Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
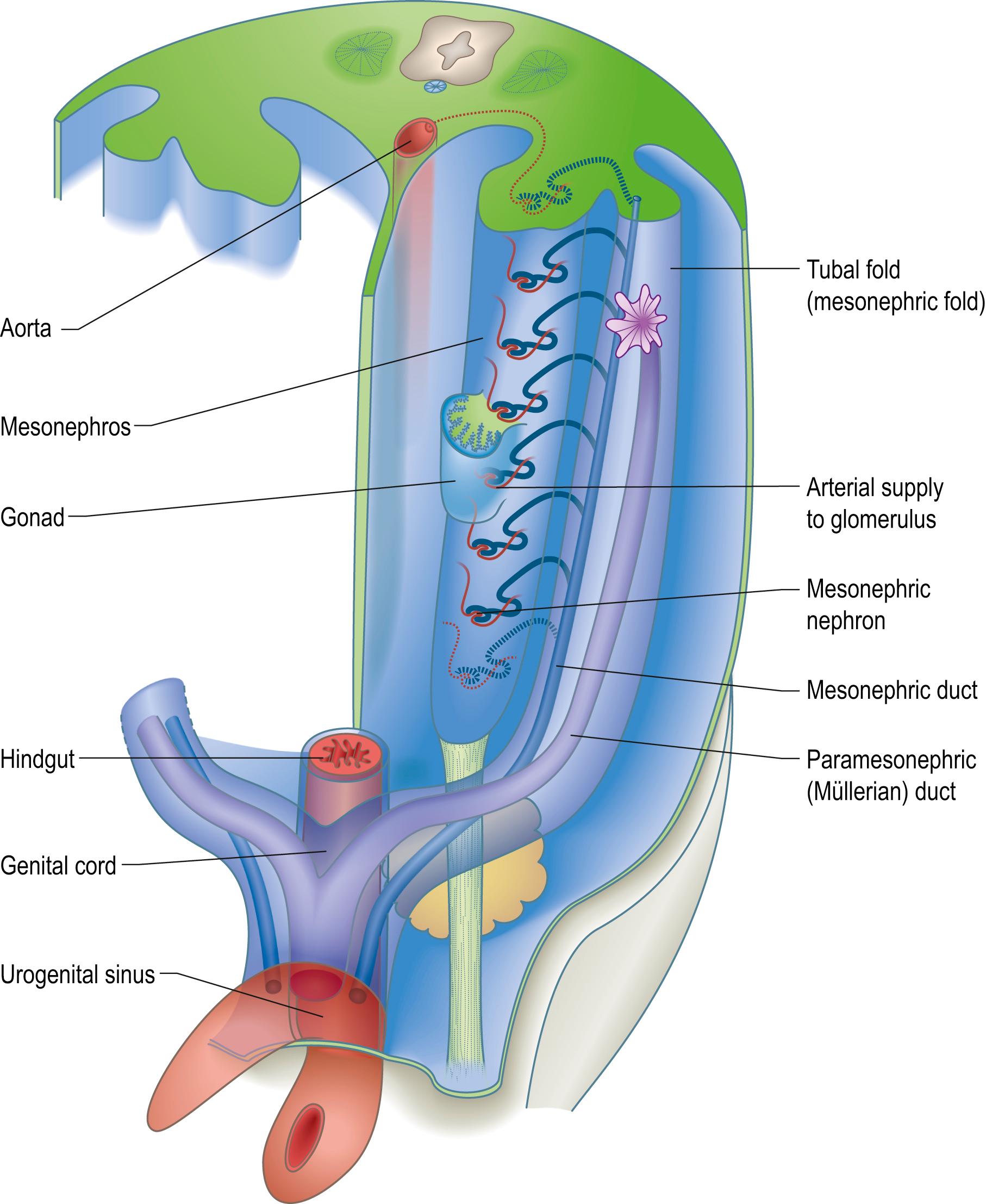
From stage 11 (29–30 days postfertilization; see Fig. 23.3 for comparison of postfertilzation days and the clinical scale of postmenstrual weeks) the dorsal region of the wall of the intraembryonic coelom is composed of a mesenchymal population termed intermediate mesenchyme. Predictive fates of epiblast cells that transform to mesenchyme on ingression through the primitive streak show temporally specified induction of cells that ingress later than those that form the lateral halves of the somites and earlier than mesoblast forming the lateral plate, and become epithelial coelomic wall at the end of their migration (see Fig. 10.3 ). The rostral limit of the intermediate mesenchymal population, in stage 10 embryos, is lateral to somite 6. It lengthens caudally during development, and is sited (in the folded embryo) at the junction between the splanchnopleuric mesenchyme (surrounding the gut medially) and the somatopleuric mesenchyme (subjacent to the ectoderm laterally). Ultimately it blends with splanchnopleuric mesenchyme around the cloaca ( Fig. 22.1 ). After the early migration of mesoblast, further intermediate mesenchyme is formed by proliferation of the overlying coelomic epithelial wall to give a ventrally projecting urogenital ridge (previously termed the nephrogenic cord). Interaction between the coelomic epithelium and its mesenchymal progeny give rise, in a rostro-caudal progression, to the kidneys, suprarenal glands, gonads and reproductive ducts.

