mainly Tpo), (2) the proliferation of MK progenitors, (3) the differentiation and maturation of MKs through a unique process of endomitosis, and finally (4) the production and release of platelets into the circulation. The first part of this section is dedicated to reviewing the biology of adult megakaryopoiesis. The second half is focused on fetal and neonatal megakaryopoiesis, highlighting the key developmental differences.
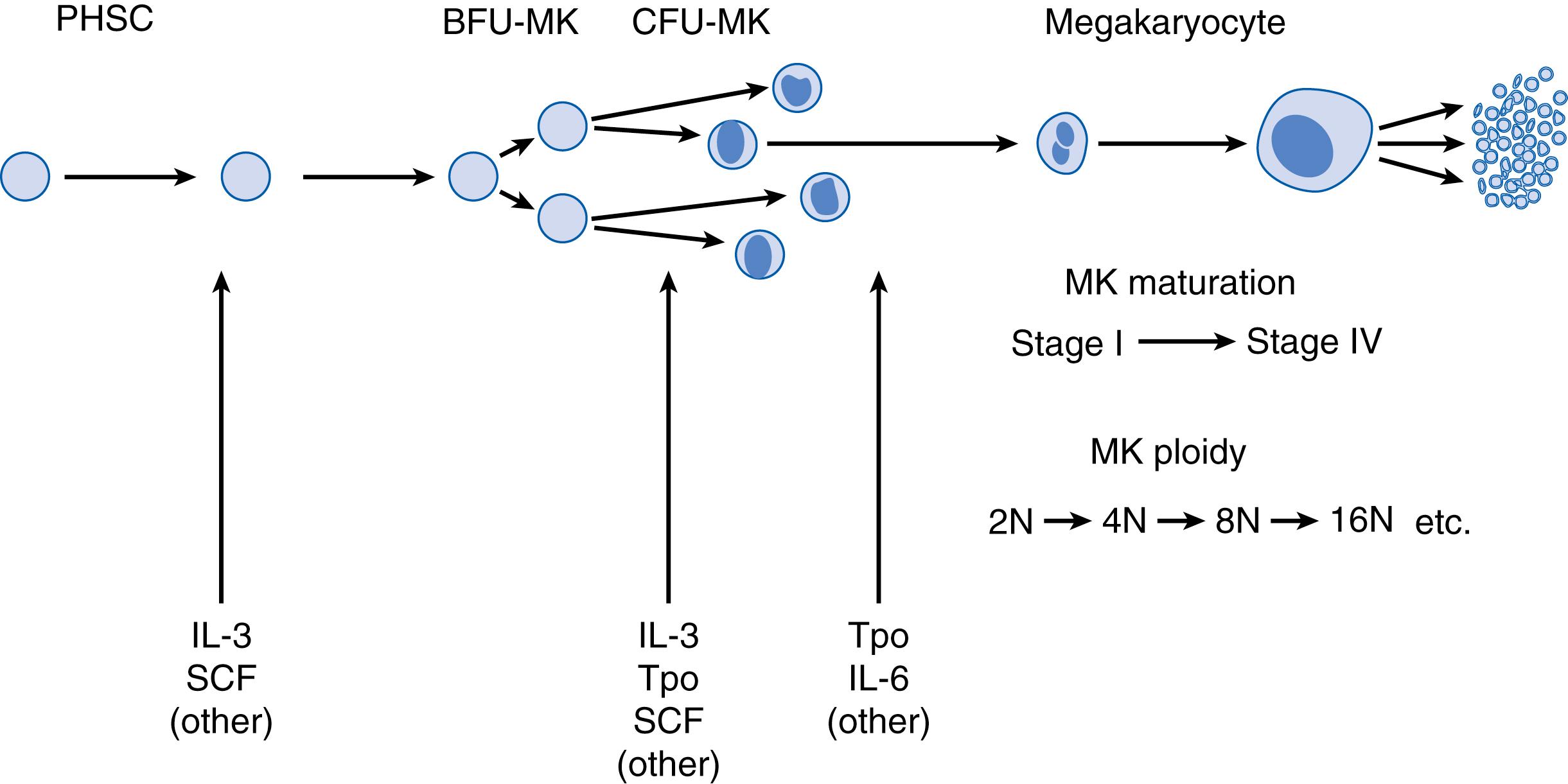
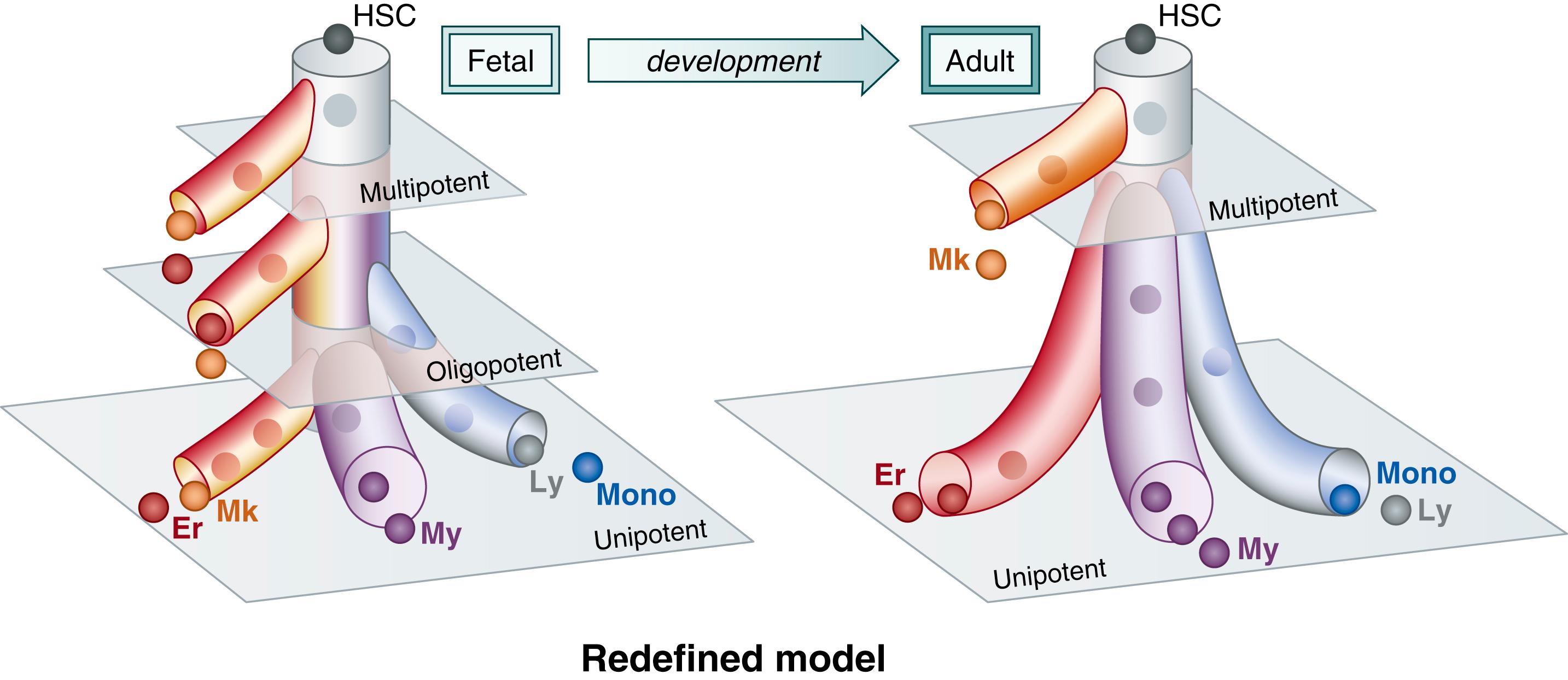
The development of the megakaryocytic lineage from pluripotent hematopoietic stem cells (HSCs) is not clearly understood. The classical model states that HSCs give rise to a common lymphoid progenitor, which produces lymphocytes, and a common myeloid progenitor, which gives rise to the myeloid, macrophage, eosinophil, erythroid, and megakaryocytic lineages. In this model, the erythroid and MK lineages arise from a common oligopotent MK-erythroid progenitor (MEP). , However, this classical pathway of hematopoiesis has been challenged by several recent discoveries. Studies examining the heterogeneity within the human hematopoietic progenitor/stem cell (CD34 + ) compartment found that the blood hierarchy in adult BM was mainly composed of two tiers: a top tier containing multipotent cells such as HSCs and multipotent progenitors (MPPs), and a bottom tier composed of committed unipotent progenitors with primarily myeloid or erythroid potential. Interestingly, this study found that the MK lineage in the adult BM emerged directly from the HSC compartment, supporting the hypothesis that MK branching in the adult human BM occurs directly from a multipotent cell and that there might be a subset of human HSCs primed for platelet production, similar to the von Willebrand factor (vWF)-positive platelet-primed stem cells described in the mouse. , The specific signals that regulate the separation into the different lineages are not well understood and are the subject of active research efforts.
