Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The skeleton of the head and pharynx is made up of the neurocranium —the bones that support and protect the brain and sensory organs (olfactory organs, eyes, and inner ears)—and the viscerocranium —the bones of the face and pharyngeal arches. The neurocranium can be subdivided into cranial base (the bones underlying the brain), cranial vault (the bones covering the brain), and sensory capsules (the bones encapsulating the sensory organs). There are two types of bone in the head. One type, endochondral bone , forms from a cartilaginous intermediate and ossifies through the process of endochondral ossification . The bones of the cranial base are formed by endochondral ossification and are collectively called the chondrocranium . The other type of bone develops from an ossification directly in the mesenchyme through the process of intramembranous ossification ; this type of bone is known as membrane or dermal bone . The facial skeleton and the skull vault are formed almost entirely of membrane bone. Many of the skeletal structures in the head are unusual in that they are formed from neural crest cells rather than from mesoderm, as they are in the rest of the body.
In humans, five pairs of transient pharyngeal arches (also called branchial arches, especially in the older literature) form on either side of the pharyngeal foregut, starting on day 22. These arches are numbered 1 through 5 in craniocaudal sequence (in older literature, arch 5 is numbered as arch 6). Each pharyngeal arch has an outer covering of ectoderm, an inner lining of endoderm, and a core of mesenchyme derived from paraxial and lateral plate mesoderm and neural crest cell–derived ectomesenchyme . Pharyngeal arches contain an aortic arch artery and an arch-associated cranial nerve, and pharyngeal arches 1 through 3 also contain a cartilaginous supporting element. The pharyngeal arches are separated externally by ectodermal-lined pharyngeal clefts (also called grooves ) and internally by endodermal-lined pharyngeal pouches .
The skeletal elements in pharyngeal arches 1 through 3 are derived from neural crest cells, whereas the skeletal elements associated with pharyngeal arches 4 and 5 are derived from both neural crest and mesoderm. In all pharyngeal arches, the striated musculature and endothelial cells are derived from the mesoderm. The first pharyngeal arch (and more cranially associated mesenchyme) initially forms the transient Meckel’s and palatopterygoquadrate cartilages , which ultimately give rise to the malleus and incus of the middle ear, respectively. The second pharyngeal arch initially forms Reichert’s cartilage, and later the stapes, stylohyoid , the lesser horns , and part of the body of the hyoid . The third pharyngeal arch forms the greater horns and part of the body of the hyoid . The fourth and fifth pharyngeal arches form the cartilages of the larynx.
The mesoderm of the first pharyngeal arch forms the muscles of mastication , and the mesoderm of the second pharyngeal arch forms the muscles of facial expression . The mesoderm in pharyngeal arches 3 through 5 form the muscles of the pharynx and larynx. The muscles derived from each pharyngeal arch are innervated by a corresponding cranial nerve (first, second, third, and fourth and fifth arches are innervated by cranial nerves V, VII, IX, and X, respectively). The extraocular muscles form from the most rostral paraxial mesoderm and prechordal plate mesoderm. These are innervated by the oculomotor (III), trochlear (IV), and abducens (VI) nerves.
The human face is formed between the 4th and 10th weeks by the fusion of five facial prominences : the frontonasal prominence , a pair of maxillary prominences , and a pair of mandibular prominences . During week 5, a pair of thickened ectodermal nasal (olfactory) placodes develops on the frontonasal prominence and then invaginates to form the nasal pits and simultaneously divide part of the frontonasal prominence into the medial and lateral nasal processes . The facial primordia join to form the face ; failure to do so results in facial clefts . During normal development, the medial nasal processes merge to generate the bridge of the nose , the philtrum , and the primary palate ; the lateral nasal processes give rise to the side of the nose; and the maxillary processes generate much of the cheeks. The secondary palate is formed from shelves that grow out from the maxillary swellings and fuse across the midline. The mandibular processes fuse to form the lower jaw.
Each of the pharyngeal pouches forms an adult structure. The first pouch forms most of the tympanic cavity and all of the auditory (Eustachian) tube . The second pouch gives rise to the palatine tonsils . The third pouch forms the thymus gland and inferior parathyroid glands , and the fourth pouch forms the superior parathyroid glands . The fifth pouch forms the ultimobranchial body . The thymus, ultimobranchial glands, and parathyroid glands migrate to their final position in the neck.
The thyroid gland forms as a midline, ventral endodermal evagination of the pharynx; its point of evagination is marked in the adult by the foramen cecum on the upper surface of the tongue. This primordium of the thyroid gland elongates after its evagination, detaches from the pharyngeal endoderm, and finally migrates to its definitive location just inferior and ventral to the larynx. Cells from the ultimobranchial body become incorporated into the thyroid gland to form the calcitonin producing C-cells .
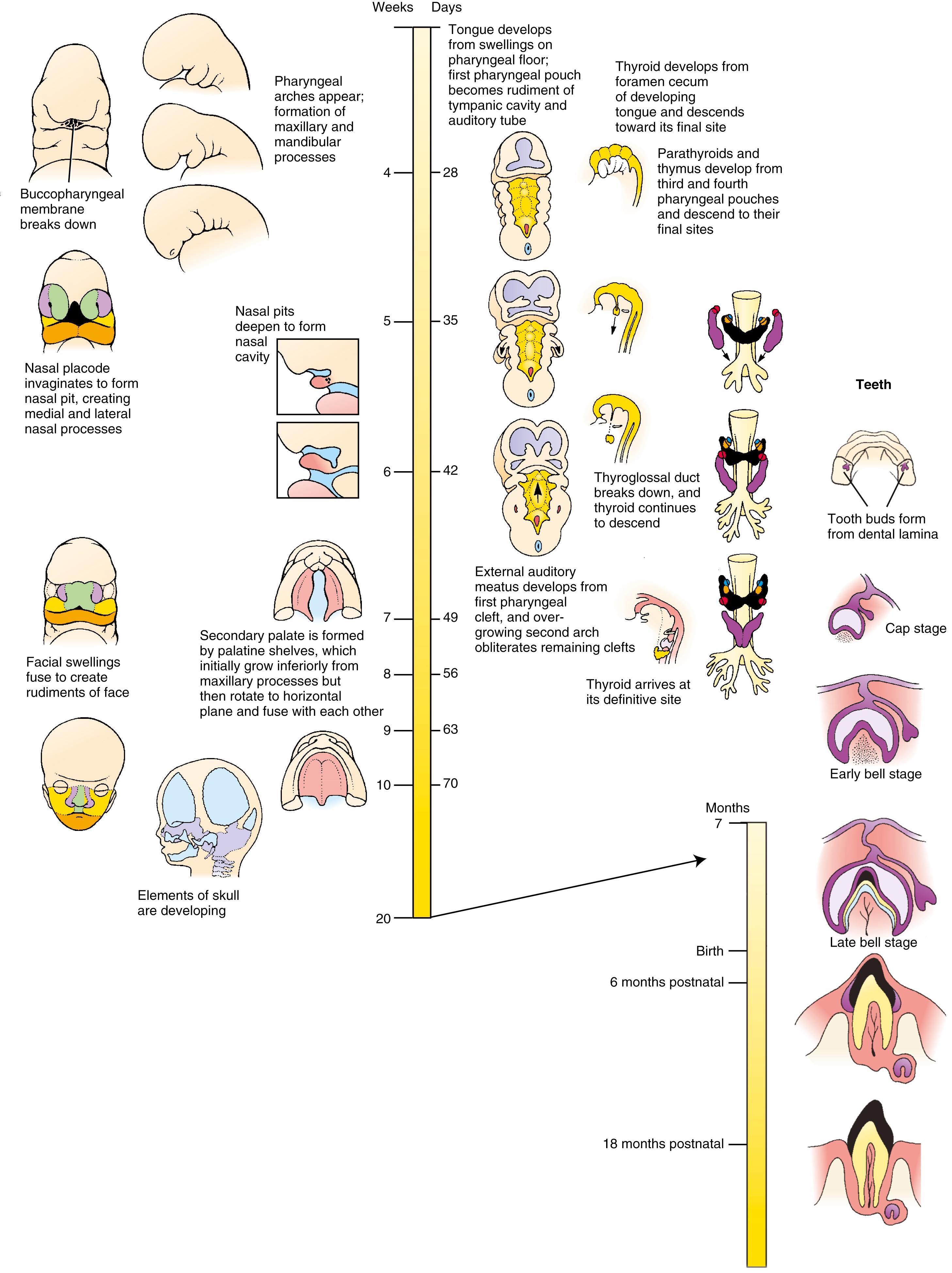
The tongue develops from endodermal-covered neural crest cell–derived mesenchymal swellings on the floor of the pharynx. The anterior two-thirds of the tongue mesenchyme is derived from first-pharyngeal arch swellings, whereas the posterior one-third receives contributions from the mesenchyme of the third and fourth pharyngeal arches. In contrast, most tongue muscles are formed from myocytes arising from occipital somites and are innervated by the hypoglossal nerve . For this reason, the motor and sensory nerve fibers of the tongue are contained within separate sets of cranial nerves.
With the exception of the first pharyngeal cleft (the cleft separating the first and second pharyngeal arches), which forms the external auditory meatus , all other pharyngeal clefts are obliterated by overgrowth of the second pharyngeal arch, although they occasionally persist as abnormal cervical cysts or fistulae.
Teeth arise from ectoderm and neural crest cell–derived mesenchyme. The first sign of tooth development is the formation of a U-shaped epidermal ridge, called the dental lamina , along the crest of the upper and lower jaws. Twenty dental lamina downgrowths, which induce condensation of the underlying neural crest cell–derived mesenchyme, together form the tooth buds of the primary (deciduous) teeth. The secondary, permanent teeth are formed by secondary tooth buds that sprout from the primary buds. Soon after each tooth bud forms, its mesenchymal component forms a hillock-like dental papilla that indents the epithelial enamel organ formed from the bud. This stage of dental development is called the cap stage because the enamel organ sits on the papilla like a cap. By the 10th week, the dental lamina becomes a bell-shaped structure that completely covers the dental papilla. At the late bell stage , the cells of the enamel organ differentiate into enamel-producing ameloblasts , which begin to secrete organic matrix that mineralizes to form radially arranged prisms of enamel between themselves and the underlying papilla. The outermost cells of the papilla differentiate into odontoblasts , which secrete the dentine of the tooth. The inner cells of the dental papilla give rise to the tooth pulp. Nerves and blood vessels gain access to the pulp through the tips of the tooth roots.
An 18-year-old woman was taken to the emergency room complaining of a “heart attack.” She was noticeably sweaty, out of breath, and experiencing chest pain. The symptoms began an hour earlier, while she was jogging. She became especially alarmed when her jogging mate said, “I can see your heart beating in your neck!”
