Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development and structure of the human haemochorial placenta
The development of the uteroplacental and fetoplacental circulations
This chapter highlights areas in which developmental placental biology directly impinges on clinical practice. Pregnancies complicated by the pathologies of stillbirth, severe preeclampsia and intrauterine growth restriction (IUGR), especially those that deliver before 34 weeks, are associated with significant gross and microscopic placental pathology (covered in Chapter 9 ). Prenatal diagnosis of pregnancies at risk for these severe complications is now possible by identifying markers of ‘placental insufficiency’ at the 20-week stage. Unfortunately, at the current time, new biomarkers derived from maternal blood samples do not show adequate power of prediction in the first or second trimester. According to the American College of Obstetricians and Gynecologists, ‘current predictive tests for preeclampsia may harm more women than they benefit because of their low positive predictive value (PPV)’ ( https://www.acog.org/Womens-Health/Preeclampsia-and-Hypertension-in-Pregnancy ). This situation underscores the need for a greater understanding of normal placental development and thus a better appreciation of the developmental origins of placental pathology. More detailed information can be found in a review by Dunk and colleagues.
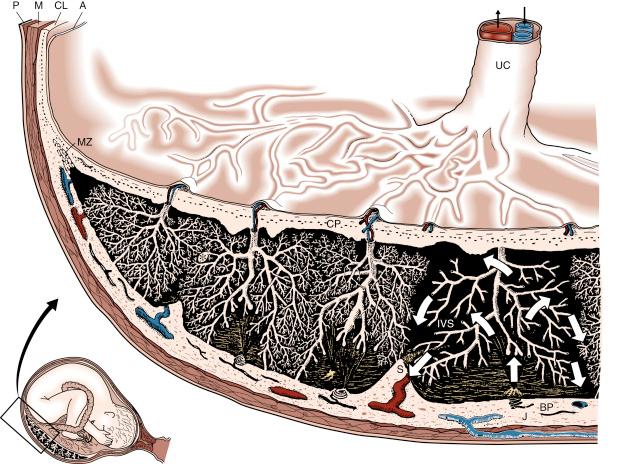
A full-term human placenta is a disclike, circular organ commonly inspected in its ‘inverted’ state from the fetal, or chorionic plate, surface. The chorionic plate is a fibrous disc into which the umbilical arteries ramify as chorionic plate vessels, branching in a dichotomous fashion, each penetrating the plate to enter the stem (truncus) of a villous tree, and accompanied by a vein draining oxygenated blood back towards the umbilical vein. The chorionic plate is covered by the amniotic membrane, which is normally glossy and can easily be peeled off. At the placental margin, the chorionic plate thickens to form a ring and continues and fuses with the basal plate to form the chorion laeve, the fetal membranes. The gross structure of a full-term placenta is illustrated in cross section in Fig. 7.1 .
Macroscopic abnormalities, such as succenturiate lobes, velamentous cord insertion or circumvallate margin (in which the ring is undergrown by villous trees and the intervillous space and the membranes insert medial to the edge of the chorionic plate) are identified from this aspect and occur in approximately 10% of cases at term. In isolation these abnormalities have weak associations with low birth weight or preeclampsia and may therefore be ignored if other aspects of placentation are normal. Nevertheless, experience in second trimester ultrasonographic examination of the placenta is increasing, with rare but clinically relevant problems, such as vasa praevia, being identified by ultrasound resulting in dramatic improvements in perinatal survival.
The fetal surface of the placenta may also be inspected at delivery for evidence of intraamniotic infection, such as cloudy yellow malodorous discolouration, or chronic staining by meconium, when clinically relevant. Successful diagnosis of chorioamnionitis at delivery is facilitated by collection of fluid or pus (for gas chromatography), a membrane roll (from rupture point to edge of placenta) and a sample of umbilical cord into sterile containers before transport of the placenta to the pathology department. Cloudy nodular discolourations of the amnion, termed amnion nodosum , are indicative of prolonged rupture of the membranes and oligohydramnios.