The first cells fully committed to the MK lineage are the MK progenitors. By definition, these cells have proliferative potential and are characterized by their ability to form clusters of pure MKs (MK colonies) when cultured. Two different types of MK progenitors have been identified. The most primitive MK progenitor is the burst-forming unit–MK (BFU-MK). A later, more mature progenitor has been designated the colony-forming unit–MK (CFU-MK). These two progenitor types can be differentiated on the basis of their immunologic markers and the characteristics of the colonies that they form in vitro ( Table 110.1 ). Overall, CFU-MK-derived colonies are smaller (usually <50 cells) and unifocal, compared with the large and multifocal appearance of BFU-MK colonies. CFU-MK progenitors are also the predominant MK progenitor type in the bone marrow, whereas BFU-MK progenitors predominate in the blood. Finally, although both progenitors express CD34, only CFU-MKs express the human leukocyte antigen (HLA)-DR.
| Characteristic | BFU-MK | CFU-MK |
|---|---|---|
| Appearance in culture | Later | Earlier |
| Cells/colony | >50 | 3–50 |
| Foci of development | ≥2 | 1 |
| Immunologic phenotype | CD34 + , HLA-DR – | CD34 + , HLA-DR + |
| Site of predominance | Peripheral blood and bone marrow | Bone marrow |
Unlike MK progenitors, which possess a high proliferative potential, MKs are cells that have lost the ability to proliferate but instead undergo a unique process known as endomitosis , in which chromosomes duplicate without nuclear division, resulting in increased cell ploidy. The transition from MK progenitors to mature MKs is characterized by a significant level of overlap, which gives rise to a population of cells known as transitional cells , MK precursors , or promegakaryoblasts , which have increasing ploidy (4N to 8N) and decreasing proliferative potential. These cells are thought to represent the intermediate step between progenitors and MKs and are difficult to identify morphologically. Immunologic studies have suggested that transitional cells express both CD34 and CD61 (GPIIIa). During maturation, the cells accumulate various α-granule proteins including platelet factor 4 (PF4), thrombospondin, β-thromboglobulin, and vWF, and accumulate surface markers such as platelet GPIb and the vWF receptor ( Fig. 110.2 ).