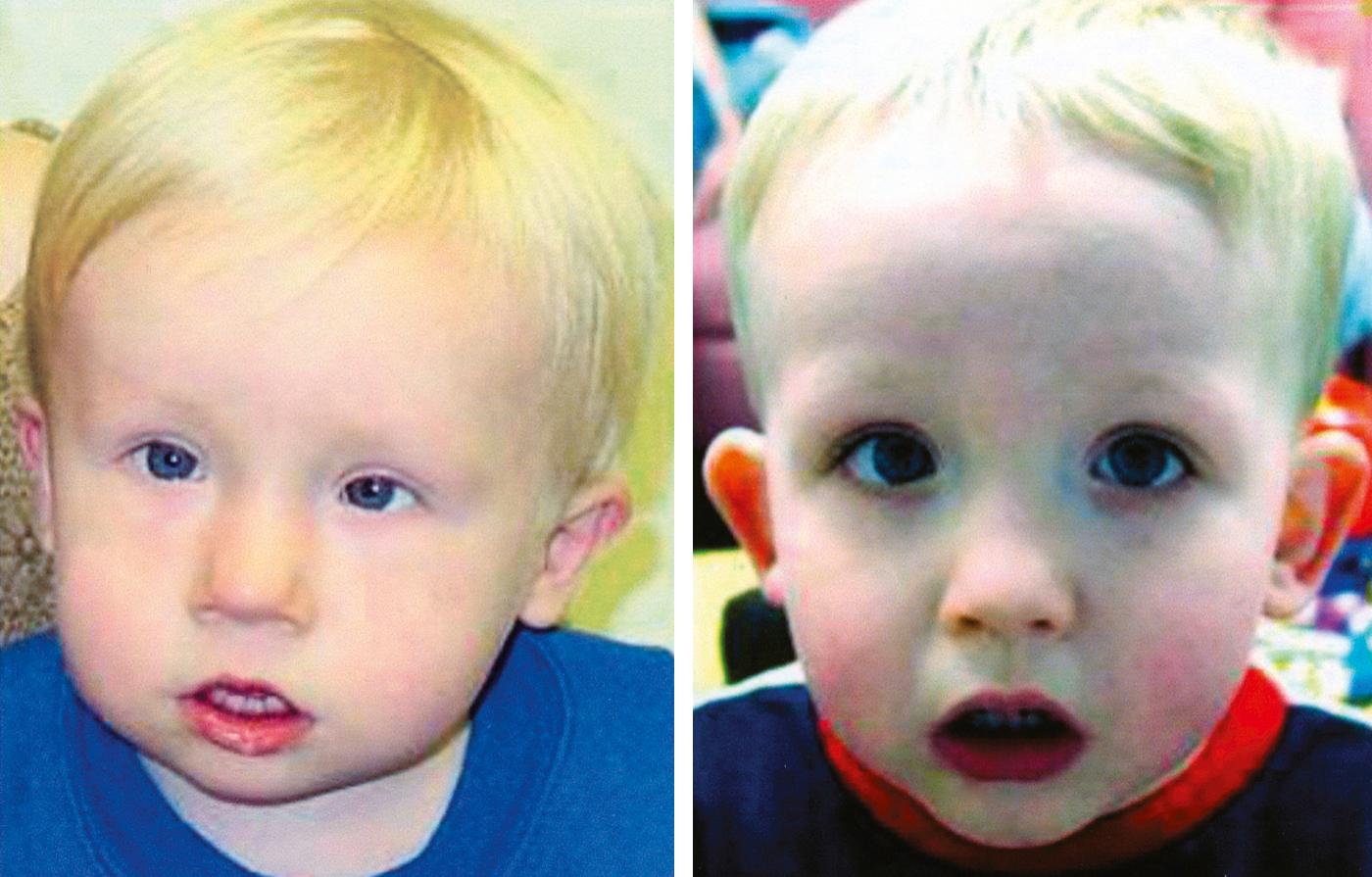
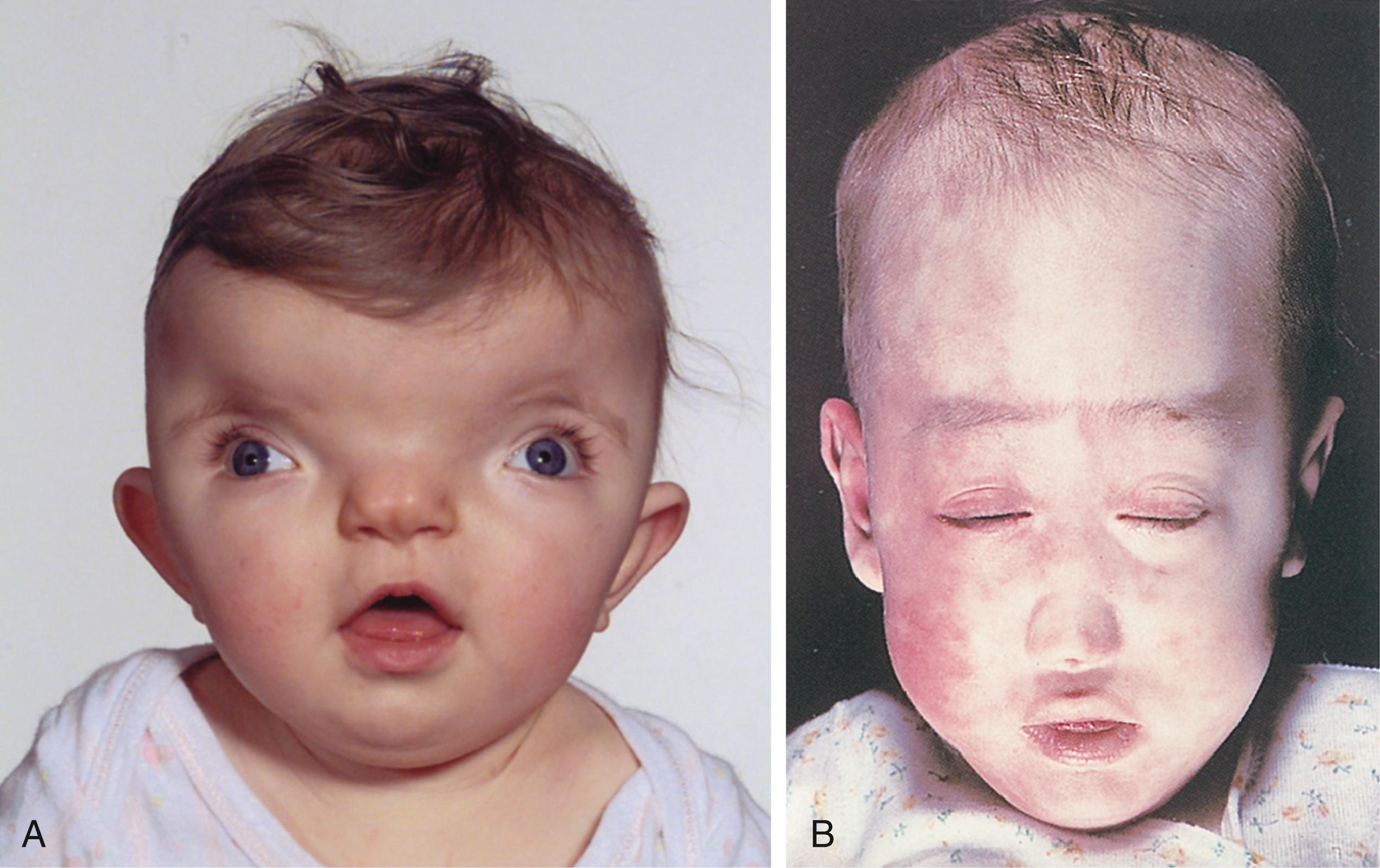
A normal ECG, normal laboratory tests, and improvement without therapy ruled out a heart attack. Physical findings of costochondritis (inflammation of the rib joints) provided a likely cause for the chest pain, which, in turn, led to a panic attack. However, the ER physician continued to hear inspiratory stridor (a sound made by partial obstruction of a large airway) well after the panic attack had subsided. This, combined with visible arterial pulsations at the base of the patient’s neck, suggested a vascular anomaly. The doctor also noticed that the woman had minor craniofacial anomalies, including small, abnormally shaped ears, retrognathia (a setback jaw), a short philtrum (the structure between the upper lip and nose), and a broad nasal tip. In addition, the patient had a bifid uvula and hypernasal speech, suggestive of palatal dysfunction.
Magnetic resonance angiography (MRA) done 1 week later revealed a cervical aortic arch (CAA) that was impinging on, and partially obstructing, the trachea, causing stridor during deep inhalation. CAA results from abnormal development of the aortic arches, with regression of the left fourth aortic arch and enlargement of the left third aortic arch. Normally, as covered in Chapter 13 , the left fourth aortic arch persists and contributes to the arch of the aorta. CAA and related vascular anomalies, especially when accompanied by craniofacial defects and velopharyngeal insufficiency (dysfunction of the palate and pharynx; “velo” refers to palate), commonly occur in chromosome 22q11.2 deletion syndrome (also known as velocardiofacial or DiGeorge syndrome ). Genetic testing confirmed this deletion. 22q11.2 deletion syndrome is characterized by a wide range of abnormalities, often subtle in infancy ( Fig. 17.1 ), affecting the craniofacial and pharyngeal arch derivatives.
In humans, the bones of the head can be divided into the neurocranium and viscerocranium . These bones arise from more than 100 ossification centers that consolidate to form 45 bones in the neonatal skull. Fusion of these bones postnatally reduces this number to 22 bones in the adult skull. The neurocranium encompasses the bones surrounding and protecting the brain and sensory organs: the bones of the cranial base, sensory organs, and skull vault ( Fig. 17.2A ). The viscerocranium encompasses the bones of the face and larynx (see Fig. 17.2A ). The neurocranium and viscerocranium consist of two types of bones: those formed through ossification of a cartilaginous intermediate are known as endochondral bones , and those formed through direct ossification in the mesenchyme are known as membrane or dermal bones (covered in Chapter 8 ). In humans, dermal bones, with the exception of part of the clavicle , are found only in the head.