In lower vertebrates, intermediate mesenchyme typically develops serial, segmental, epithelial diverticula termed nephrotomes. Each nephrotome encloses a cavity, the nephrocele, which communicates with the coelom through a peritoneal funnel, the nephrostome ( Fig. 22.2 ). The dorsal wall of a nephrotome evaginates as a nephric tubule. The dorsal tips of the cranial nephric tubules bend caudally and fuse to form a longitudinal primary excretory duct, which grows caudally and curves ventrally to open into the cloaca. The more caudally placed, and therefore chronologically later, tubules open secondarily into this duct or into tubular outgrowths from it. Glomeruli, specific arrangements of capillaries and overlying coelomic epithelium, arise from the ventral wall of the nephrocele (internal glomeruli) or the coelomic wall adjacent to the peritoneal funnels (coelomic or external glomeruli), or in both situations (see Fig. 22.2 ). External glomeruli, composed of recognizable podocytes, facing the intraembryonic coelom, and the lateral openings of ciliated peritoneal funnels, have been described in turtle embryos ( ): it is doubtful whether external glomeruli develop in human embryos.

The renal excretory system is described as forming from three organs, the pronephros, mesonephros and metanephros, which succeed each other in time and space, such that the last to develop is retained as the permanent kidney (see Figs 22.1 – 22.2 ). It is difficult to provide reliable criteria by which to distinguish these stages or to define their precise limits in human embryos. Much of the knowledge on development of the meso- and meta-nephros comes from experimental studies of mouse embryos and more recently from in vitro study of human stem cell-derived kidney organoids. Although kidney development is evolutionarily conserved in mammals, there are significant heterochronic differences between murine and human development that make direct extrapolation between the species unwise; similarly, extrapolation of in vitro conditions to in vivo development should be treated with caution.
A pronephros is present in human embryos only as clusters of cells in the most cranial portions of the urogenital ridge (see Figs 22.1 – 22.2 ). More caudally, similar groups of cells appear and become vesicular. The dorsal ends of the most caudal of the vesicles join a primary excretory duct. Their central ends are connected with the coelomic epithelium by cellular strands that probably represent rudimentary peritoneal funnels. Glomeruli do not develop in association with these cranially situated nephric tubules, which ultimately disappear.
In stage 11 embryos of approximately 14 somites, a primary excretory duct can be seen as a solid rod of cells in the dorsal part of the urogenital ridge. Its cranial end is about the level of somite 9 and its caudal tip merges with the undifferentiated mesenchyme of the ridge. It differentiates before any nephric tubules and, when the latter appear, it is at first unconnected with them. In older embryos, the duct has lengthened and its caudal end becomes detached from the urogenital ridge to lie beneath the ectoderm. From this level, it grows caudally, independent of the nephrogenic mesenchyme, and then curves ventrally to reach the wall of the cloaca. The lumen develops progressively from its caudal end to form a true duct, which opens into the cloaca in stage 12 embryos.
From stage 12 mesonephric tubules, which develop from the intermediate (now nephrogenic) mesenchyme between somite levels 8 and 20, begin to connect to the primary excretory duct, which is now renamed the mesonephric duct. More caudally, a continuous ridge of nephrogenic mesenchyme extends to the level of somite 24. The mesonephric tubules (nephrons) are not metameric and there may be two or more mesonephric tubules opposite each somite.