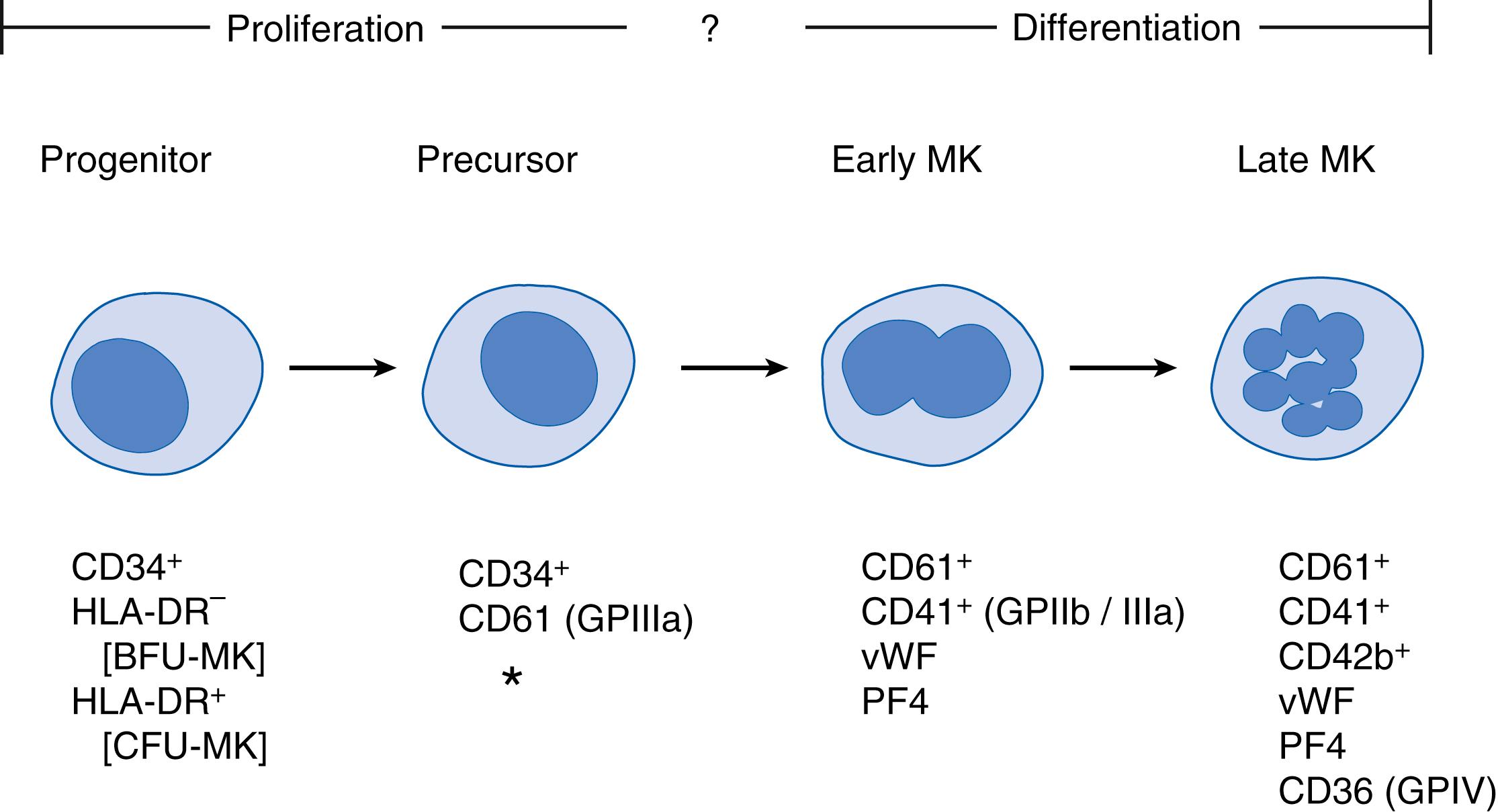
MKs are cells that have lost their proliferative abilities and undergo a complex process of maturation. Through this process, they evolve from small, mononuclear cells that are indistinguishable from those of other lineages, to very large, polyploid cells that are easily recognized as MKs. The process of maturation involves both nuclear and cytoplasmic changes, as well as a corresponding increase in size. At the nuclear level, MKs undergo endoreduplication or endomitosis, which leads to increased ploidy. The ploidy level of MKs can be assessed on stained individual cells in a cytospin or by flow cytometry. Using the latter technique, the modal ploidy in adult marrow has been shown to be 16N. Increasing ploidy levels correlate with increased platelet production by MKs, at least in vitro .
The patterns of endomitosis are somewhat different between high and low ploidy MKs. In the transition from a 2N to a 4N MK, the cell elongates while creating two separated nuclear masses, forming a cleavage furrow; however, due to a halt in cytokinesis, the cells do not separate and instead move backwards to reassemble a 4N cell. The clear cleavage furrow created through this process is seldom seen in higher ploidy MKs, as chromosomes segregate into 3 to 5 groups containing multiple territories, without exhibiting a clear furrow.
Based on a combination of size and nuclear and cytoplasmic features, the maturational stages of the MKs have been classified as stages I to IV. This morphologic classification is often difficult, however, particularly because megakaryoblasts and stage I and II MKs can easily be missed on microscopic examination. For these reasons, other criteria have been established for MK differentiation, and for defining various maturational stages, including the development of surface glycoproteins and the appearance of specific granules. , For example, GPIIIa (CD61) and GPIIb/IIIa (CD41/61) are present in very early MKs, whereas GPIb (CD42b), GPIV (CD36), and the GP1b-V-IX (CD42) complexes appear later during MK maturation (see Fig. 110.2 ). , The development of the invaginated membrane system, formerly known as the demarcation membrane system , and of α-granules has been documented by electron microscopy. Contents of the α-granules, such as fibrinogen, thrombospondin, and vWF, also correlate with MK maturation. , ,
In the course of human as well as murine development, hematopoiesis transitions from the yolk sac to the liver and then to the BM. In humans, MKs have been observed in the yolk sac by 5 weeks’ gestation, and the first platelets appear in the circulation at 8 to 9 weeks. The transition to hepatic hematopoiesis is thought to involve the migration of stem cells from the yolk sac to the liver. MKs are first found in the human liver at 10 weeks’ gestation. Every stage of MK development is present in the fetal liver, although MKs at every stage are significantly smaller than their adult counterparts. , The transition from hepatic to BM hematopoiesis occurs progressively throughout gestation. BM hematopoiesis starts at 11 weeks’ gestation, and it becomes the dominant site of hematopoiesis by 20 weeks. , The contribution of the liver, in contrast, decreases throughout fetal development. At 16 weeks, 50% to 70% of all liver cells are hematopoietic cells, but this percentage decreases after 27 to 32 weeks’ gestation to 25% to 30% at term. Thus, in the human, the BM is the primary hematopoietic site in preterm and term neonates, but the liver is still active as a secondary hematopoietic site.