The maternal surface of the placenta (referred to as the basal plate) is an artificial surface in that delivery of the ‘placenta’ requires the organ to be cleaved from the uterine wall through the basal plate (see Fig. 7.1 ). In this sense, part of the trophoblast remains undelivered in the placental bed, normally regressing over the ensuing weeks. The basal plate is a therefore heterogeneous mixture of trophoblastic and decidual cells, embedded in large amounts of extracellular debris, fibrinoid and blood clot. The basal surface is divided by a system of grooves into 10 to 40 elevated areas referred to as the maternal lobes of the placenta. These correspond approximately to the underlying arrangement of villous trees with three or four trees per lobe in the centre; small lobes at the periphery of the placenta are generally occupied only by a single villous tree. Only the latter correspond to what has been described as a placentome : a villous tree together with its surrounding part of the intervillous space and its corresponding uteroplacental vessels. Terms such as ‘fetal placenta’ for the chorionic plate (including villous trees) and ‘maternal placental’ for the basal plate (including uteroplacental vessels invaded by trophoblast) are tempting from a clinical perspective, as will be discussed in relation to Doppler studies. However, because it is impossible to physically separate the fetal and maternal cellular constituents, this concept must be viewed with caution.
Many academic departments take an active interest in placental genetics, structure and function as they relate to development, pathology and the new science of perinatal programming. Sampling of the placenta at delivery is thus an increasingly important task. Because the organ is inherently heterogeneous, a strategy of systematic random block sampling, used in studies that use stereology methods, is preferred. When the focus is on vascular structure and villous development, the best approach is to immediately clamp the cord root (to prevent fetoplacental vascular collapse) and allow the organ to fix for several days in formaldehyde. However, because both mRNA’s and proteins are rapidly degraded after delivery, especially in the metabolically active villous trophoblast, when studies at a protein, molecular, or ultrastructural level are undertaken, a better approach is to open the placenta at random sites from the basal plate to excise samples of villous tissue that can be divided and rapidly frozen (for protein extraction) or placed into RNA fixative.
Sampling of the placental bed is far more challenging than that of the delivered placenta. Nevertheless, the introduction of punch biopsy of the placental bed now permits several samples to be taken, including samples from women after vaginal deliveries and earlier gestation miscarriages.
The human placenta is termed haemochorial because it provides direct contact between maternal blood and the chorionic (fetal) villi in the intervillous space (see Fig. 7.1 ). Maternal blood leaves the openings of the transformed spiral arteries and circulates around the villi. Some villi anchor the villous trees to the basal plate, but the bulk of the term placenta comprises trees of gas-exchanging terminal villi floating in maternal blood. The classic injection studies of spiral arteries by Wigglesworth indicated that a majority of the 60 to 70 villous trees at term, branching from the chorionic plate, are maternally perfused from their centres, thus creating hollow-centred structures. This concept was substantiated by Schuhmann’s description of the 50 to 100 maternal arterial inlets as being located near the centres of the villous trees. The 50 to 200 maternal venous outlets per placenta at term are thought to be arranged around the periphery of the villous trees such that each villous tree is perfused in a centrifugal manner. The radioangiographic studies conducted in humans support the placentome concept which can now be imaged using colour-flow Doppler imaging to study intraplacental blood flow relationships. The maternal arterial jet flows rapidly upwards through the central cavity, dispersing in a centrifugal manner to perfuse the surrounding well developed and more densely packed villi of mature intermediate and terminal type (see later). Short-term reduced intervillous perfusion of a placentome results in temporary hypoxia because fetal perfusion continues to remove oxygen bound to fetal haemoglobin. In response, the stem villous arterioles constrict to reduce the rate of removal of oxygen until intervillous oxygen tension equilibrates again. In this way, the individual placentomes self-regulate to maximise maternal–fetal exchange. Persistent lack of intervillous perfusion can cause stem villous arterial constriction or thrombosis. However, the central portions of the placenta have the largest placentomes and the most-transformed spiral arteries, so central vascular pathology is therefore rare in normal pregnancy. By contrast, spiral artery thrombosis and villous infarction are often found at the margins of the placental disc after a term delivery, with no apparent ill effects.
It is the uniquely invasive properties of the extravillous trophoblast (EVT) that transform the uterine spiral arteries, which result in the haemochorial arrangement that characterises human placentation. The process of invasion is precisely controlled, and it is thought that the disordered EVT proliferation and migration, in combination with the placental vascular pathology, relates to major clinical conditions such as preeclampsia and IUGR (inadequate invasion) or placenta percreta (excessive invasion) and is discussed in detail in Chapter 9 . Please see the online video files associated with Chapter 9 for more information.