Within the mesonephros, each tubule first appears as a condensation of mesenchyme cells that epithelialize and form a vesicle. One end of the vesicle grows towards and opens into the mesonephric duct, while the other dilates and invaginates. The outer stratum forms the glomerular capsule, while the inner cells differentiate into mesonephric podocytes that clothe the invaginating capillaries to form a glomerulus. The capillaries are supplied with blood through lateral branches of the aorta. It has been estimated that 70–80 mesonephric tubules and a corresponding number of glomeruli develop. However, these tubules are not all present at the same time: it is rare to find more than 30–40 in an individual embryo because the cranial tubules and glomeruli develop and atrophy before those situated more caudally have developed.
By stage 17 each mesonephros is an elongated, spindle-shaped organ that projects into the coelomic cavity, one on each side of the dorsal mesentery, from the level of the septum transversum to the third lumbar segment. This whole projection is called the mesonephric ridge, mesonephros or Wolffian body (see Fig. 22.1B–C ; Fig. 22.3 ). It develops subregions, and the suprarenal gland and gonad develop on its medial surface. There are striking similarities in structure between the mesonephros and the permanent kidney or metanephros, but the mesonephric nephrons lack a segment that corresponds to the descending limb of the loop of Henle. The mesonephros is believed to produce urine by stage 17. A detailed comparison of the development and function of the mesonephros and metanephros in staged human embryos is not available.
In stage 18 embryos the mesonephric ridge extends cranially to about the level of rib 9. In both sexes, the cranial end of the mesonephros atrophies. In stage 19 embryos a mesonephros is found only in the first three lumbar segments, although it may still possess as many as 26 tubules. The most cranial one or two tubules persist as rostral aberrant ductules (see Fig. 22.13 ); the succeeding five or six tubules develop into either the efferent ductules of the testis and lobules of the head of the epididymis (male), or the tubules of the epoophoron (female); the caudal tubules form the caudal aberrant ductules and the paradidymis (male), or the paroophoron (female).
Once mesonephric nephrons connect to the primary excretory duct, it is renamed the mesonephric duct. This runs caudally in the lateral part of the urogenital ridge, and projects into the cavity of the coelom in the substance of a mesonephric fold at the caudal end of the ridge (see Fig. 22.3 ). As the mesonephric ducts from each side approach the urogenital sinus (see below) the two mesonephric folds fuse, forming a transverse partition across the cavity of the pelvis, somewhat inappropriately called the genital cord (see Fig. 22.3 ). The insertion of the mesonephric duct into the urogenital sinus occurs at stage 13 and remains associated with segmental levels 31–32 (S2–S3) as the embryo lengthens ( ). In the male, the mesonephric duct itself becomes the canal of the epididymis, ductus deferens and ejaculatory duct, whereas it involutes in the female.
The early cloaca consists of a dorsal, tubular, enteric hindgut region extending from the caudal intestinal portal to the cloacal membrane, and a ventral, blind-ending allantois extending from the connecting stalk. It is a slightly dilated cavity, lined by endoderm ( Fig. 22.1 ). The allantois and enteric hindgut portions of the cloaca are separated, from stage 15, by the proliferation of the urorectal septum, a partition of mesenchyme and endodermal cloacal wall in the angle of the junction of enteric hindgut and allantois ( Fig. 22.4 ; see Fig. 22.7 ). Molecular markers of the urorectal septum mesenchyme, also termed the intercloacal mesenchyme, include Six1 and Six2 ( , ). The endodermal epithelium beneath the mesenchyme of the urorectal septum moves towards the cloacal membrane and fuses centrally, separating the intraembryonic urogenital sinus and extraembryonic allantois (ventrally), bounded now by a urogenital membrane, from the presumptive rectum and upper anal canal (dorsally), bounded by the anal membrane ( ). Both membranes break down during stages 18–20. The urogenital sinus is continuous cranially with the allantoic duct within the connecting stalk. During development it forms the vesico-urethral canal with a middle, narrow channel, the pelvic portion, and a caudal, deep, phallic section that is open to the amniotic cavity. It receives the mesonephric and paramesoneprhic ducts and will become the urinary bladder and urethra ( Fig. 22.4A ).

The pronephros and mesonephros are linear structures. They both contain stacks of tubules distributed along the craniocaudal axis of the embryo, an arrangement that results in the production of hypotonic urine in the embryo and fetus. In the mesonephric kidney, development proceeds in a craniocaudal progression, and cranial nephrons degenerate before caudal ones are produced. In the metanephric kidney, development is patterned radially such that the outer cortex is the last part to be formed, and nephrons are arranged concentrically with the loops of Henle directed towards the renal pelvis. This arrangement allows different concentration gradients to develop within the metanephric kidney and results in the production of hypertonic urine.