In the mouse, primitive MK/erythroid progenitors appear at embryonic day 7.25 (E7.25), along with pure MK and pure primitive erythroid progenitors, which indicates that primitive hematopoiesis is bilineal. The first platelet-forming cells of the embryonic yolk sac are not polyploid MKs, but diploid platelet-forming cells (DPFCs), similar to the 2N MKs that produce platelets in the human fetus (see “Fetal and Neonatal Megakaryocytes”). DPFCs appear in the yolk sac at E8.5 and produce the first platelets at E9.5. Highly polyploid MKs (achieving 8N) are first seen in the fetal liver at E11.5 and progressively achieve greater ploidy levels, a process associated with a significant expansion of the fetal platelet mass. In contrast to humans, the liver is the main murine hematopoietic organ at birth, and the transition from liver to BM megakaryopoiesis occurs largely during the first 2 weeks of postnatal life, with the spleen providing hematopoietic support during this period.
Recent studies challenging the traditional paradigms of hematopoiesis also unveiled important developmental differences between human fetal and adult hematopoiesis. Compared to the two-tier model in adult BM (see “Adult Megakaryocyte Progenitors), the fetal liver exhibits a three-tier hierarchy, composed of multipotent HSCs and MPPs at the top, oligopotent progenitors with myeloid-erythroid-MK and erythroid-MK activity in the middle, and unipotent progenitors in the bottom ( Fig. 110.3 ) . An additional recent study investigating blood and immune cell development in the human liver between 7 and 17 weeks post-conception (when the liver is the main site of hematopoiesis), found that HSCs/MPPs in the fetal liver have different intrinsic potential according to gestational age, with samples from early stages exhibiting an erythroid lineage bias and lymphoid and myeloid lineages being more represented at later stages. This study also supported the presence of a fetal liver–shared MK-erythroid-mast cell progenitor (MEMP) immediately downstream of the HSC/MPP cell, and identified F11R , PBx1, and MEIS as the genes dynamically modulated in the specification of the fetal MK lineage.
Most studies assessing the quantity and clonogenic potential of MK progenitors during fetal development have been performed using umbilical CB, from which large quantities of MK progenitors can be readily obtained. Olson and associates found a significantly higher concentration of CFU-MK progenitors in term and preterm CB than in adult peripheral blood (PB). Furthermore, when stimulated with aplastic canine serum, CB CFU-MK-derived colonies contained significantly more MKs than adult colonies. Zauli and co-workers cultured hematopoietic progenitors (CD34 + cells) obtained from the blood of fetuses between 18 and 22 weeks’ gestation, or from adult bone marrow in a fibrin clot assay, and found that, as in adult blood, BFU-MKs predominate in fetal blood, with a CFU-MK/BFU-MK ratio of 0.4:1. Fetal and adult MK progenitors had similar immunologic profiles with regard to the expression of CD34 and HLA-DR. , However, there were significant differences in the morphology and size of the BFU-MK-derived colonies: the fetal BFU-MK-derived colonies were significantly larger than the adult colonies and were usually composed of only one or two foci of development, whereas the adult BFU-MK-derived colonies were always multifocal.
Other investigators have also reported the existence of a MK progenitor with an unusually high proliferative potential exclusively found in the human fetal bone marrow. Bruno and colleagues described a high proliferative potential cell–MK (HPPC-MK) in fetal bone marrow that gives rise to large, unifocal colonies with more than 300 cells. This cell, not observed in adult bone marrow cultures, may represent a more primitive MK progenitor only present during fetal life. Nishihira and associates cultured CB hematopoietic progenitors (26 to 41 weeks’ gestation) with optimal concentrations of recombinant Tpo (rTpo) and found that all colonies consisted of more than 50 cells and that 50% had more than 500 cells. It is unclear whether these colonies should be classified as BFU-MK- or HPPC-MK-derived colonies.
Comparing circulating MK progenitors between preterm and term neonates, Murray and collaborators found that, in the first day of life, preterm neonates (24 to 36 weeks’ gestation) had higher circulating concentrations of MK progenitors than those born at term. This finding was in concordance with the previously described gestational age-related decrease in the concentration of other committed hematopoietic progenitors, deemed to reflect the migration of progenitors from the fetal liver to the bone marrow over gestation. Saxonhouse and associates also observed an inverse relationship between the concentration of circulating MK progenitors and the postmenstrual age (gestational age + postnatal age in weeks) of growing preterm infants being cared for in the neonatal intensive care unit (NICU), suggesting that the decrease in circulating MK progenitors is a process that follows the same developmentally regulated pattern regardless of premature birth.
In addition to these phenotypic differences, at the transcriptional level fetal and neonatal MK progenitors continue to express erythroid lineage markers, suggesting incomplete lineage separation in fetal/neonatal compared to adult progenitors. In a study comparing the gene expression profiles of purified MK progenitors from murine fetal livers and murine adult BM, 7 out of the 122 transcripts upregulated in fetal liver were erythroid transcripts. Similarly, human CB-derived MKs (CD41 + cells) express the erythroid antigen glycophorin A (GPA), while MKs derived from adult BM do not. ,
Using different cell sources and techniques, several investigators demonstrated that MKs in the fetal and neonatal BM, in CB, or cultured from CB progenitors were consistently smaller and of lower ploidy than MKs from adults, , , and documented a progressive shift to higher ploidy classes and larger MKs during fetal development. , Despite this shift, however, 78% of MKs in the BM of fetuses at 7 to 8 months’ gestation had a ploidy of 8N or less, compared with only 33% of the MKs in adult BM.