Placental development begins with blastocyst attachment to the uterine wall. At this stage, the first extraembryonic cell lineage differentiates and is termed the trophoblast . Signals from the embryo (inner cell mass), including fibroblast growth factor 4 (FGF4), expand the population of trophoblast stem (TS) cells that are capable of differentiating along both the extravillous and villous pathways of development. Considerable knowledge of trophoblast differentiation has been obtained using transgenic mice and mouse TS cells and is summarised in Knott and Paul 2004. Blastocyst symmetry is directed by the inner cell mass because only those trophoblast cells overlying the inner cell mass make direct contact with the uterine epithelium ( Fig. 7.2A ). Abnormal orientation of these structures likely causes abnormalities in the site of umbilical cord insertion into the placental disc and are more common in pregnancies arising from in vitro fertilisation (IVF).
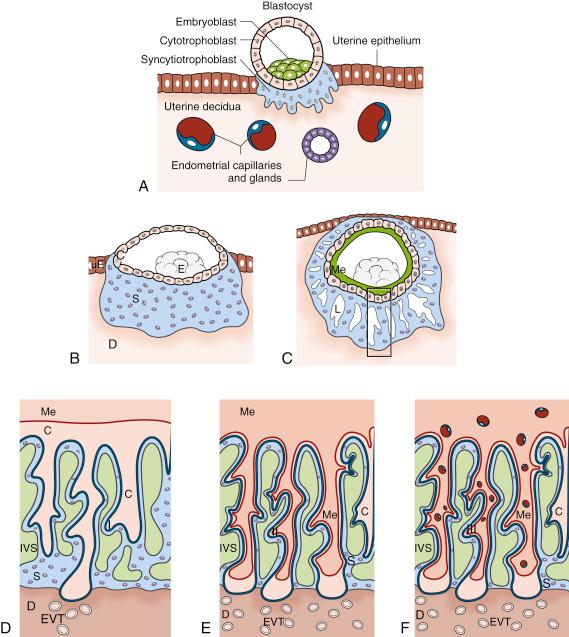
The prelacunar stage of development ( Fig. 7.2B ) is characterised by the formation of an outer shell of syncytiotrophoblast that is distinct from the later villous syncytiotrophoblast by being able to penetrate the uterine epithelium and embed the conceptus in the uterine stroma. The more proximal trophoblast cell population is referred to as cytotrophoblast and is positioned between the syncytiotrophoblast and embryoblast. The cytotrophoblast layer is assumed to be a multipotent stem cell population capable of subsequently producing each type of trophoblast, as in mice. Around day 14 postconception, the conceptus is fully embedded within uterine tissues, and the syncytiotrophoblast starts to develop fluid-filled spaces termed lacunae ( Fig. 7.2C ). The lacunae gradually coalesce to form one large intervillous space of the placenta. This lacunar stage results in the formation of syncytiotrophoblast columns, referred to as trabeculae , which reach from the embryonic side of the placenta to the maternal decidual tissues. The development of the lacunae and the establishment of the intervillous space compartmentalise the growing placenta as follows:
The site of attachment, comprising anchoring villi and the basal plate
The lacunar spaces, forming the intervillous space
Branches that derive from the trabeculae develop into floating villi
The embryonic side develops into the chorionic plate
Further invasion of the maternal tissues by placental trophoblast cells is necessary so as to transform the spiral arteries of the uteroplacental circulation as well as the uterine glands. The first step is the streaming of cytotrophoblast cells down the centre of the syncytiotrophoblast trabeculae, creating a new lineage of extravillous cytotrophoblast that is in direct contact with maternal tissues beyond the initial wave of syncytiotrophoblast invasion ( Fig. 7.2D–F ). Cytotrophoblast at the tips of the trabeculae, their maternal ends now referred to as anchoring villi, form trophoblast cell columns. The proximal cells proliferate as the source of all subsequent subtypes of invasive EVT cells. Initially, the invasion of maternal decidual tissues by cytotrophoblast begins in the connective tissue (interstitial EVT) followed by the walls of the spiral arteries and uterine veins (endovascular trophoblast cells) or the epithelium of uterine glands.
Trophoblast invasion into maternal tissues is not restricted to the process of implantation and early placentation but is a continuous process throughout pregnancy, serving a number of purposes. The trophoblastic cell columns at the base of the anchoring villi provide the cellular sources for this invasive process. The cell columns do not contain stroma because they were not excavated by mesenchyme during formation of the villous trees. The cytotrophoblast cells composing these cell columns, together with trophoblast cells in the placental bed, basal plate, chorionic plate and membranes, are collectively described as EVT cells.