The metanephric kidney develops from three sources from stage 13. An evagination of the mesonephric duct, the ureteric bud, and a local condensation of mesenchyme, the metanephric blastema, form the nephric structures ( Fig. 22.5 ). Angiogenic mesenchyme migrates into the metanephric blastema slightly later to produce the glomeruli and vasa recta. It is possible that an intact nerve supply is also required for metanephric kidney induction. A range of intra- and extracellular factors potentially involved in metanephric development have been discussed ( ).

The following interactions occur sequentially in the development of the metanephric kidney (see Fig. 22.5 ). Although the timing of human development differs from that in other mammalian species that have been described, similar growth and transcription factors are thought to underpin kidney development ( , , , ). The ureteric bud bifurcates when it comes into contact with the metanephric blastema in response to extracellular matrix molecules synthesized by the mesenchyme. Both chondroitin sulphate proteoglycan synthesis and chondroitin sulphate glycosaminoglycan processing are necessary for branching of the ureteric bud. The proximal part of the ureteric bud elongates to form the developing ureter and the first generation of branches form the major and minor calyces of the renal pelvis (see Fig. 22.6 ). Further ureteric branches form the collecting ducts and as they elongate the metanephric mesenchyme condenses adjacent to each of them. An adhesion molecule, syndecan, can be detected between the mesenchymal cells in the condensate. The cells switch off expression of neural cell adhesion molecule (N-CAM), fibronectin and collagen I, and start to synthesize liver cell adhesion molecule (L-CAM; also called E cadherin) and the basal lamina constituents, laminin and collagen IV. The mesenchymal clusters are thus converted to small groups of epithelial cells that undergo complex morphogenetic changes. Each epithelial group elongates, initially forming a comma-shaped, and then an S-shaped, body, with a dilated sac at its proximal end and an elongating distal end that subsequently fuses with a collecting duct (see Fig. 22.5 ). Each dilated sac invaginates to form a cup: the outer cells become parietal, glomerular capsule cells, and the inner ones become visceral, epithelial podocytes. The podocytes develop in close proximity to the angiogenic mesenchyme of invading capillaries which give rise to the endothelial and mesangial cells of the glomerulus. Both cell lines contribute to components of the glomerular basement membrane. The direct mutual support of two epithelial layers, rather than support of an epithelium by an underlying lamina propria, is unusual and seen only in the lung and brain (see Fig. 11.2 . Podocytes and endothelial cells contribute to the generation of mutual basal laminal proteins and the formation of the glomerular basement membrane. The isoforms of type IV collagen within this layer follow a specific programme of maturation as the filtration of macromolecules from the plasma becomes restricted. Platelet-derived growth factor (PDGF) β-chain and the PDGF receptor β-subunit (PDGFR β) have been detected between stage 23 and the end of the first trimester; PDGF β-chain has been localized in the dilated epithelial sac during its comma- and S-shaped stages and PDGFR β is expressed in the undifferentiated metanephric blastema, vascular structures and interstitial cells. Both PDGF β-chain and PDGFR β are expressed by mesangial cells, which may promote further mesangial cell proliferation. Innervation is also necessary for metanephric development: nephrogenesis is completely blocked if developing kidney rudiments are incubated with antisense oligonucleotides that neutralize nerve growth factor receptor (NGF-R) mRNA. With continuing elongation of the collecting ducts and adjacent coalescence of mesenchymal cells, all stages of nephron differentiation can be seen concurrently (see Fig. 22.5C ). A number of genes involved in the development of the renal medulla and vasa recta in the mouse have been identified ( ).

Metanephric mesenchyme will develop successfully in vitro , which makes experimental perturbation of non-human kidney development comparatively easy to evaluate. Early experimental studies demonstrated that other mesenchymal populations, and spinal cord, were able to induce ureteric bud division and metanephric development. Culture of hiPSC along a kidney lineage has resulted in the generation of nephron progenitor cells and kidney organoids which are being used to study aspects of podocyte development ( , ). Time-lapse study of cultured mouse embryonic kidney has shown that the original branching morphogenesis of the collecting ducts (from the ureteric bud) changes during maturation such that the branching point moved centripetally. This may promote elongation of outer cortical collecting ducts, resulting in a renal pattern rather than one that would result from repeated dichotomous branching morphogenesis. Biomechanical factors generated by the outer renal capsule may contribute to these temporal specifications of epithelial mesenchymal interactions ( ).