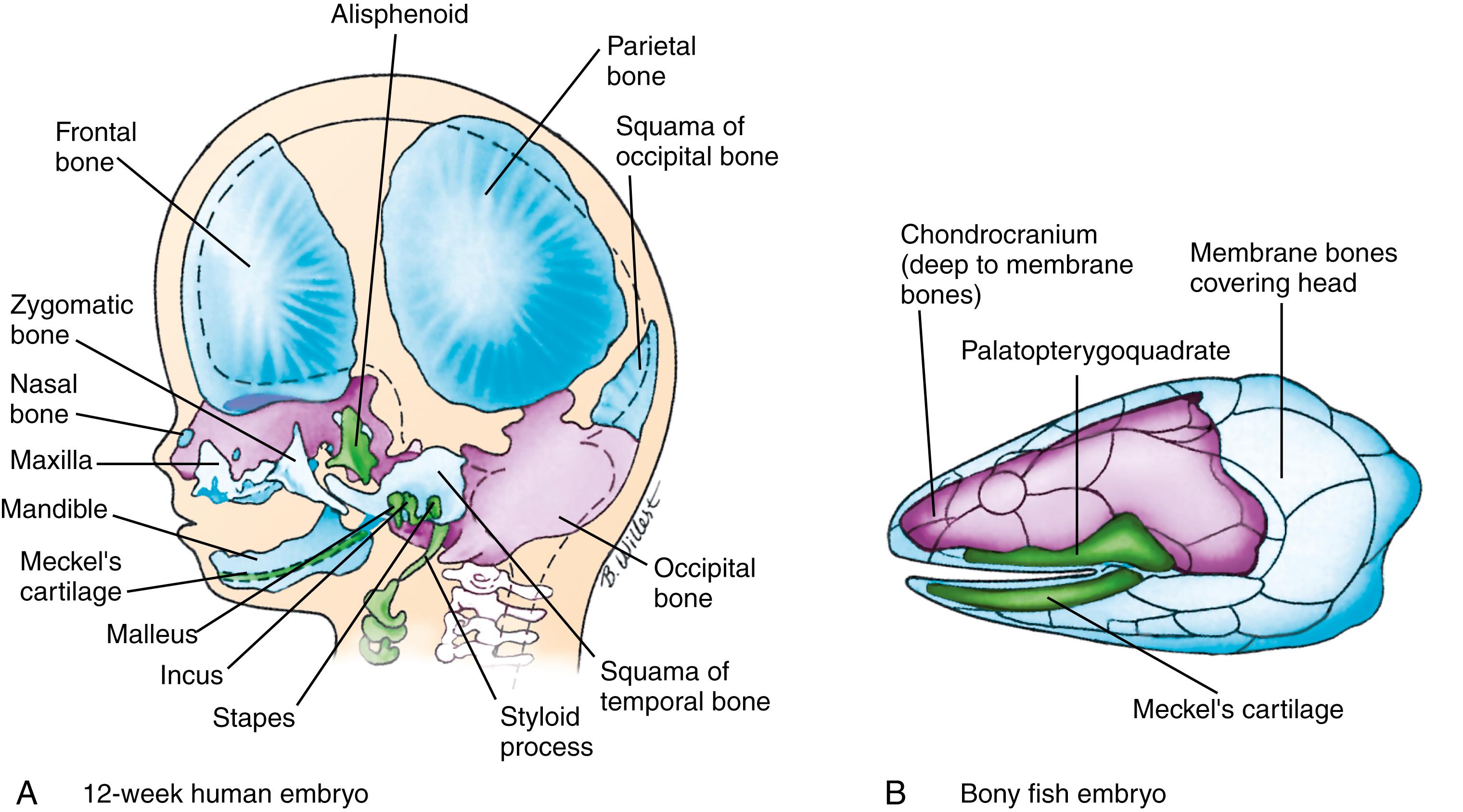
The presence of these two types of bones in the head was conserved during evolution. The cranial skeleton of fishes is composed of (1) the chondrocranium , which encloses and protects the brain and helps to form the sensory capsules that support and protect the olfactory organs, eyes, and inner ears; (2) an external armor of membrane (dermal) bones , which overlie the chondrocranium; and (3) the visceral skeleton or viscerocranium , which supports the gill bars and jaws (see Fig. 17.2B ). The bones of the chondrocranium (as the name indicates) are preformed in cartilage. These three components of the cranial skeleton of fishes can still be distinguished in the development of the human skull (compare Fig. 17.2A,B ). However, during evolution, with establishment of the novel temporomandibular joint, the pharyngeal arch cartilages (i.e., the viscerocranium) were modified to form the cartilages of the larynx and bones of the middle ear. Also, as the brain expanded, the chondrocranium came to underlie, rather than surround, the developing brain, forming the cranial base, whereas the dermal bones expanded to form the cranial vault.
In humans, the chondrocranium is also defined as that portion of the neurocranium formed by endochondral ossification. The chondrocranium, in part, develops from three pairs of cartilaginous precursors that were also present in our early ancestors— prechordal cartilages ( trabeculae cranii ), hypophyseal cartilages , and parachordal cartilages ( Fig. 17.3A ). These cartilages contribute to the cranial base and, together with the cartilage from the occipital somites (see next paragraph) and the cartilaginous elements that develop around the eye, otic pits, and nasal pits, form the chondrocranium, which helps to protect the brain and sensory organs.
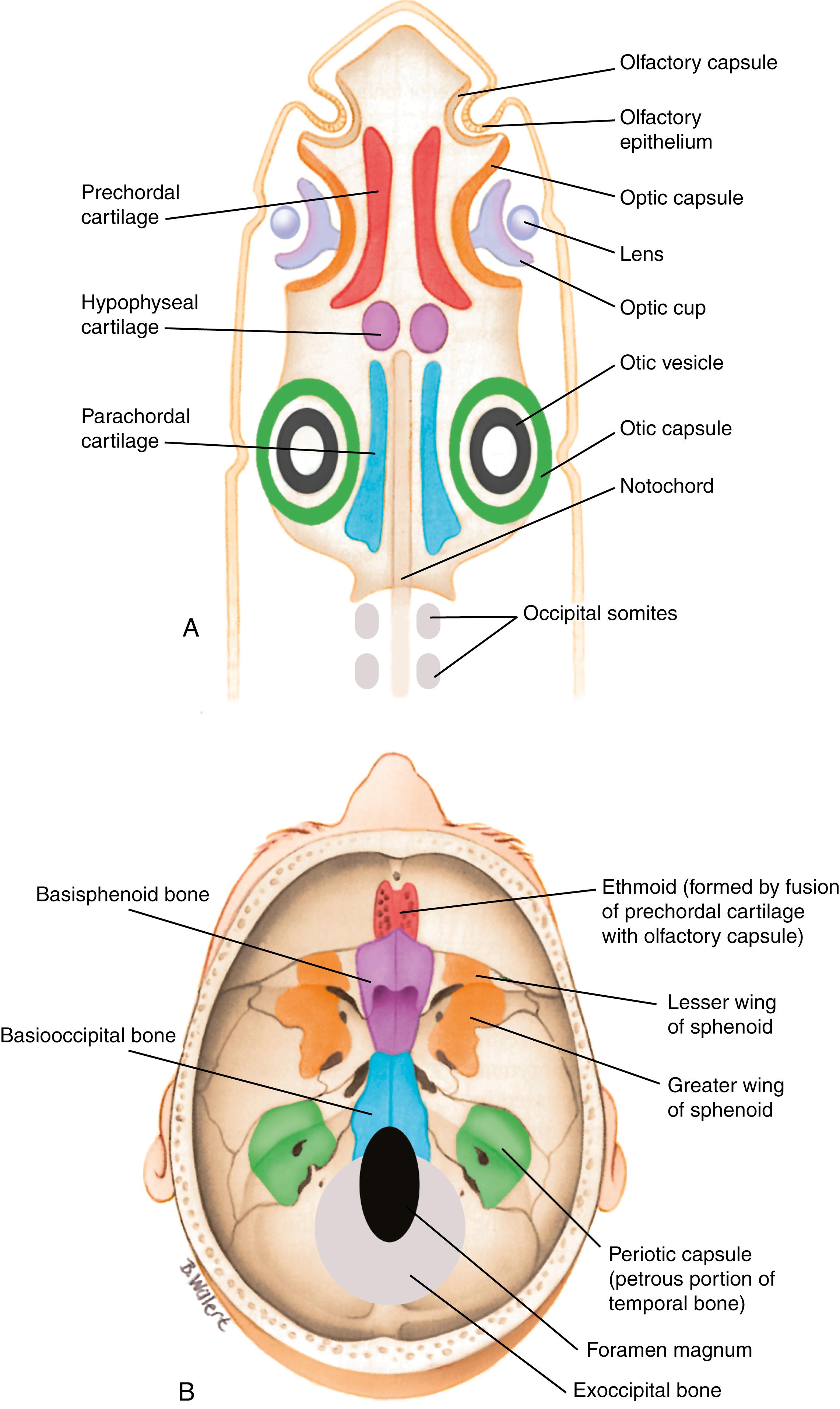
The most caudal pair of elements that contributes to the chondrocranium, the parachordal cartilages , is derived from unsegmented paraxial mesoderm and is the first pair of elements to develop. The two cartilages will fuse across the midline to form the base of the occipital bone (i.e., the basioccipital bone ; see Fig. 17.3B ). The occipital somites (see Fig. 17.3A ) form the exoccipital bone (see Fig. 17.3B ), which is, therefore, a modified vertebral element derived from segmented paraxial mesoderm (covered in Chapter 8 ). The periotic capsules are derived from the primitive otic capsule ossification centers (see Fig. 17.3A ) which will eventually form the petrous and mastoid regions of the temporal bone (see Fig. 17.3B ) . The basioccipital, exoccipital, and periotic capsule derivatives will eventually unite to form the occipital bone encircling the foramen magnum (see Fig. 17.3B ).
The hypophyseal cartilages , the middle cartilages that contribute to the cranial base, fuse to form the body of the sphenoid bone (i.e., the basisphenoid bone ; see Fig. 17.3B ). Similarly, the posterior region of the prechordal cartilages , the most anterior pair of elements that contributes to the chondrocranium, forms the presphenoid bone ( Fig. 17.4A ). These bones will unite with three cartilages that develop around the eye: the ala temporalis cartilage (which together with the membranous laminal obturans bone will form the alisphenoid bone) (see Fig. 17.2A ), the hypochiasmatic cartilage (see Fig. 17.4A ), and the orbitosphenoid ( ala orbitalis ) cartilage . The alisphenoid cartilage will ultimately contribute to the greater wing of the sphenoid bone , whereas the orbitosphenoid and hypochiasmatic cartilages contribute to the lesser wing of the sphenoid bone (see Fig. 17.3B ). In addition, the prechordal cartilages form the ethmoid bone , which, together with the nasal and turbinate bones , encapsulates the nasal cavity (see Figs. 17.2A, 17.3 ).