The trophoblastic cell columns resting on the basal plate should be viewed as a rapidly proliferating zone from which EVT cells migrate continuously into maternal tissues ( Fig. 7.3 ). However, as soon as the EVT cells leave this proliferative zone, they leave the cell division cycle and change to an invasive phenotype. Their pattern of integrin expression, secretion of proteolytic enzymes and production of extracellular matrix (ECM) proteins is strikingly similar to that of malignant tumour cells. Fortunately, they differ in one fundamental aspect: they do not proliferate during invasion. This is one of the reasons why the depth of invasion into maternal tissues is limited. If deportation into the maternal circulation occurs, metastatic growth is impossible because these cells have lost their generative potency.
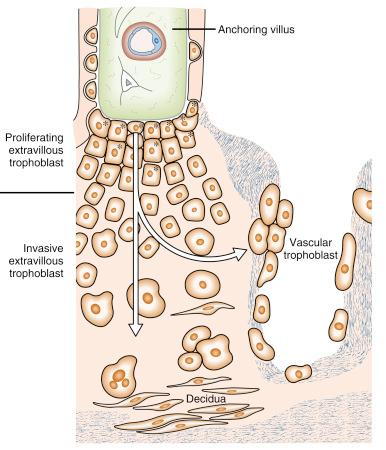
Invasion by EVT serves three very different purposes, namely adhesion of the placenta to the uterine wall, adaptation of uterine glands to enable histotrophic nutrition during the first trimester of pregnancy and adaptation of uteroplacental arteries and veins, enabling haemochorial nutrition to meet the fetal requirements later in gestation.
Among the invasive trophoblast cells, a subset of cells secretes huge amounts of ECM (composed of laminins, collagen IV, fibronectins, vitronectin and heparan sulphate) known as matrix-type fibrinoid into which they are embedded. The EVT cells adhere to the ECM via the surface expression of molecules known as integrins. Likewise, the endometrial stromal cells adhere via similar mechanisms; root-like projections of EVT, together with its associated matrix, penetrate into the inner third of the myometrium, thereby ensuring anchoring of the placenta. Adhesion of the placenta by the gluelike ECM depends on the viability of the EVT cells expressing integrins; in this sense, it is reversible at delivery. This anchoring process is essential; otherwise, entry of maternal blood into the intervillous space at high velocity would shear off the whole placenta.
Along the invasive pathway through the decidual interstitium, EVT cells show morphologically and functionally different phenotypes. These different phenotypes display a varying behaviour regarding contact to maternal cells, secretion of matrix and invasiveness. At present, the molecular basis of these different phenotypes is not known, although it has been described in the related mouse placenta. Such knowledge may in the future help understanding of the pathways that prevent this physiologic process from occurring in certain specific diseases, such as abruption (premature separation), preeclampsia and IUGR.
In a normal intrauterine pregnancy, EVT cells that are invading the decidual interstitium can be subdivided into three morphologically and functionally different subtypes.
This subtype of large polygonal EVT cells corresponds to the well described former X-cells. Compared with the subset of small spindle-shaped cells, the relative number of this subtype increases from 45% at weeks 9 to 12 to 69% at weeks 16 to 24 and makes up about 89% in weeks 31 to 39. Hence, this subtype is the prevailing phenotype of EVT at the time of delivery. These cells are evenly distributed throughout the basal plate as well as along the route of invasion reaching the inner third of the myometrium. Morphologically, these cells are large, polygonal, uninucleated EVT cells displaying big, irregularly shaped, intensely staining nuclei. No other subtype of all trophoblast cells displays a stronger immunoreactivity for cytokeratin 7 than these large polygonal cells. At the same time, they are always immunonegative for proliferation markers such as anti-Ki 67.
These cells secrete the typical ECM of EVT, the matrix-type fibrinoid. This basement membrane-like ECM comprises three different patches of matrix molecules: (i) collagen IV and laminin, (ii) an amorphous ground substance containing heparan sulphate and vitronectin, and (iii) fibronectins and fibrillin embedded in the same amorphous ground substance. The large cells fix themselves within their self-secreted matrix by expression and exposition of the respective integrins, such as α5/β1, α1/β1 and α-v/β3/5 integrins, reviewed by Harris and colleagues. These cells organise themselves in clusters, which are usually void of maternal tissue components but filled with matrix-type fibrinoid. These features do not support the classical thinking that the large polygonal cells (‘X-cells’) are highly invasive cells. Rather, it appears as if this subtype of interstitial EVT may have a function in fixing and adhering the placenta to the uterine wall by secreting matrix-type fibrinoid, which has been termed the ‘trophoblast glue’.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here