Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As complex as the human nervous system is, it starts out embryonically as a simple, tubular, ectodermal structure. An understanding of the development of the nervous system helps make sense of its adult configuration and organization. Similarly, congenital malformations of the central nervous system (CNS) are more easily understood in light of its embryological development; such malformations provide clues that aid in the understanding of normal development.
The focus in this chapter is on the events that lead to the formation of the CNS and the configuration of its major components. There is clearly much more to building a nervous system than this—neurons must proliferate in enormous numbers, migrate from their places of birth to their final destinations, and establish appropriate connections with other neurons. Some of these aspects are touched on in Chapter 24 .
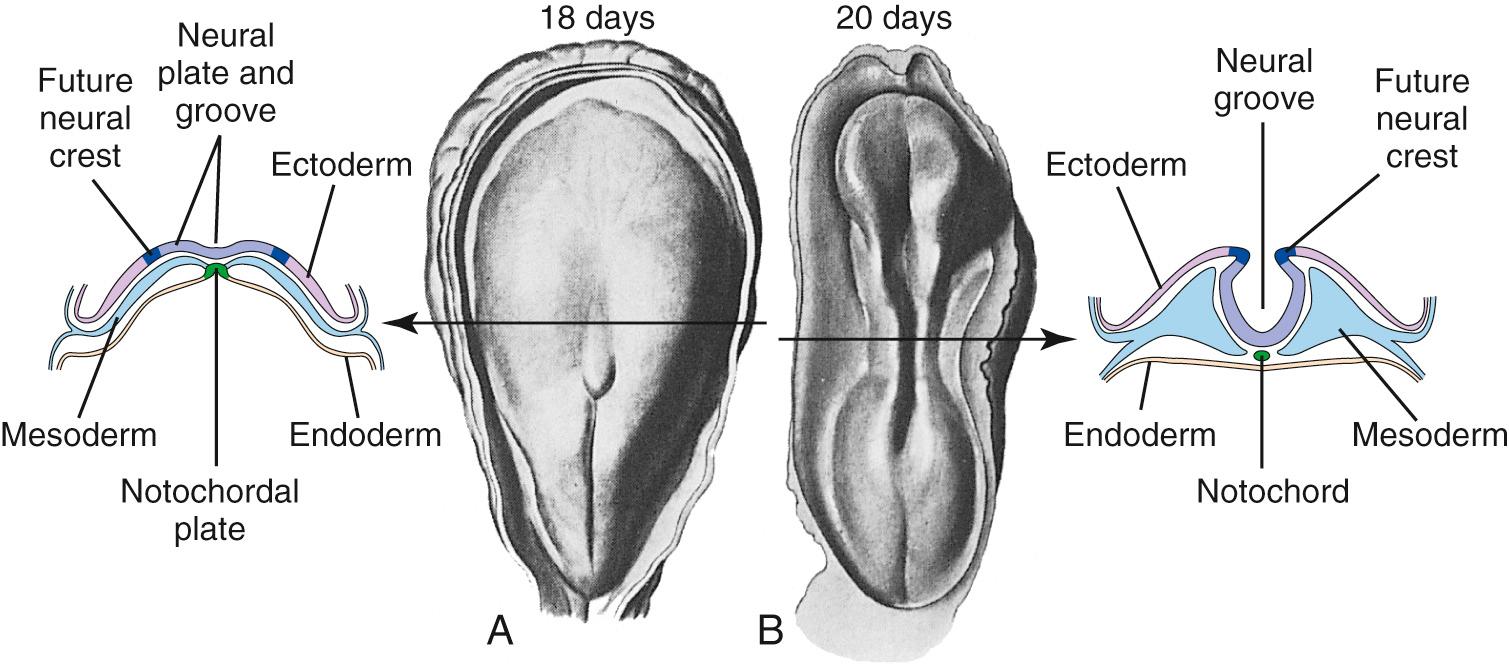
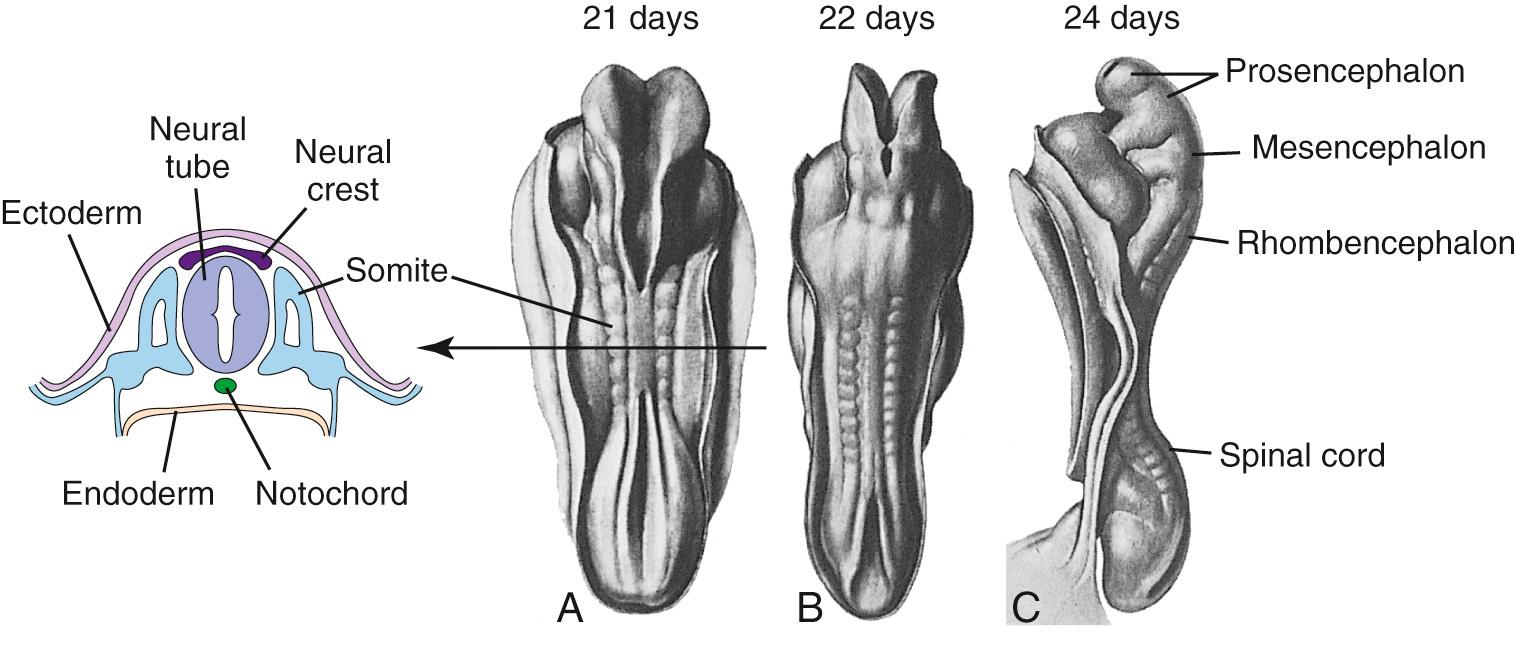
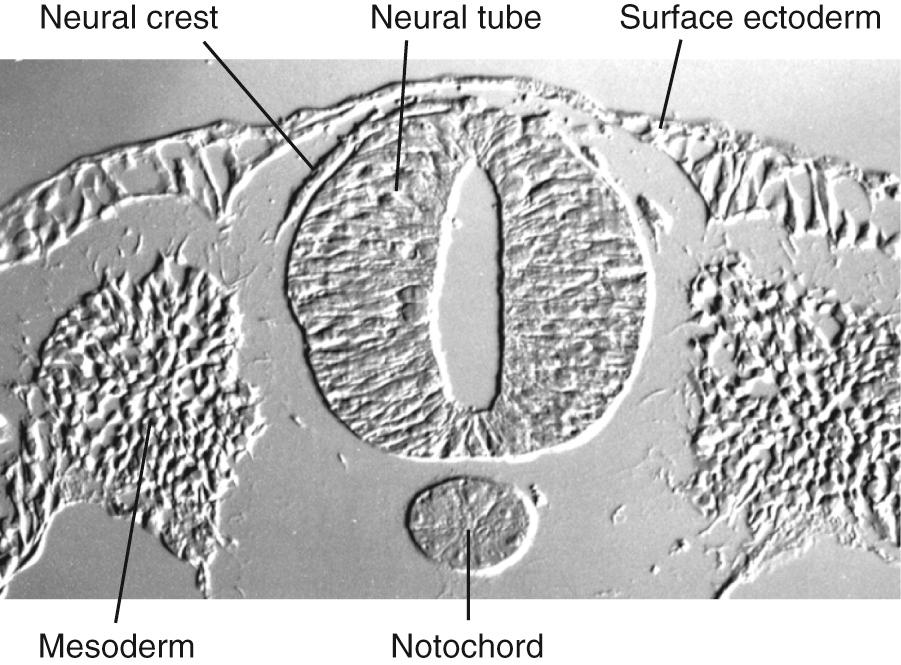
During the third week of embryonic development, in response to chemical signals released by the underlying midline mesoderm, a longitudinal band of ectoderm thickens to form the neural plate. Shortly thereafter, the neural plate begins to fold inward, forming a longitudinal neural groove in the midline flanked by a parallel neural fold on each side ( Figs. 2.1 and 2.2 ). The neural groove deepens, and the neural folds approach each other in the dorsal midline. At the beginning of the fourth week, the two folds begin to fuse midway along the neural groove at a level corresponding to the future cervical spinal cord, starting the formation of the neural tube ( Fig. 2.3 ), the open ends of which are the cranial and caudal neuropores. Fusion proceeds