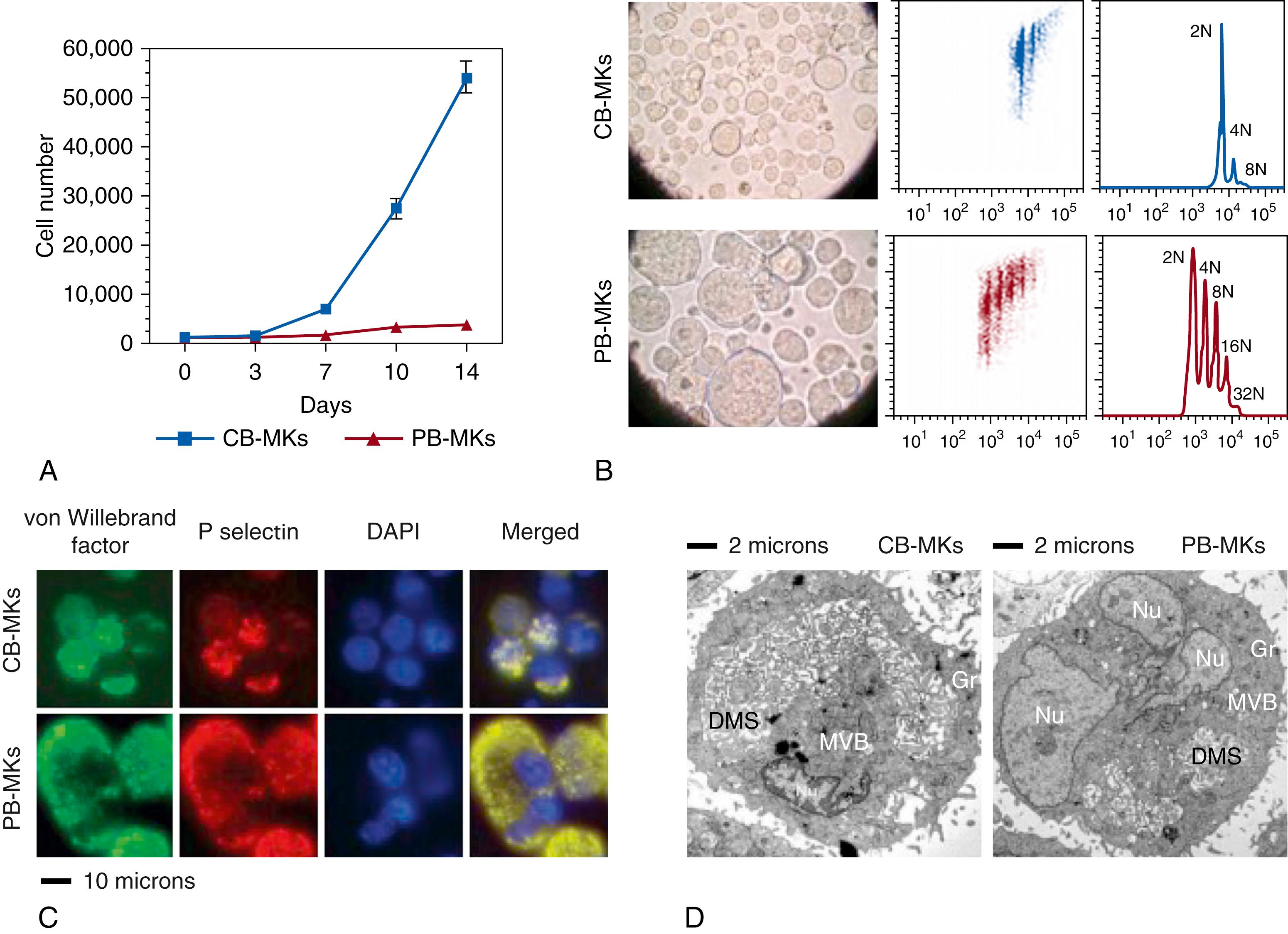
Applying ultrastructural techniques, Hegyi and collaborators evaluated the maturational stage of small fetal liver MKs and found that, despite their low ploidy, they had all the cytoplasmic components of a mature cell, with multiple granules and a well-developed demarcation system. Consistently, our group found that MK progenitors from CB cultured in liquid culture medium generated 10 times more MKs than progenitors from adult PB. The neonatal MKs, however, were significantly smaller and of lower ploidy than adult MKs, but were cytoplasmically mature based on surface markers, immunofluorescence, and electron microscopy ( Fig. 110.4 ). Taken together, these studies demonstrated a developmentally unique uncoupling of proliferation, polyploidization, and cytoplasmic maturation in neonatal MKs. In terms of platelet production, however, the small size and low ploidy of CB MKs is associated with decreased levels of platelet production (per MK), compared to adult MKs. Thus, the current evidence suggests that platelet production in fetuses and neonates is highly dependent on the increased proliferative rate of their MK progenitors.