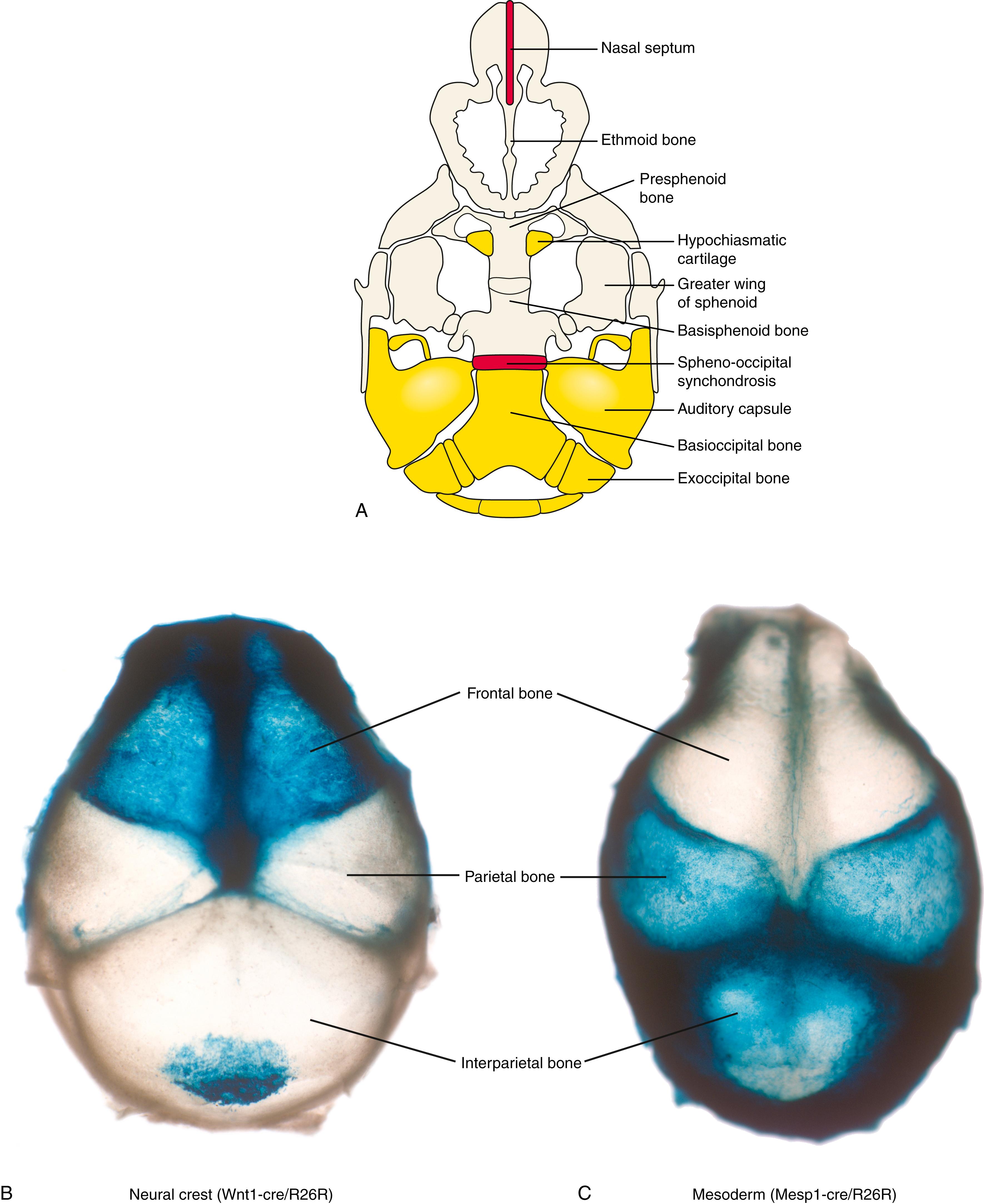
By birth, most of the cranial base has ossified, but two key regions of cartilage persist that are important for postnatal growth: nasal septum and spheno-occipital synchondrosis ( SOS ) (see Fig. 17.4A ). The nasal septum is important for growth of the upper face until the age of 7, whereas the SOS contributes to growth along the anterior-posterior axis of the cranial base until age 13 to 15 in girls and age 15 to 17 in boys. The SOS consists of a modified epiphyseal growth plate that grows in two directions (see Chapter 8 ).
Defects in development of the chondrocranium are rare, although a mutation in a factor that controls endochondral bone formation will also affect development of the cranial base (see Chapter 8 for additional coverage of endochondral bone formation). As the cranial base is linked to the upper jaw, this will have a secondary consequence on upper jaw development, resulting in malocclusion of the upper molars. This is illustrated in Chapter 8 ( Fig. 8.1 ), which shows a girl with achondroplasia. In addition to shortening of the long bones, a defect in development of the cranial base results in midface hypoplasia .
The membrane-bone armor that covered the skull of our piscine ancestors (bony fishes) is represented in humans by the membrane bones of the skull, consisting of the flat bones of the cranial vault , or calvaria , as well as many bones of the face ( Fig. 17.5 , also see Fig. 17.2 ). The mesenchyme from which they develop is derived from both neural crest cells and mesoderm (see the following “In the Research Lab” entitled “Origin of Vertebrate Head”).
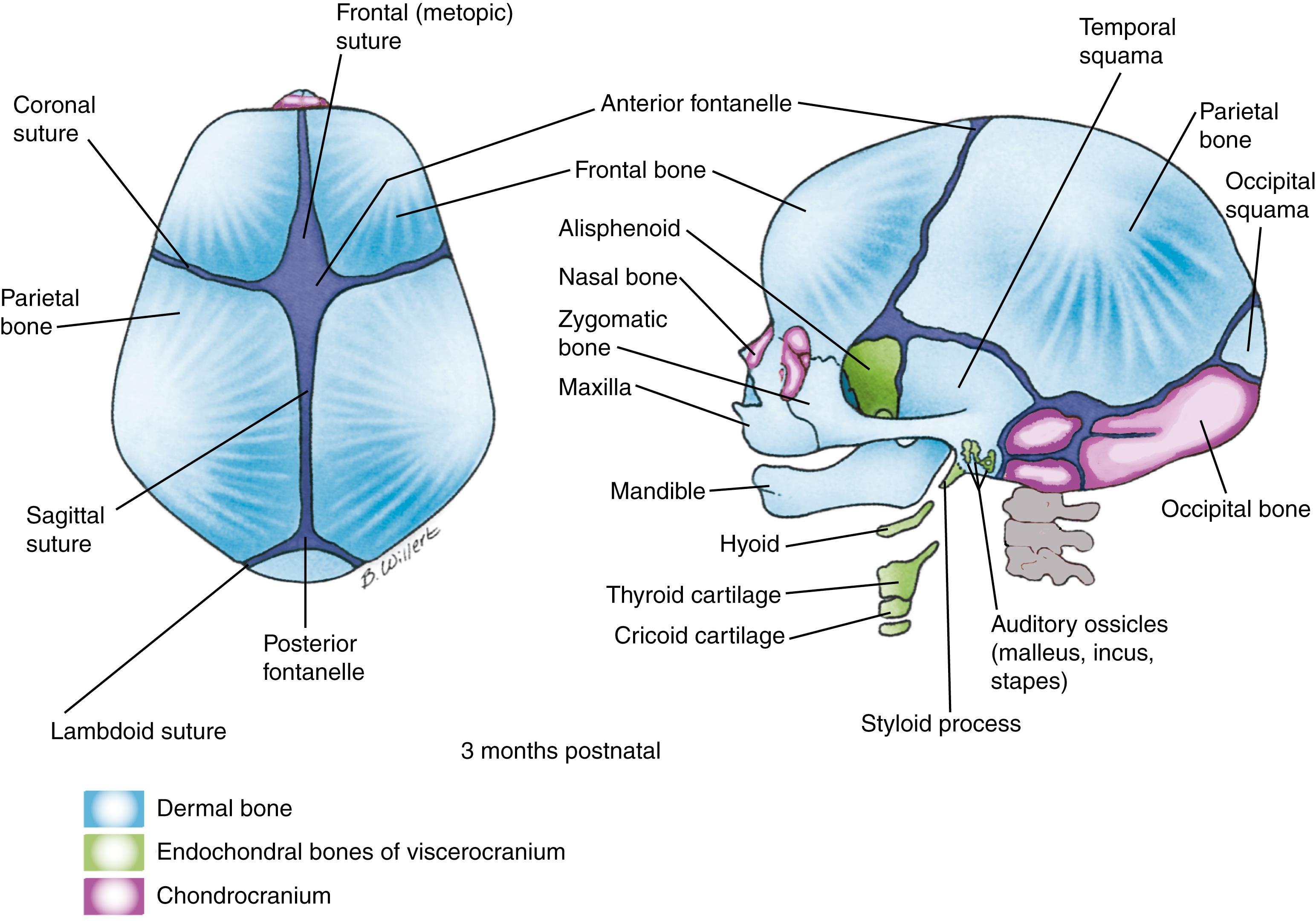
The bones of the cranial vault do not complete their growth during fetal life. The soft, fibrous sutures that join them at birth permit the skull vault to deform as it passes through the birth canal and allow it to continue to grow throughout infancy and childhood. Six such membranous sutures or fontanelles occupy the areas between the corners of cranial vault bones at birth (see Fig. 17.5 ). The posterior and anterolateral fontanelles , the smaller of the fontanelles, close by 3 months after birth, whereas the anterior and posterolateral fontanelles , which are larger, normally close during the second year. Palpation of the anterior fontanelle can be used to detect elevated intracranial pressure or premature closure of the skull sutures.
The “new head hypothesis” proposed that the vertebrate head evolved by adding structures, derived from neural crest cells, cranial to the notochord. Cell lineage tracing, for example, using genetically modified mice to label neural crest cell–derived and mesoderm-derived structures, has confirmed this hypothesis. In the cranial base, the structures that develop around and caudal to the cranial tip of the notochord (i.e., the occipital bone) are derived from the mesoderm (yellow; see Fig. 17.4A ). Structures that form rostral to the notochord (i.e., the sphenoid and ethmoid bones) are derived from the neural crest (tan; see Fig. 17.4A ). Therefore, the neural crest–mesoderm boundary lies at the sphenoid-occipital interface, with one exception—the hypochiasmatic cartilage , which provides the attachment for the extraocular muscles, contributes to the sphenoid bone, and is derived from mesoderm, even though it lies rostral to the notochord (yellow islands; see Fig. 17.4A ).
These lineage-tracing studies have revealed the contributions of neural crest and mesoderm to the mammalian cranial vault and pharyngeal arches. Neural crest–derived cells contribute to the frontal bone and to a portion of the interparietal bones in the cranial vault, whereas the mesoderm forms the parietal bones and the lateral part of the interparietal bones (see Fig. 17.4B,C ). Neural crest cells are also found between paired parietal bones. Therefore, two sutures ( coronal and sagittal sutures ) are formed at a neural crest–mesoderm interface. This is significant, as mutations affecting formation of these boundaries result in craniosynostosis (see the following “In the Clinic” entitled “Craniosynostosis”). Neural crest cells also give rise to cartilages formed within pharyngeal arches 1 to 3.
The chondrocranium is induced to form in response to signals from surrounding tissues. In the trunk, the notochord, which expresses sonic hedgehog (Shh), is essential for formation of the axial skeleton (see Chapter 8 ). However, the structures of the anterior cranial base develop cranial to the notochord. Therefore, they must form in response to signaling from different tissues. Shh signaling from the developing brain and/or facial ectoderm is required for development of the midline structures of the cranial base, whereas FGF signals from the otic and nasal epithelium induce the formation of the otic and nasal capsules, respectively. Consequently, treatment of zebrafish embryos with cyclopamine (a chemical inhibitor of hedgehog signaling) results in loss of the trabecular bones. Similarly, ablation of the hedgehog receptor, smoothened, in the cranial neural crest in mice results in loss of the structures of the anterior cranial base. Therefore, it seems that the brain and ectoderm induce formation of the cranial base, and that similar signaling networks control development of the trunk and cranial axial structures.