Nephrons are added to the metanephric kidney throughout the fetal period with different temporal patterning. The earliest ureteric bud branches are absorbed into the renal pelvis so that an average of 44 collecting ducts open into 8 papillae. After the 13th to 15th branching, several metanephric mesenchymal to epithelial transformations can occur along adjacent collecting ducts, forming arcades of juxtaglomerular nephrons in the inner renal cortex. The mid and outer renal cortices are induced by collecting tubules that elongate towards the renal capsule. Addition of new nephrons in the outermost cortex occurs by metanephric mesenchymal induction at collecting duct tips, at a constant average of 83 μm from the inner side of the renal capsule, with only two layers of mesenchyme intervening ( ). This subcapsular region of the developing kidney is called the nephrogenic zone. It contains angiogenic mesenchymal populations forming embryonic blood vessels, which means that the tissues are at lower oxygen tension than more central regions where endothelial glomeruli are established. It has been suggested, on the basis of animal experimentation, that increased oxygen tension promotes glomerular differentiation, whereas low oxygen tension maintains stem cell populations and continued developmental interactions ( ). The addition of new nephrons normally ceases after postmenstrual week 38, although the mechanism is not known ( ). Nephrogenesis has been reported as complete by postmenstrual week 34, presumably in fetuses with appropriate weight ( ). Mature renal capsules contain a constant number and proportion of podocytes relative to other glomerular cell types, whether formed early or later between postmenstrual weeks 20 and 40 ( ).
The metanephric kidney is lobulated throughout fetal life, but this condition usually disappears during the first year after birth (see Fig. 22.8 ). Varying degrees of lobulation occasionally persist throughout life.
The growth of left and right kidneys is well matched during development: fetal kidney volume increases most during the second trimester in both sexes. Male fetuses show greater values for renal volume than female fetuses from the third trimester onwards ( ). In neonates and infants, total renal blood flow is determined by clearance of p -aminohippurate (PAH), which measures effective renal plasma flow (ERPF); flow correlates with postmenstrual age. ERPF increases in infants born preterm from 20 ml/min/1.73m 2 (the latter unit is a standard measure of body surface area) at postmenstrual week 30, to 45 ml (same units) at postmenstrual week 35 and 83 ml at postmenstrual week 40. In full-term infants, during postnatal months 1–3, ERPF increases to 300 ml/min/1.73 m 2 and thereafter reaches values of 650 ml/min/1.73 m 2 by postnatal months 12–24. The full-term neonatal kidney has a greater percentage of blood flow to inner cortical and medullary areas than the adult kidney ( ).
At delivery of preterm and low birth weight babies nephrogenesis is still active. It has been shown to continue for up to 3 postnatal weeks in experimental non-hominids, however, there is evidence of nephrogenic perturbation resulting in oligonephropathy in 8–24% of preterm deliveries ( ). Dilated Bowman’s space and shrunken glomerular tufts have been identified in the outer renal cortex. It may be that the general increase in oxygen tension after birth causes rapid differentiation of all formed nephrons but inhibition of further development of immature outer cortical mesenchymal populations. Kidney weight is lower in infants with low (< −2 SDs) birth weight, reflecting a decreased number of nephrons. This relative smaller kidney size continues into early childhood and may be a factor in adult kidney pathology ( ). Preterm neonates achieve a similar estimated glomerular filtration rate as term neonates soon after birth, possibly by glomerular hyperfiltration in the nephrons present ( ).
The kidney functions not only as an excretory organ, but also as an endocrine organ, secreting hormones that are concerned with renal haemodynamics. Before birth, homeostasis is controlled by the placenta. The fetal kidney produces amniotic fluid. The kidneys of premature babies of less than 36 postmenstrual weeks are immature and contain incompletely differentiated cortical nephrons that compromise their ability to maintain homeostasis. Problems of immaturity are further compounded by the effects of hypoxia and asphyxia, which modify renal hormones.
Renal hormones include the renin–angiotensin system, renal prostaglandins, the kallikrein–kinin system and renal dopamine. Renin is found in the smooth muscle cells of arterioles, interlobular arteries and branches of the renal artery, and has also been described in the distal convoluted tubule cells. Renal dopamine is produced (mainly) by the enzymatic conversion of l -dopa to dopamine in the early segments of the proximal convoluted tubule, and is also sourced locally from dopaminergic nerves. Other renal hormones include an antihypertensive lipid produced in the interstitial cells of the renal medulla, and, possibly, histamine and serotonin. Growth factors produced by human embryonic kidney cells include erythropoietin and interleukin β (which stimulate megakaryocyte maturation) and transforming growth factor-β. For reviews of the various factors in this aspect of kidney development see and . Prostaglandins may be important in the fetus in the regulation of renal blood flow ( ).