The mechanisms underlying the small size of neonatal MKs are not clearly understood but likely involve a combination of cell-intrinsic factors and factors in the fetal and neonatal microenvironment. This was demonstrated by Slayton and associates, who transplanted neonatal liver cells or adult bone marrow cells from green fluorescent protein (GFP) transgenic mice into wild-type adult recipients and evaluated the size and ploidy of the donor-derived (GFP + ) MKs. MKs derived from neonatal stem and progenitor cells, placed in an adult environment, were significantly larger and of higher ploidy than neonatal MKs in their original environment (neonatal liver). However, they were significantly smaller than post-transplantation MKs derived from adult bone marrow cells. In vitro studies culturing CB CD34 + cells in adult bone marrow stromal cell–conditioned media also yielded MKs with higher ploidy levels than those cultured in fetal bone marrow stromal cell–conditioned or in unconditioned serum-free media. Comparison of MK size in bone marrow biopsy specimens obtained from children transplanted with either CB - or adult bone marrow–stem cells revealed that MKs in the post-engraftment bone marrow samples were significantly smaller in the CB group than in the bone marrow group, demonstrating that the attainment of adult size in CB-derived MKs is delayed after human CB transplantation. Taken together, these findings suggest that the environment influences the size and ploidy of MKs (with the adult environment being more conducive to MK maturation) but that cell-intrinsic factors limit the ultimate size and ploidy that neonatal MKs achieve.
To establish the timing of the transition from a neonatal to an adult phenotype, Fuchs and collaborators measured MK diameters (using a combination of immunohistochemistry and image analysis) in 72 BM samples from patients aged 3 days to 80 years. This study found that neonates had MKs of uniform small sizes, which diverged into separate clusters of smaller and larger cells beginning at 2 years, and finally transitioned to larger (adult-like) MKs by 4 years.
The process of megakaryopoiesis is carefully coordinated by a number of transcription factors and transcriptional regulators responsible for regulating the expression of MK-specific genes. Of current interest is how such factors may be responsible for stage-specific differences noted between neonatal and adult megakaryopoiesis. In the first comprehensive study comparing the transcriptome of MKs at different stages of development (human embryonic stem cells, fetal livers, full-term CB, and adult PB), 253 genes were found to be up-regulated in MKs through development, which revealed a progression toward increased polyploidization, proplatelet formation, and platelet function. Genes enriched in fetal MKs, on the contrary, were mostly related to angiogenesis, the extracellular matrix, transforming growth factor receptor, and bone morphogenic protein (BMP) signaling. Some of the best studied transcription factors that play a role in megakaryopoiesis are summarized here.
The GATA family of transcription factors plays key roles in normal hematopoiesis. GATA - 1, a 47-kDa protein with the corresponding DNA sequence mapped to the X-chromosome, is required for terminal differentiation of both erythroid and megakaryocytic cell lines. GATA - 1 contains three functional domains: two zinc fingers (N- and C-) and an N-terminal activating domain. The C-finger binds to GATA - 1 binding sites. The N-finger provides stability during DNA binding and recruits the cofactor Friend of GATA - 1 (FOG-1). Interaction with FOG-1 is necessary for normal hematopoiesis, as demonstrated by the presence of anemia and thrombocytopenia in individuals with GATA1 mutations that interfere with the binding of FOG-1. The N-terminal activating domain confers transcriptional activity.
Specific GATA-1 knockout mice models have demonstrated the necessity of GATA-1 in normal hematopoiesis. GATA-1-null mice die during gestation from severe anemia. Mice with MK-specific deficiency of GATA-1 have thrombocytopenia without anemia, with platelet counts approximately 15% of normal. Morphologically, GATA1-deficient MKs are also abnormal, characterized by small size, large segmented nuclei, scant cytoplasm, and decreased ploidy compared with wild-type cells. , Several GATA1 mutations have been described in patients with thrombocytopenia, usually characterized by the presence of large platelets (macrothrombocytopenia). Recently, Liu and colleagues showed a threefold increase in GATA-1 protein levels in CB—compared to PB–derived MKs.
Calligaris and associates first described a short isoform of GATA-1, termed GATA-1 short (GATA-1s). This isoform is 40 kDa in size, compared with the 47-kDa full-length protein, and is produced by the initiation of translation of the GATA-1 transcript at amino acid 84. The resultant protein lacks the N-terminal transactivation domain. Both full-length and short GATA-1 transcripts are normally found in murine fetal liver cells, in human MKs, and in K562 cells (a human erythroleukemia cell line with the ability to differentiate into erythroid and megakaryocytic cells). GATA-1s plays an important role in the pathogenesis of Down syndrome (trisomy 21)–associated transient myeloproliferative disorder (DS-TMD) and acute megakaryoblastic leukemia (DS-AMKL). DS-TMD is a disorder characterized by increased proliferation and maturational arrest of erythromegakaryocytic cells, which resolves spontaneously within the first few months of life, thus the name transient myeloproliferative disorder. The abnormality in megakaryopoiesis is thought to originate in the fetal liver, as suggested by its spontaneous resolution within months of birth (as hematopoiesis in the fetal liver ceases) and by the progressive megakaryoblast infiltration of hepatocytes with relative sparing of the BM. Two cytogenetic changes, or “hits,” occur prenatally that are thought to give rise to this condition. The first is the presence of trisomy 21, which by itself is associated with increased frequency and clonogenicity of MEPs in the fetal liver, and the second is a mutation in GATA-1 (within exon 2 of the GATA-1 transcript) that results in the exclusive production of GATA-1s, which lacks the N-terminal transactivation domain. Consistent with the transient nature of DS-TMD, a knock-in mouse engineered to exclusively express GATA-1s showed striking developmental stage-specific effects on megakaryopoiesis. Specifically, murine MK progenitors from yolk sac and fetal liver were markedly hyperproliferative compared to wild-type progenitors, but this phenotype disappeared at later fetal stages and postnatally, even though the mice still exclusively produced GATA-1s. This observation indicated that the effects of GATA1s on MK hyperproliferation were sensitive to the molecular developmental differences between fetal and adult MK progenitors, potentially only affecting megakaryopoiesis from the fetal liver.