Craniosynostosis , the premature closure of sutures, affects approximately 1 in 2500 children. Sutures, which occur where two membrane bones meet, contain the progenitor cells that will give rise to new bone cells, the osteoblasts. These are the sites where growth of membrane bone occurs. Craniosynostosis can be caused by gene mutations or chromosomal abnormalities and occurs in many syndromes, including Crouzon , Apert , Pfeiffer , Muenke , and Saethre-Chotzen syndromes . Craniosynostosis can also be a consequence of environmental factors such as a restricted intrauterine environment or exposure to teratogens.
The sagittal suture is the most commonly affected. Closure at one suture causes increased growth at other sutures, thereby deforming the brain and skull, which develop a characteristic deformation depending on the suture(s) that is closed. This is shown in Fig. 17.6A . Here, the coronal suture has closed, so that the head cannot grow in length, and now the developing brain forces growth along the other sutures. The result is a skull deformity, with possible changes in neurologic function, increased intracranial pressure, and inability to feed and close the eyes, together with respiratory and hearing abnormalities. Dolichocephaly and scaphocephaly are the terms used to describe long and narrow skulls. Acrocephaly describes a towered skull; trigonocephaly describes a skull that is triangular at the front; brachycephaly describes a skull that is broad and flattened; and plagiocephaly describes a skewed skull (see Fig. 17.6B ).
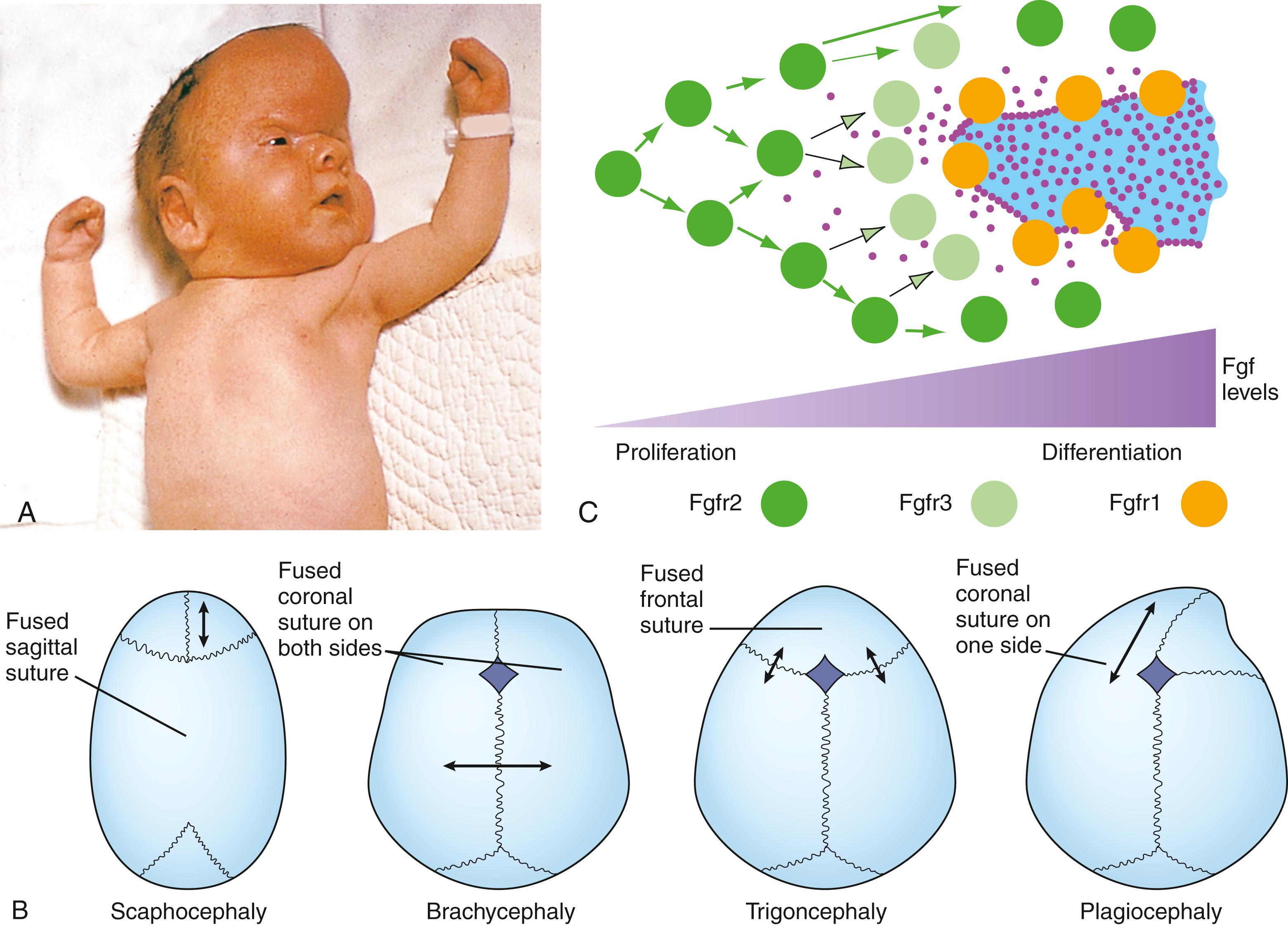
The most common genetic causes of craniosynostosis syndromes are mutations in genes coding for (1) Fgf receptors 2 ( Apert , Crouzon , and Pfeiffer syndromes ) and 3 ( Muenke and Crouzon syndromes with acanthosis nigricans ); (2) the transcription factor, Twist ( Saethre - Chotzen syndrome ); and (3) a membrane-bound ligand, Ephrin B1 (EfnB1) ( craniofrontonasal syndrome ; Fig. 17.7A ). Pfeiffer syndrome is highly variable, but the most severe form is characterized by multiple synostoses (union of separate bones to form a single bone) that are associated with high mortality. Distinguishing characteristics of the hand and face can be used to help with an initial diagnosis of the syndrome. For example, Pfeiffer syndrome is defined by a broad thumb and a big toe, Apert syndrome by syndactyly, and craniofrontonasal syndrome by split nails.
As was just covered, mutations in genes coding for Fgf receptors (Fgfr1, 2, and 3) result in craniosynostosis. All of these mutations cause a gain-of-function in Fgf signaling owing to the constitutive activation of receptors (i.e., these mutations result in an increase in Fgf signaling). Fgfs are expressed in the osteoblastic front of membrane bones (see Fig. 17.6C ). Therefore, the highest levels of signaling are found in the cells adjacent to the bone, whereas lower levels are found in the sutural mesenchyme (see Fig. 17.6C ). The cognate receptors are expressed in the sutural mesenchyme (Fgfr2) and in differentiating osteoblasts (Fgfr1 and 3; see Fig. 17.6C ).
Fgf signaling has two roles in suture development. Low levels of Fgfs in the sutural mesenchyme promote cell proliferation whereas high levels promote osteoblast differentiation. Therefore, the rate of osteogenesis is carefully controlled by balancing the level of Fgf signaling (see Fig. 17.6C ). Increased Fgf signaling through constitutive activation of the receptors decreases cell proliferation and accelerates osteoblast differentiation, resulting in synostosis across the sutures (i.e., premature closure of the fontanelles). In mouse models of craniosynostosis, administration of pharmacologic inhibitors against the Fgf pathway during pregnancy and postnatally prevents premature suture closure. Such approaches may eventually allow non-surgical or reduced surgical intervention for the treatment of craniosynostosis in humans. Curiously, FGFR2 and 3 mutations are usually inherited as spontaneous new mutations through the paternal lineage, increasing in frequency with paternal age. One such mutation in FGFR2 (a mutation that causes Apert syndrome ) increases the clonal expansion of mutant spermatogonia, thereby increasing the number of sperm carrying the mutation.
The EFNB1 and TWIST mutations specifically affect the coronal suture. Long-term lineage tracing studies in mice have shown that the coronal suture arises at a neural crest cell–mesoderm interface (see Fig. 17.4B,C ). The frontal bone is derived from neural crest, whereas the adjacent parietal bone and the coronal sutural mesenchyme are derived from mesoderm. The neural crest–derived mesenchyme and the mesoderm have different cell adhesion properties and do not mix, thereby creating a sharp boundary. These distinct properties are controlled by the differential expression of the membrane-bound ephrin ligands and their Eph receptors in the neural crest– and mesoderm-derived tissues (also see Fig. 8.5 in Chapter 8 and Fig. 20.29 in Chapter 20 , as well as Chapter 13, Chapter 14, Chapter 9 , for other examples of the differential expression of ephrin ligands and Eph receptors inhibiting cell mixing). Mutations in the ephrin signaling pathway and in twist, a transcription factor that regulates ephrin expression, will affect the formation of this boundary. Twist also directly inhibits Runx2, a master transcriptional regulator of bone differentiation (covered further in Chapter 8 ). Therefore, reduction in twist expression will result in bone formation across the suture.
Craniofrontonasal dysplasia , caused by an EfnB1 mutation, is an unusual X-linked syndrome in that heterozygote females show more severe phenotypes than hemizygous males. The ephrin ligand EfnB1 is specifically expressed in the neural crest and prevents mixing of neural crest–derived cells with mesodermal cells. Due to random X-inactivation, in heterozygote females, the frontal bone will consist of a mixture of cells that either express one copy of the functional EFNB1 gene or express the mutated non-functional EfnB1 protein. Cells that express the normal EFNB1 gene have different cell adhesion and signaling properties than cells expressing the non-functional EFNB1 gene, such that the two populations will clump and segregate: the result is that some of these cells cross and abolish the sutural boundary. Additional features of craniofrontonasal dysplasia include severe hypertelorism (widely spaced eyes) and a bifid nasal tip in females (see Fig. 17.7A ).
Holoprosencephaly (HPE) is the most common developmental defect of the forebrain, affecting 1 in 16,000 births and an estimated 1 in 250 fetuses. It results from a disturbance in early patterning of the forebrain (see Fig. 17.7B ). The spectrum of phenotypes is wide and there is variable expressivity, which means that the same mutation can produce different phenotypic outcomes. This indicates the possibility of modifier genes and/or environmental effects, and the fact that HPE should be viewed as a multigenic or multifactorial disorder . It is estimated that approximately 30% of people with HPE mutations have no phenotype.
HPE can be classified into five forms: alobar , lobar , semilobar , microform , which are classic forms of HPE, and middle interhemispheric form ( MIH ; also known as syntelencephaly ). In its most severe form, alobar HPE, only a single cerebral lobe forms (hence the name of the condition) rather than paired right and left hemispheres. Defects of the olfactory nerves, olfactory bulbs, olfactory tracts, basal olfactory cortex, and associated structures including the limbic lobe, hippocampus, and mammillary bodies can also occur. The corpus callosum is sometimes affected; the hindbrain is usually normal. Patients with alobar HPE typically die within the first year. In the milder forms of HPE, semilobar and lobar HPE, partial separation of the single cerebral lobe is noted. In MIH, the anterior forebrain develops normally, but the hemispheres fail to separate in the posterior parietal and frontal regions.