The metanephric kidney is initially sacral, but as the ureteric outgrowth lengthens and the body curvature diminishes, it becomes positioned progressively more cranially. The metanephric pelvis lies on a level with the fourth lumbar vertebra at about stage 18 ( ). During this period the ascending kidney receives its blood supply sequentially from arteries in its immediate neighbourhood, i.e. the middle sacral and common iliac arteries. The definitive renal artery is not recognizable until the beginning of the third month. It arises from the most caudal of the three suprarenal arteries, all of which represent persistent mesonephric or lateral splanchnic arteries. Additional renal arteries are relatively common, and may enter at the hilum or at the upper or lower pole; they also represent persistent mesonephric arteries.
At first, the distal end of the ureter is connected to the dorsomedial aspect of the mesonephric duct but, as a result of differential growth, this connection comes to lie lateral to the duct. The wall of the early ureter is initially highly permeable. Its lumen later becomes obliterated and is subsequently recanalized. Both of these processes begin in intermediate portions of the ureter and proceed cranially and caudally. Recanalization is not associated with metanephric function, but perhaps reflects the rapid elongation of the ureter as the embryo grows. Two fusiform enlargements appear at the lumbar and pelvic levels of the ureter at 5 and 9 months, respectively (the pelvic enlargement is inconstant). As a result, the ureter shows a constriction at its proximal end (pelviureteric region) and another as it crosses the pelvic brim. A third narrowing is always present at its distal end and is related to the growth of the bladder wall. Ureteric smooth muscle differentiation occurs from the ureterovesical junction to the ureteropelvic junction. The processes that occur during the development of typical and atypical smooth muscle cells that generate ureteric peristaltic waves are unknown. Interstitial cells of Cajal-like cells have been identified in mouse embryos before ureteric pacemaker activity first occurs ( ).
The urinary bladder develops from the cranial vesico-urethral canal, which is continuous above with the allantoic duct (see Fig. 22.4 ; Figs 22.6 – 22.7 ). The mesonephric ducts open into the urogenital sinus during stage 13. The ureters develop as branches of the mesonephric ducts, which gain their own access to the developing bladder, and their orifices open separately into the bladder on the lateral side of the opening of the mesonephric ducts during stage 17. Later, the two orifices become separated still further and, although the ureter retains its point of entry into the bladder, the mesonephric duct opens into that part of the urogenital sinus that subsequently becomes the prostatic urethra ( ) (see Fig. 22.6E ). The remainder of the vesico-urethral canal forms the body of the bladder and urethra, and its apex is prolonged to the umbilicus as a narrow canal, the urachus.

For many years, it was believed that the absorption of the mesonephric ducts into the urogenital sinus contributed a mesodermal epithelium into the endodermal bladder, limited to the trigone and the dorsal wall of the proximal half of the prostatic urethra extending to the opening of the prostatic utricle and ejaculatory ducts, or its female homologue, the whole female urethral dorsal wall. Studies on transgenic mice have demonstrated that the region of the combined ducts close to the urogenital sinus, referred to as the common nephric duct, undergoes local apoptosis as a part of the normal development of the trigone region of the bladder and the establishment of separate entry points for the ureters and mesonephric ducts ( ). Retinoic acid is required for ureteric insertion into the urogenital sinus ( ). There is no mesodermal epithelial contribution to the bladder at the trigone. This is supported by the observation that in vitro , combination of fetal urogenital sinus mesenchyme with epithelial mesoderm or endoderm produces different outcomes. Tissue recombined with endoderm gives rise to prostatic epithelium, whereas the same mesenchyme combined with epithelial mesoderm forms seminal gland epithelium. Trigone epithelium differentiates into prostatic epithelium, confirming an endoderm lineage ( ).
The previous mechanism for trigone development also suggested that the muscle in that region was derived from the ureter and contributed to the valve-like entry point that prevents urinary reflux. Studies have now shown that the ureter passes through a tunnel in the bladder wall in parallel with blood vessels and that a tunnel forms even in the absence of ureters ( ). Study of smooth muscle progenitors shows that the bulk of the trigone is derived from bladder muscle (detrusor); there is a limited contribution from ureteric longitudinal smooth muscle fibres at the lateral edges. Eponymous muscular tissues such as Mercier’s bar (between the ureteric orifices) and Bell’s muscle (extending caudally from the ureteric orifices to the apex of the trigone) that were previously thought to arise from the ureter, have been shown to be derived from urinary bladder muscle ( ).