Two studies have shed light on the developmental stage-specific factors that mediate the unique sensitivity of fetal MK progenitors to GATA-1s. First, fetal (but not adult) MK progenitors are highly sensitive to insulin-like growth factor (IGF) signaling, which activates the E2F transcriptional network through mTOR. In normal fetal MK progenitors, full-length GATA-1 restricts the IGF-mediated activation of the E2F transcription network to coordinate proliferation and differentiation. In the absence of full-length GATA-1 (i.e., in mutated GATA1s ) the overactive IGF signaling is “unchecked” and leads to unrestricted proliferation of fetal progenitors ( Fig. 110.5 ) . In a separate study, type 1 interferon (IFN)-responsive genes were found to be upregulated in murine BM versus fetal liver–derived MK progenitors, as well as in human BM versus fetal liver MKs (thought to be secondary to production of IFN by osteoblasts and osteoclasts in the BM). Importantly, exogenous IFN-α markedly reduced the hyperproliferation of fetal liver MKs obtained from GATA-1s mice. Conversely, genetic or pharmacologic neutralization of IFN signaling increased the proliferation of MK progenitors in the BM of adult GATA-1s mice. Taken together, these observations suggest that the insensitivity of adult MK progenitors to IGF signaling and the increased type 1 IFN signaling in the adult BM contributes to the spontaneous resolution of DS-TMD.
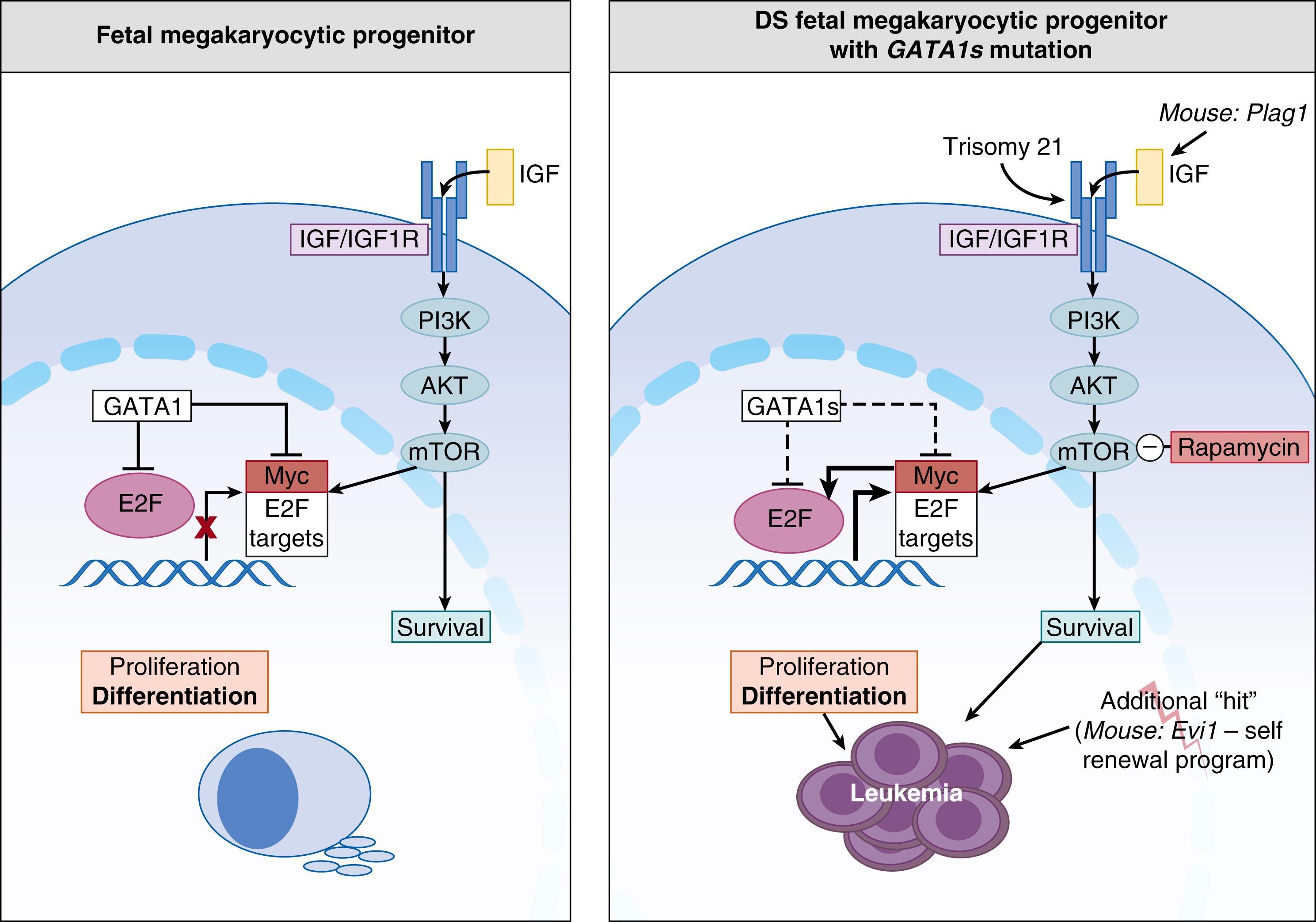
Despite 85% to 90% of newborns with DS-TMD demonstrating resolution of disease, approximately 20% to 30% subsequently develop acute megakaryoblastic leukemia (DS-AMKL), typically within 1 to 2 years after resolution of the TMD. Analysis of paired samples from patients with both TMD and DS-AMKL have found identical GATA-1 gene mutations in both the TMD and DS-AMKL, suggesting that additional genetic and/or epigenetic transforming events or hits are involved in leukemic progression. In one study, 44% of DS-AMKL samples showed additional genetic mutations aside from trisomy 21 and GATA-1, with major mutational targets involving multiple cohesin components and common signaling pathways, such as JAK family kinases, MPL, SH2B3 (LNK), and multiple RAS pathway genes.