Except for MIH, forebrain defects are accompanied by a spectrum of facial abnormalities that often, but not always, reflect the severity of the forebrain defect. Facial anomalies typical of HPE include a flat nose, ocular hypotelorism (closely spaced eyes), deficient philtrum or cleft lip, high arched or cleft palate, and microcephaly (small skull) (see Fig. 17.7B ). Particularly severe cases involve dramatic defects of the facial structures arising from the frontonasal prominence, most notably the nasal placodes (development of the face is covered in detail later in this chapter). Failure of the medial nasal processes to form results in agenesis of the intermaxillary process and reduction or absence of other midfacial structures such as the nasal bones, nasal septum, and ethmoid. The consequence may be cebocephaly (a single nostril; see Fig. 17.7B ) and, at the most extreme, cyclopia (a single, midline eye). Mild cases of HPE are characterized by relatively minimal midface anomalies and by trigonocephaly , a triangular skull shape that develops as a result of premature closure (synostosis) of the suture between the frontal bones and causes compression of the growing cerebral hemispheres (see Fig. 17.6B ). Occasionally, the presence of a single, central incisor and the loss of the maxillary midline frenulum are the only indications of a holoprosencephalic phenotype. In microform HPE , mild facial abnormalities occur in the absence of forebrain defects.
Mutations in at least 17 genes have been implicated in HPE in humans, and mutations in nine genes in these loci have been identified. Of these, four are components of the sonic hedgehog signaling pathway (SHH, PTC1, GLI2, and DISP1: DISPATCHED HOMOLOG 1). Mutations in SHH and in ZIC2, SIX3, and TRANSFORMING GROWTH FACTOR INTERACTING FACTOR (TGIF) all of three of which encode transcription factors, are the leading genetic causes of non-syndromic HPE.
HPE is also a feature of more than 25 syndromes and is seen in 5% of Smith-Lemli-Opitz syndrome patients. Smith-Lemli-Opitz syndrome affects about 1 in 9000 births (live and stillborn) and is the result of a mutation in the DHCR7 gene, which encodes 7-dehydrocholesterol reductase, an enzyme involved in the penultimate step of cholesterol synthesis. A cholesterol modification of Shh is necessary for full Shh activity (see Chapter 5 ); hence, it is believed that some Smith-Lemli-Opitz phenotypes are due to loss of hedgehog signaling.
Environmental agents associated with HPE include maternal diabetes. Infants of diabetic mothers have a risk of HPE that may be as high as 1%. Maternal exposure to alcohol , excess vitamin A , or statins , which disrupt Shh signaling, have also been implicated.
Conversely, some syndromes have hypertelorism (widely spaced eyes) as a component. This is seen in craniofrontonasal dysplasia , resulting from mutations in EFNB1 (EPHRIN-B1; see Fig. 17.7A ); Gorlin syndrome resulting from mutations in PTC1; and Greig cephalopolysyndactyly , resulting from mutations in GLI3. As Gli3 and Ptc1 are components of the Shh pathway and can repress Shh signaling, it is believed that the hypertelorism observed in Gorlin and Greig cephalopolysyndactyly is due to a gain in Shh signaling (see the following “In the Research Lab” entitled “Cranial Midline and HPE”).
Establishment of the cranial midline involves three major steps during development ( Fig. 17.8A ): (1) formation of the prechordal plate during gastrulation; (2) signaling from the prechordal plate to the overlying forebrain, which initially consists of a single cerebral lobe (covered in Chapter 9 ), to divide the forebrain into two cerebral lobes; surgical ablation of the prechordal plate results in cyclopia, owing to loss of this signaling; and (3) signaling by the forebrain to the facial ectoderm in post-neurulation stages to induce and/or maintain gene expression in this area. Signaling by the facial ectoderm regulates the outgrowth of the frontonasal prominence during subsequent formation of the midline of the face (development of the face is covered later in this chapter).
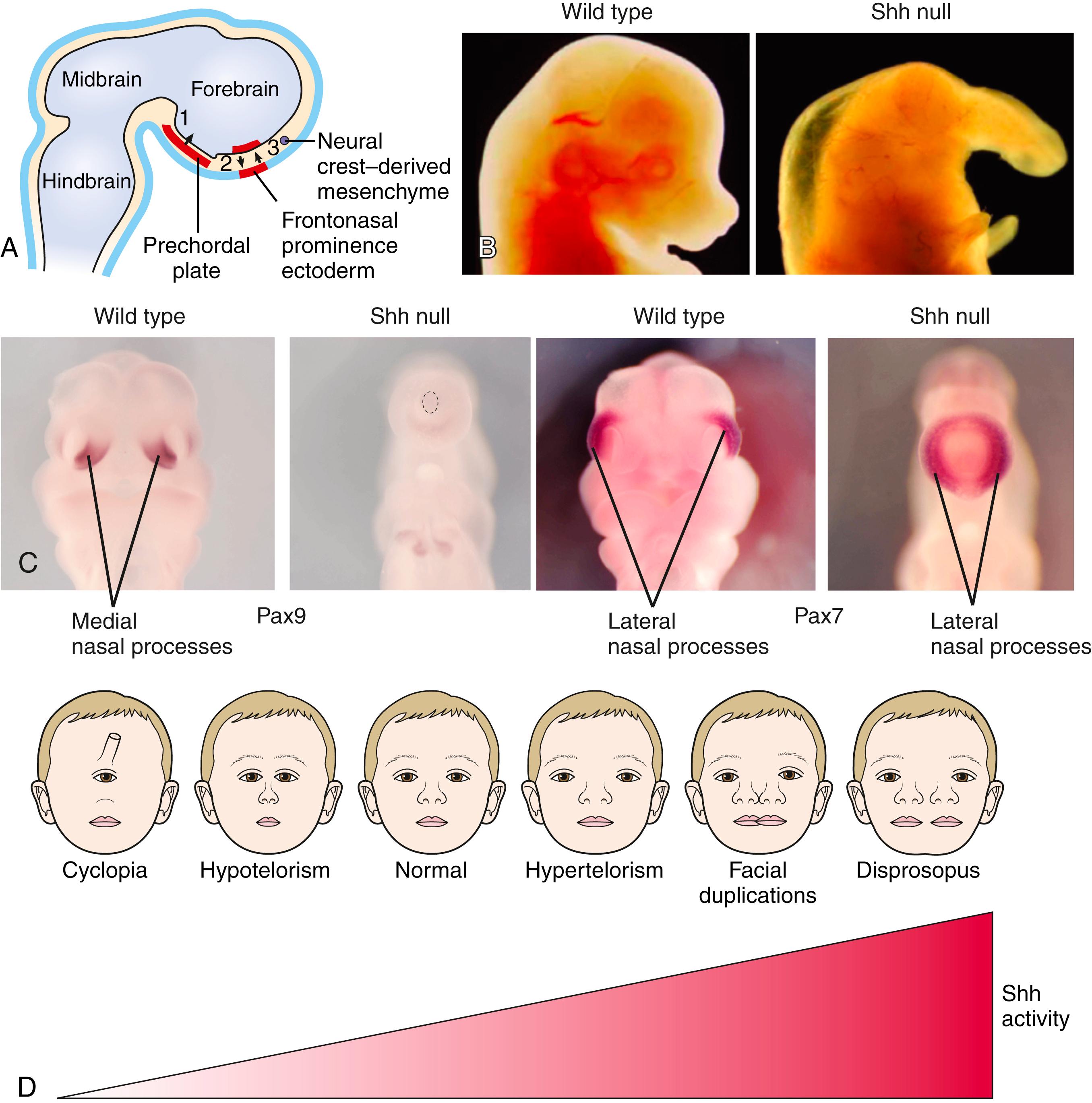
In humans, SHH mutations are the leading genetic cause of non-syndromic HPE. In animal models, Shh is expressed, and is required, in the prechordal plate, forebrain, and facial ectoderm to establish the midline (see Fig. 17.8A ). Clearly emphasizing the importance of the Shh pathway, Shh−/− mutant mice exhibit cyclopia and develop a proboscis (see Fig. 17.8B ). The developing upper face normally consists of the medial nasal and lateral nasal processes (see Fig. 17.8C ; also see Fig. 17.18 ). In Shh mutants, a total collapse of the midline is shown by loss of the medial nasal processes (see Fig. 17.8C ). With loss of these processes, the lateral nasal processes become positioned at the midline of the upper face (see Fig. 17.8C ).
During the establishment of the midline, Shh signaling is first required in the prechordal plate region to divide the single midline eye primordium in the forebrain into eye (Pax6 expressing) and non-eye (non-Pax6 expressing) territories (see Fig. 17.8A ). Shh expression also maintains cell survival of the prechordal plate and activates Shh expression in the developing diencephalon (see Fig. 17.8A ). Subsequently, Shh signaling from the diencephalon activates the expression of Shh in the ectoderm of the frontonasal prominence and creates a unique zone called the frontonasal ectodermal zone (FEZ; see later “In the Research Lab” entitled “Patterning of Facial Prominences”), which is required for proximodistal outgrowth and dorsoventral patterning of the upper face (see Fig. 17.8A ). Application of Shh-blocking antibodies in the diencephalon or frontonasal prominence of developing chick embryos results in hypotelorism, showing that both domains of Shh expression are needed for appropriate midline development.
HPE is due to haploinsufficiency (i.e., the presence of one mutated gene and one wild-type allele) and two aspects of the syndrome should be emphasized. First, mice with heterozygous deletion of Shh do not show a phenotype. Second, there is extreme phenotypic variability from cyclopia to normality between individuals carrying the same gene mutation. Treatment of developing chick embryos with cyclopamine, an inhibitor of hedgehog signaling, has shown that loss of Shh function earlier in development (e.g., in the prechordal plate) has a more severe effect than loss of Shh function later in development (e.g., in the facial ectoderm). This could, in part, explain the spectrum of phenotypes observed as a result of Shh mutations and why phenotypes are observed in humans with one copy of mutated Shh. An environmental insult, such as in utero exposure to alcohol or retinoic acid, would downregulate the expression of the wild-type allele of Shh, further decreasing the levels of Shh. An early environmental insult would have the most severe consequences, as this would affect both the forebrain and subsequently the facial prominence, whereas later insults would affect only the facial structures, thereby resulting in milder forms of HPE.
Mutations in silent modifier genes may also determine the phenotypic spectrum of particular Shh mutations. The severity of the HPE defect would be dependent on when and where these silent modifier genes are expressed. Modifier genes would include components or regulators of the Shh pathway, such as the cell-surface molecules Gas1 and Cdo, which are expressed at sites distant to the source of Shh and are thought to increase levels of Shh signaling.
Mutations in TGIF, ZIC2, and SIX3 also result in loss of Shh signaling, explaining why their loss of function causes HPE. Tgif and Zic2 are required for development of the prechordal plate. Therefore, mutations in these factors results in early loss of Shh signaling. Six3 directly regulates Shh expression in the forebrain and, hence, affects later stages of midline patterning. Conversely, gain of function of Shh signaling results in expansion of the midline. Misexpression of Shh in the diencephalon or the ectoderm of the frontonasal prominence in chick embryos results in an expansion of the midline. Hence, it is believed that the levels of Shh signaling may define a spectrum of facial disorders from cyclopia to diprosopus—the duplication of facial structures (see Fig. 17.8D ).