Differentiation of the bladder mucosal epithelium to transitional epithelium can be seen from about postmenstrual week 10 ( ). Urothelium develops from both the original mesonephric duct epithelium of the ureteric bud and the endodermal epithelium of the urogenital sinus which becomes the bladder, and in places it is responsive to different local osmolarity. The cells express uroplakin, which is thought to be involved in signalling during the epithelial/mesenchyme interactions that are involved in urinary tract development. The differentiation of the urothelium is a significant element in the development of the blood–urine barrier; genetic modification of uroplakin genes suggests their perturbation is associated with the development of congenital anomalies of the urinary tract ( ).
Bladder filling and emptying cycles are required for normal bladder remodelling during fetal development; in this process, detrusor smooth muscle cells undergo cyclical apoptosis and proliferation. Mechanical stretching promotes proliferation. Bladders in which the fluid is diverted do not show this cycle, and do not enlarge or undergo normal remodelling. The detrusor stops growing, producing a low-volume, low-compliance bladder, although the serosal connective tissues continue to expand ( ). As yet, there is little information on the development of bladder interstitial cells of Cajal, which are present within detrusor, bladder microvessels and the mucosal lamina propria ( ).
The fetal bladder may be identified by ultrasound examination at postmenstrual weeks 9–11 in transverse and sagittal section. Filling and emptying over a 30–45 minute cycle can be demonstrated. The absence of a bladder image at postmenstrual week 13 or later is considered abnormal. At postmenstrual week 20, the kidneys are best visualized in a section of the abdomen caudal to that used for abdominal circumference estimation. The diameter of the renal pelvis is reported in the anterior posterior view; 7 mm or less is considered normal around postmenstrual week 20 ( ).
The routine use of ultrasound as an aid to in utero diagnosis of anomalies has revealed a prevalence of 1–2 abnormal fetuses per 1000 ultrasound procedures, of which 20–30% are anomalies of the genitourinary tract, detectable as early as postmenstrual weeks 12–15. The decision to be made after such a finding is by no means clear. Urinary obstruction is considered an abnormality, yet transient modest obstruction is considered normal during canalization of the urinary tract, and has been reported in 10–20% of fetuses in the third trimester. A delay in canalization, or in the rupture of the cloacal membrane, can produce a dilation. Similarly, the closure of the urachus at postmenstrual week 32 may be associated with high-resistance outflow for the system, which again produces transient obstruction. Distension of the fetal bladder, which may indicate lower urinary tract obstruction, affects 2.2/10,000 births, more commonly in males ( , ). Over half of such cases are caused by the development of posterior urethral valves (a congenitally obstructing posterior urethral membrane); there may also be a dilated posterior urethra and thickened bladder wall
The kidneys function early in development and produce the amniotic fluid that surrounds the fetus. At full-term (postmenstrual week 40), the two kidneys weigh approximately 23 g and the lobulated appearance of fetal kidneys is still present ( Fig. 22.8 ; see Fig. 23.14C ). Addition of new cortical nephrons continues in the first few months of postnatal life, and after this time, general growth of the glomeruli and tubules results in the disappearance of lobulation. The renal blood flow is lower in the neonate; adult values are attained by the end of the first year. The glomerular filtration rate at birth is approximately 30% of the adult value, which is attained by 3–5 months of age.

The neonatal urinary bladder is egg-shaped and the larger end is directed downwards and backwards ( Figs 22.9 – 22.10 ; see Figs 23.14A, F–I ). Although described as an abdominal organ, nearly one-half of the neonatal bladder lies below a line drawn from the promontory of the sacrum to the upper edge of the pubic symphysis, i.e. within the cavity of the true pelvis. From the bladder neck, the bladder extends anteriorly and slightly upwards in close contact with the pubis, until it reaches the ventral abdominal wall. The apex of the contracted bladder, which has a distinct interureteric fold, lies at a point midway between the pubis and the umbilicus. When the bladder is filled with urine, the apex may extend up to the level of the umbilicus: pressure on the lower abdominal wall will express urine from an infant bladder. The bladder remains connected to the umbilicus by the obliterated remains of the urachus (see Figs 22.19 and 23.15A ), and so stimulation of the umbilicus can initiate micturition in babies. The elongated shape of the bladder in neonates means that the ureters are correspondingly reduced in length and they lack a pelvic portion. The bladder does not gain its adult, pelvic, position until about the sixth year.