FOG-1 is an essential cofactor for proper megakaryopoiesis. Single-point mutations (V205M, D218G D218Y, G208S) have been described, which result in reduced GATA-1 and FOG-1 interaction and a phenotype of macrothrombocytopenia with anemia. Mice engineered with complete absence of FOG-1 have a complete absence of MK progenitors. PF4 and GPIIb (early MK transcripts) are detectable in these models, implying an impaired differentiation and replication of early MK lineage cells. These data taken together suggest that FOG-1 plays multiple roles in megakaryopoiesis at both early and later stages. ,
The core binding factor (CBF) is a group of heterodimeric transcription factors composed of a non-DNA-binding CBFβ chain and one of three DNA-binding CBFα chains: RUNX1, RUNX2, or RUNX3. RUNX1 is the predominant CBF expressed in hematopoietic cells. Knockout of RUNX1 in mice leads to a complete absence of all definitive hematopoiesis due to a failure of definitive HSCs to emerge from specialized hemogenic endothelium during mid-embryonic development. Hematopoietic selective deletion of RUNX1 in adult mice using a MX1-Cre model results in moderate thrombocytopenia, T cell defects, and a mild myeloproliferative disorder. RUNX1–deficient MKs are small, contain scant cytoplasm, and have hypo-lobulated nuclei with low DNA ploidy compared to wild-type MKs. CBPβ deficiency likewise impairs MK development. In humans, germline haploinsufficiency of RUNX1 causes familial platelet disorder with propensity to develop AML (FPD/AML). This is a rare autosomal dominant disorder characterized by moderate thrombocytopenia, platelet dysfunction, and a high incidence of developing leukemia (approximately 35% lifetime risk). A number of RUNX1 MK direct target genes have been identified, including GPIIb, c-mpl, myosin light chain regulatory polypeptide (MYL9), PKC-theta, and platelet 12-lipoxygenase gene (ALOX12).
NF-E2 is a transcription factor found in erythroid, megakaryocytic, and mast cells. In MKs, NF-E2 regulates β-tubulin, a major component of microtubulin. Microtubulin formation is required for proplatelet formation and subsequent release of platelets from the MK. Loss of NF-E2 results in increased numbers of MKs with a disorganized internal membrane and impaired release of proplatelets, leading to thrombocytopenia and a lethal coagulopathy in the neonatal period, with only a mild decrease in erythropoiesis. ,
The Ets family of transcription factors is characterized by the presence of a highly conserved winged helix-turn-helix DNA binding domain (Ets domain) that allows recognition of purine-rich DNA sequences with a core GGA (A/T) consensus, designated EBS (Ets binding sequence). Ets-1 is up-regulated in megakaryopoiesis, and Ets binding sites have been identified in many MK-specific gene promoters. Overexpression of Ets-1 in cultured MK cells results in a state of increased proliferation with larger MKs that have an increased number of nuclear lobes. Gene expression studies in these same cells reveals increased GATA-2, GPIIb, and PF4, indicating that Ets-1 promotes MK differentiation.
Friend leukemia integration-1 (Fli-1) is a member of the Ets family of transcription factors. It is expressed in hematopoietic cell lines as well as vascular endothelial cell lines and regulates expression of multiple MK proteins, including GATA-1, GPIb, IIb, VI, and XI, and c-mpl. In K-562 cells, expression of Fli-1 results in increased CD41 and CD61 expression. In murine knockout models, loss of Fli-1 results in embryonic death due to hemorrhage (related to a state of defective vasculature) as well as dysmegakaryopoiesis with immature-appearing MKs that express early genes (αIIb and c-mpl) but diminished expression of late genes (GPIX). Disruption of Fli-1 expression caused by a terminal deletion in chromosome 11 underlies Paris-Trousseau syndrome, which is characterized by thrombocytopenia with platelets that display large α-granules. This clinical phenotype can occur independently or as part of Jacobsen syndrome (platelet defects with cardiac anomalies, facial anomalies, and intellectual disability). In both instances, MKs are increased in number but have a diminished number of α-granules as well as a disorganized internal membrane. , In a recent study, induced pluripotent stem cell–derived MKs (iMKs) with a targeted heterozygous Fli-1 knockout (FLI1 +/− ) released fewer platelets per MK, and platelets released in vivo following infusion of these iMKs had shorter half-lives and poor functionality. Interestingly, Ets-1 was overexpressed in these Fli-1-deficient iMKs, suggesting that Fli-1 negatively regulates Ets-1 in megakaryopoiesis. Furthermore, a study of 13 unrelated index cases of thrombocytopenia with dense granule secretion disorder revealed that six had novel alterations in Fli-1 or RUNX1, further strengthening the role of Fli-1 in thrombopoiesis.
A recent study identified a potential ontogenic master-regulator responsible for the phenotypic and molecular differences between neonatal and adult MKs. It is known that adult MK development is dependent on sustained, high-level activation of P-TEFb, which, along with CDK9 and cyclin T, form the complete kinase complex. This complex releases RNA polymerase II (RNAPII) from proximal stalling, thus accelerating transcriptional elongation to meet the high transcriptional demands of adult MKs. , In most non-MK cells, a feedback loop maintains P-TEFb sequestered in an inactive state within the 7SK snRNP complex. Adult MKs behave differently by employing a specialized activation pathway through down-regulation of 7SK stabilization factors (MePCE and LARP7), which allows the irreversible release and activation of P-TEFb.
Interestingly, neonatal MKs exhibit persistent inactivation of P-TEFb despite lineage-appropriate down-regulation of the 7SK stabilizing factors. In a recent study, Elagib and colleagues identified a fetal-specific 7SK stabilizing protein, IGF2BP3, responsible for the persistent inhibition of P-TEFb by 7SK despite down-regulation of MePCE and LARP7. Genetic or pharmacologic knockdown of IGF2BP3 in neonatal MKs caused down-regulation of 7SK, which led to enhanced P-TEFb activation and subsequent development of adult MK features, such as cellular enlargement, proliferation arrest, polyploidization, and erythroid suppression. The mechanisms by which activated P-TEFb exerts these effects remain unknown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here