![]()
Animations are available online at StudentConsult.
The pharyngeal arches evolved from the gill arches of jawless fish and have been evolutionarily conserved. These arches form during the embryogenesis of all vertebrates, and based on evolutionary comparisons, all arches have historically been proposed to give rise to a cartilaginous element. Whether this is correct for the caudal arches (4 and 5) in mammals is currently unclear, as these arches are remodeled before the formation of cartilage. In jawed vertebrates, the first arch gives rise to the lower jaw and part of the upper jaw. The remaining arches form the gills in modern fish and many structures of the face and neck in humans. Historically and in previous literature, the five pharyngeal arches in humans have been numbered 1 to 4 and 6; now pharyngeal arch 6 is numbered 5.
Human embryos include four pairs of well-defined pharyngeal arches numbered 1, 2, 3, and 4. Evidence for the presence of a true fifth arch is questioned, as it cannot be readily seen externally ( Fig. 17.9 ). Like so many other structures in the body, the pharyngeal arches form in craniocaudal succession: the first arch forms on day 22; the second and third arches form sequentially on day 24; and the fourth and fifth arches form sequentially on day 29.
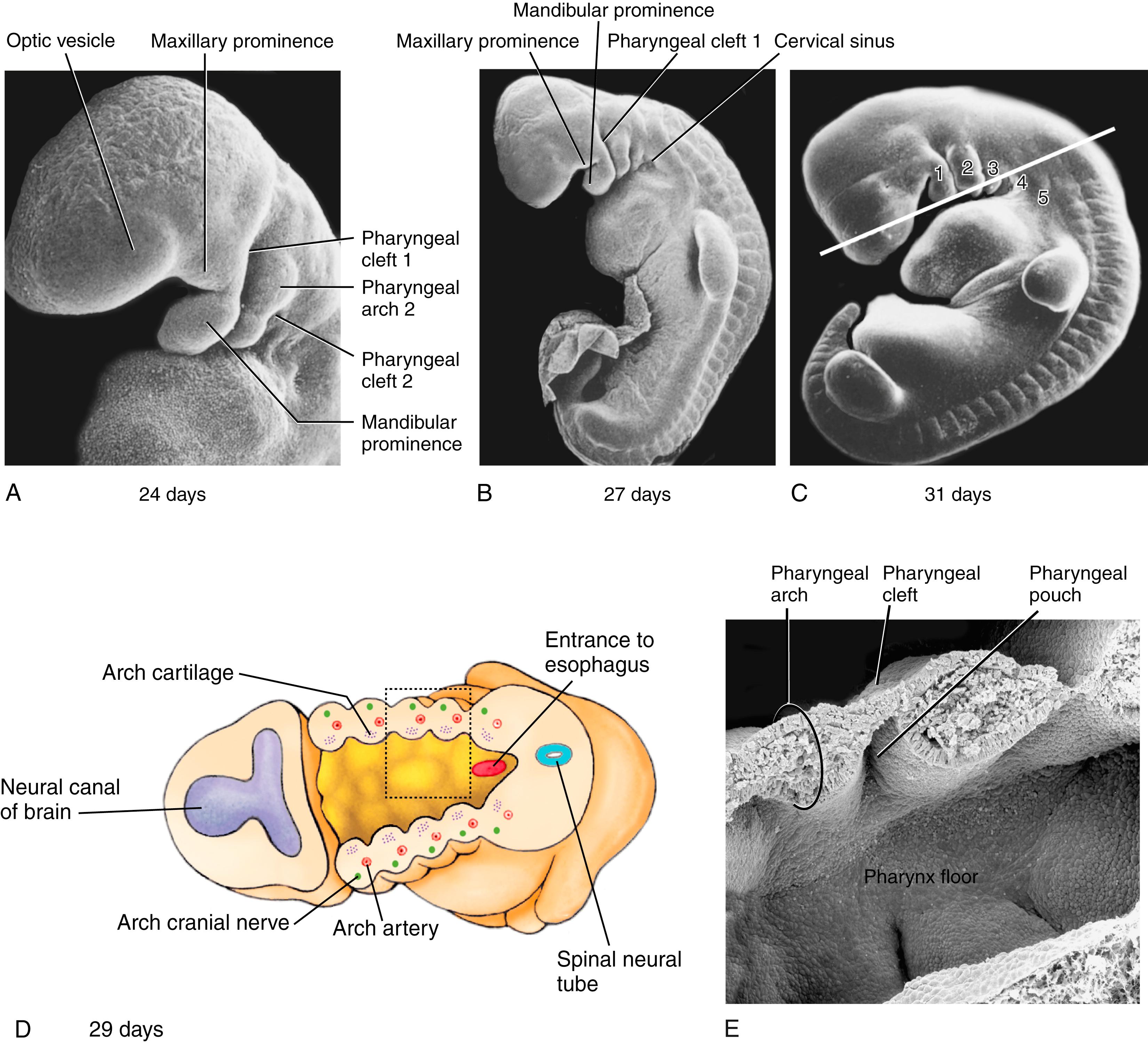
Pharyngeal arches consist of a mesenchymal core (mesoderm and neural crest cells) that is covered on the outside with ectoderm and lined on the inside with endoderm (see Fig. 17.9D,E ). Each arch contains (1) striated muscle rudiments (derived from head mesoderm) innervated by an arch-specific cranial nerve, and (2) an aortic arch artery (covered in Chapter 13 ). Additionally, pharyngeal arches contain or give rise to (after pharyngeal arch remodeling) (3) a cartilaginous skeletal element.
The pharyngeal arches of human embryos initially resemble the gill arches of fish, except that they never become perforated to form gill slits. Instead, the external pharyngeal clefts or grooves between the arches remain separated from the apposed, internal pharyngeal pouches by thin pharyngeal membranes . These membranes are initially two-layered, consisting of ectoderm and endoderm; they are later infiltrated by mesenchymal cells.
The cartilages that develop from the pharyngeal arches arise from neural crest cells originating from the midbrain and hindbrain regions (arches 1 to 4) and cranial mesoderm (arches 4 and 5). As covered in Chapter 4 , neural crest cells arise from the neural folds and migrate ventrolaterally; cranial neural crest cells are multipotent and chondrocytes are one of their potential lineages.
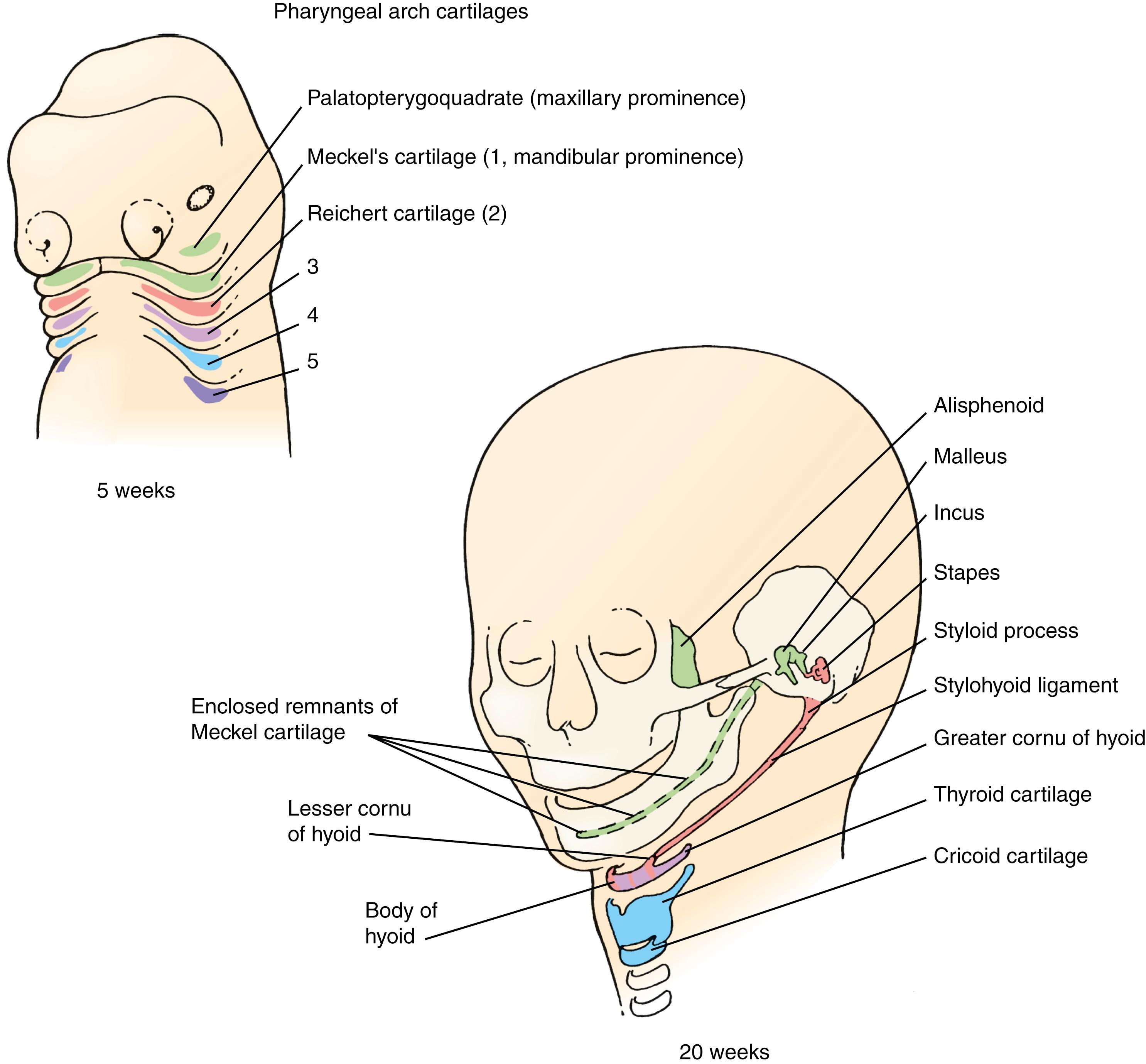
Fig. 17.10 and Table 17.1 illustrate and summarize, respectively, the skeletal elements derived from the pharyngeal arch cartilages.
| Pharyngeal Arch | Arch Artery a | Skeletal Elements | Muscles | Cranial Nerve b |
|---|---|---|---|---|
| 1 | Terminal branch of maxillary artery | Derived from arch cartilages (originating from neural crest cells): from maxillary cartilage: alisphenoid, incus From Meckel’s cartilage: malleus Derived by direct ossification from arch dermal mesenchyme: maxilla, zygomatic, squamous portion of temporal bone, mandible (originate from neural crest cells) |
Muscles of mastication (temporalis, masseter, medial and lateral pterygoids), mylohyoid, anterior belly of the digastric, tensor tympani, tensor veli palatini (originate from head mesoderm) | Maxillary and mandibular divisions of trigeminal nerve (V) |
| 2 | Stapedial artery (embryonic), caroticotympanic artery (adult) | Stapes, styloid process, lesser horns and part of body of hyoid (derived from the second-arch [Reichert’s] cartilage; originate from neural crest cells) | Muscles of facial expression (orbicularis oculi, orbicularis oris, risorius, platysma, auricularis, frontalis, and buccinator), posterior belly of the digastric, stylohyoid, stapedius (originate from head mesoderm) | Facial nerve (VII) |
| 3 | Common carotid artery, root of internal carotid | Lower rim and part of body of hyoid (derived from the third-arch cartilage; originate from neural crest) | Stylopharyngeus (originates from head mesoderm) | Glossopharyngeal nerve (IX) |
| 4 | Arch of aorta (left side), right subclavian artery (right side);original sprouts of pulmonary arteries | Thyroid and epiglottal laryngeal cartilages (derived from neural crest cells and mesoderm) | Constrictors of pharynx, cricothyroid, levator veli palatini (originate from occipital somites and head mesoderm) | Superior laryngeal branch of vagus nerve (X) |
| 5 | Ductus arteriosus (left side); roots of definitive pulmonary arteries | Remaining laryngeal cartilages (derived from mesoderm) | Intrinsic muscles of larynx (originate from head mesoderm) | Recurrent laryngeal branch of vagus nerve (X) |
a Aortic arch artery development is covered in Chapter 13 .
b Cranial nerve development is covered in Chapter 10 .