Urine can be aspirated from the neonatal bladder into a sterile syringe, via a needle inserted through the abdominal wall about 2 cm above the pubic symphysis. The success rate of this procedure is improved by the use of ultrasound scanning to visualize urine in the bladder prior to insertion of the needle ( ).
Congenital anomalies of the kidney and urinary tract are relatively common (3–6% of live births). For further reading about the genetic, epigenetic and in utero environmental factors in the pathogenesis of non-syndromic forms of human congenital anomalies of the kidney and urinary tract (CAKUT), see and .
Renal agenesis is the absence of one or both kidneys. In unilateral renal agenesis, the remaining kidney exhibits compensatory hypertrophy and produces a nearly normal functional mass of renal tissue. Atresia of the ureter during development causes a non-functional multicystic dysplastic kidney, thought to be secondary to urinary obstruction while the tubules are still forming. Problems with kidney ascent can result in a pelvic kidney. Alternatively, the kidneys may fuse together at their caudal poles, producing a horseshoe kidney that cannot ascend out of the pelvic cavity because the inferior mesenteric artery prevents its further migration. A duplex kidney arises when two ureteric buds meet the metanephros; it is characterized by two pelvicalyceal systems and is associated with ureterocele, ectopic insertion of the ureter and vesico-ureteric reflux.
It was thought that renal cysts arose from clumps of vesicular cells that persisted when the tips of branches from the ureteric diverticulum failed to fuse with metanephrogenic cap tissue. It is now believed that they are wide dilations of a part of otherwise continuous nephrons. In most cases, autosomal dominant polycystic kidney disease results from mutations of PKD1 or PKD2 genes, which are expressed in human embryos from postfertilization weeks 5–6 within the mesonephros and later the metanephros ( ). In this condition, the cystic dilation may affect any part of the nephron, from Bowman’s capsule to collecting tubules. Less common is infantile cystic renal disease, inherited as a recessive trait, in which the proximal and distal tubules are dilated to some degree but the collecting ducts are grossly affected.
Anomalies of the ventral body wall caudal to the umbilicus, especially with inappropriate siting of the genital tubercle, can result in exstrophy of the bladder ( Fig. 22.11 ) ( , ). In this condition, the urorectal septum (internal) is associated with the genital tubercle (external), which means that the urogenital and anal membranes are widely separated. When the urogenital membrane involutes, the posterior surface of the bladder is exposed to the ventral abdominal wall. The lower part of the abdominal wall is therefore occupied by an irregularly oval area, covered with mucous membrane, onto which the two ureters open. The periphery of this extroverted area, which is covered by urothelium, becomes continuous with the skin. The innervation of the underlying detrusor muscle seems to be normal, but the muscle has a decreased number of smooth muscle cells and an increased amount of collagen, which means that the bladder may have reduced contractile performance after reconstruction ( ). Bladder exstrophy is a complex condition involving the development of the pelvis, cloaca, urorectal septum, urogenital sinus, urethral plate, external genital tissues and all of the associated autonomic and somatic nerves supplying the region. For further reading about exstrophy–epispadias complex, its genetics, presentation and surgical repair, see . Absence of the bladder, low insertion of the umbilical cord and bony pelvic anomalies on routine antenatal ultrasound examination would suggest the presence of bladder exstrophy in a fetus, however, it has been reported that only 25% of those neonates born with this condition were diagnosed antenatally. Its postnatal repair is exceedingly complex and has not been completely successful in establishing continence and a bladder capable of voluntary voiding. The accumulated experience of those surgical units undertaking these reconstructions provide hope for improved patient outcomes in the future ( ).

Amniotic fluid is a dynamic fluid medium that changes throughout development ( Ch. 9 ). The volume of amniotic fluid is used as an indicator of renal function but does not reflect fetal urinary output until the second trimester. Although variation in the amount of amniotic fluid may suggest anomalies of either the gut or the kidneys, it is not always possible to correlate even severe oligohydramnios (too little amniotic fluid) with renal dysfunction. There is an important relationship between the volume of amniotic fluid, lung development and maturity: oligohydramnios has been shown to be associated with pulmonary hypoplasia ( Ch. 20 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here