The first pharyngeal arch has two pairs of prominences associated with it: the mandibular and maxillary prominences (or swellings ; see Fig. 17.9A–C ), which give rise to the lower jaw and part of the upper jaw, respectively. Although the maxillary prominences were long thought to develop from branching of the first pharyngeal arches, it is now known that the maxillary prominences arise from mesenchyme cranial to the first arch. Each pair of maxillary and mandibular prominences contains a transient central cartilaginous element. The central cartilage of each maxillary prominence is the palatopterygoquadrate bar , and the central cartilage of each mandibular prominence is Meckel’s cartilage (see Fig. 17.10 ). These cartilages arise between days 41 and 45. Both the maxillary and mandibular prominences are formed largely from neural crest cells that migrate from the neural folds of the midbrain (mesencephalon) and the cranial hindbrain (metencephalon) (covered later in this chapter).
Most of Meckel’s cartilage disappears, being resorbed or becoming encapsulated by the developing mandibular bone. However, the proximal part forms the malleus, sphenomandibular ligament , and anterior ligament of the malleus (see Fig. 17.10 ). The maxillary cartilage (palatopterygoquadrate bar) forms the incus and contributes to a small bone, called the alisphenoid , located in the orbital wall (see Fig. 17.10 ; see also Figs. 17.2A, 17.5 ). These derivatives become surrounded by the maxilla, zygomatic, and squamous portions of the temporal bones, which together with the mandible are all membrane bones. Therefore, most of the facial skeletal structures are derived from bones of dermal origin.
The second pharyngeal arch cartilage forms from neural crest cells that migrate from neural folds at the level of rhombomere 4 of the hindbrain (rhombomeres are covered further in Chapter 9 ; see Fig. 17.14 ). After the jaws evolved, the second-arch cartilages were recruited as bracing elements to help support the jaws and attach them to the neurocranium. The human second-arch cartilage, which is called Reichert’s cartilage , arises between days 45 and 48. This arch will ultimately form the stapes of the middle ear, the styloid process of the temporal bone, the fibrous stylohyoid ligament , and the lesser horns ( cornua ) and part of the body of the hyoid bone (its midline and the regions that articulate with the greater horns; see Fig. 17.10 ). The hyoid bone is stabilized by muscle attachments to the styloid process and mandible; through its muscular attachments to the larynx and the tongue, it functions in both swallowing and vocalization.
The third pharyngeal arch cartilage is formed from neural crest cells that migrate from the caudal hindbrain (myelencephalon). Ossification of this cartilage occurs endochondrally to form the greater horns (cornua) and part of the body of the hyoid bone (see Fig. 17.10 ).
Based on evolutionary comparisons that all pharyngeal arches of more primitive vertebrates contain a cartilaginous element, the fourth and fifth pharyngeal arches have been proposed to give rise to the larynx , consisting of the thyroid, cuneiform, corniculate, arytenoid , and cricoid cartilages (see Fig. 17.10 ). The thyroid cartilage is derived from the fourth-arch neural crest and mesoderm. The epiglottal cartilages do not form until the fifth month, long after the other pharyngeal arch cartilages have formed. These cartilages also arise from the fourth-arch neural crest. The remaining cartilages of the larynx arise from unsegmented cranial mesoderm.
In all jawed vertebrates except mammals, the jaw joint is formed from endochondral bones that develop from the maxillary and mandibular cartilages, even though other portions of the jaw may be formed from membrane bones. However, among the immediate ancestors of mammals, a second, novel jaw articulation developed between two membrane bones: the temporal and mandible. As this new temporomandibular joint (TMJ) became dominant, the bones of the ancient endochondral jaw articulation shifted into the adjacent middle ear and joined with the preexisting stapes to form the unique three-ossicle auditory mechanism of mammals.
The components and cavities of the TMJ are established by week 14 of gestation. The TMJ consists of a synovial joint between the mandibular condyle and the glenoid blastema (associated with the temporal bone), which are separated by an interarticular disc . The joint forms from week 9, starting with development of the condylar process on the mandible. One week later, the condylar cartilage has formed and the blastema of the temporal bones has started to develop. At this time, the condylar cartilage and temporal bone are separated by the condensation of the interarticular disc. Cavitation starts at week 10 in two waves: first between the condylar process and interarticular disc, forming the inferior joint space; and then (1 week later) between the disc and temporal bone, forming the superior joint space .
The condylar cartilage is distinct from endochondral cartilages in that it arises within the periosteum of a membrane bone. It is one of several cartilages, termed secondary cartilages , to develop in this way during facial growth. Secondary cartilages have unique properties, including that they grow in response to mechanical stimulation. Unlike the other facial secondary cartilages, the condylar cartilage persists postnatally and plays a significant role in postnatal growth of the lower jaw.
As covered in detail in Chapter 13 , the aortic arch artery system initially takes the form of a basket-like arrangement of five pairs of arteries that arise from the expansion at the end of the outflow tract called the aortic sac . These arteries connect the paired ventral aortae with the paired dorsal aortae ( Fig. 17.11 ). This system is remodeled to produce the great arteries of the thorax and the branches that supply the head and neck (illustrated in Fig. 17.11 and summarized in Table 17.1 ; see Chapter 13 for details of this remodeling).
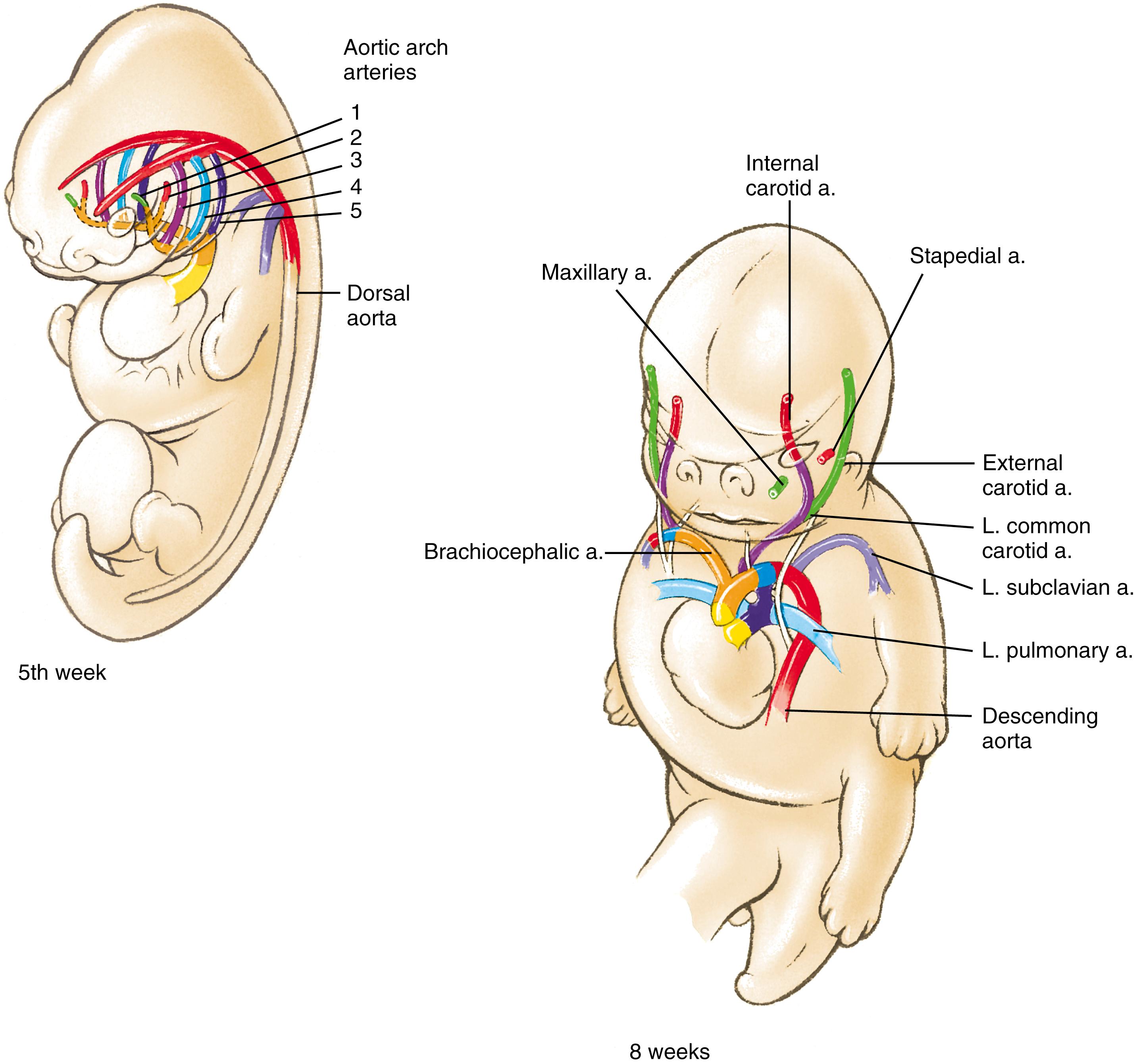
As covered in Chapter 13 , arterial blood reaches the head via paired vertebral arteries that form from anastomoses among intersegmental arteries and via the common carotid arteries . The common carotid arteries branch to form the internal and external carotid arteries . The internal carotid and vertebral arteries supply the brain, and the external carotid arteries supply the face. The common carotids and the roots of the internal carotids are derived from the third-arch arteries, whereas distal portions of the internal carotids are derived from the cranial extensions of the paired dorsal aortae. The external carotid arteries sprout de novo from the common carotids. The endothelium of the head vasculature and aortic arch arteries is derived from mesoderm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here