cranially and caudally, and the entire neural tube is closed by the end of the fourth week. This process is referred to as primary neurulation. As the neural tube closes, it progressively separates from the ectodermal (i.e., skin) surface, leaving behind groups of cells from the crest of each neural fold ( Fig. 2.4 ). These neural crest cells develop into a variety of cell types (see Fig. 2.19 ), including the peripheral nervous system (PNS). The neural tube, on the other hand, develops into almost the entire CNS; its cavity becomes the ventricular system of the brain and the central canal of the spinal cord.
The sacral spinal cord forms by a slightly different mechanism. After the neural tube closes, a secondary cavity extends into the solid mass of cells at its caudal end during the fifth and sixth weeks, in a process of secondary neurulation.
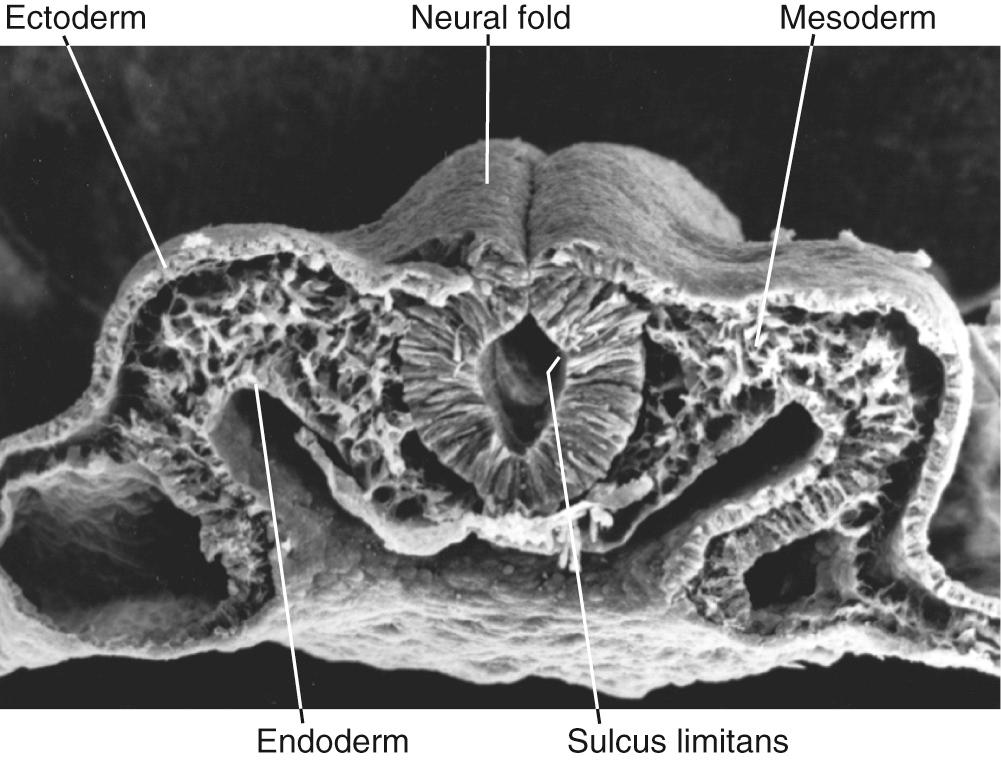
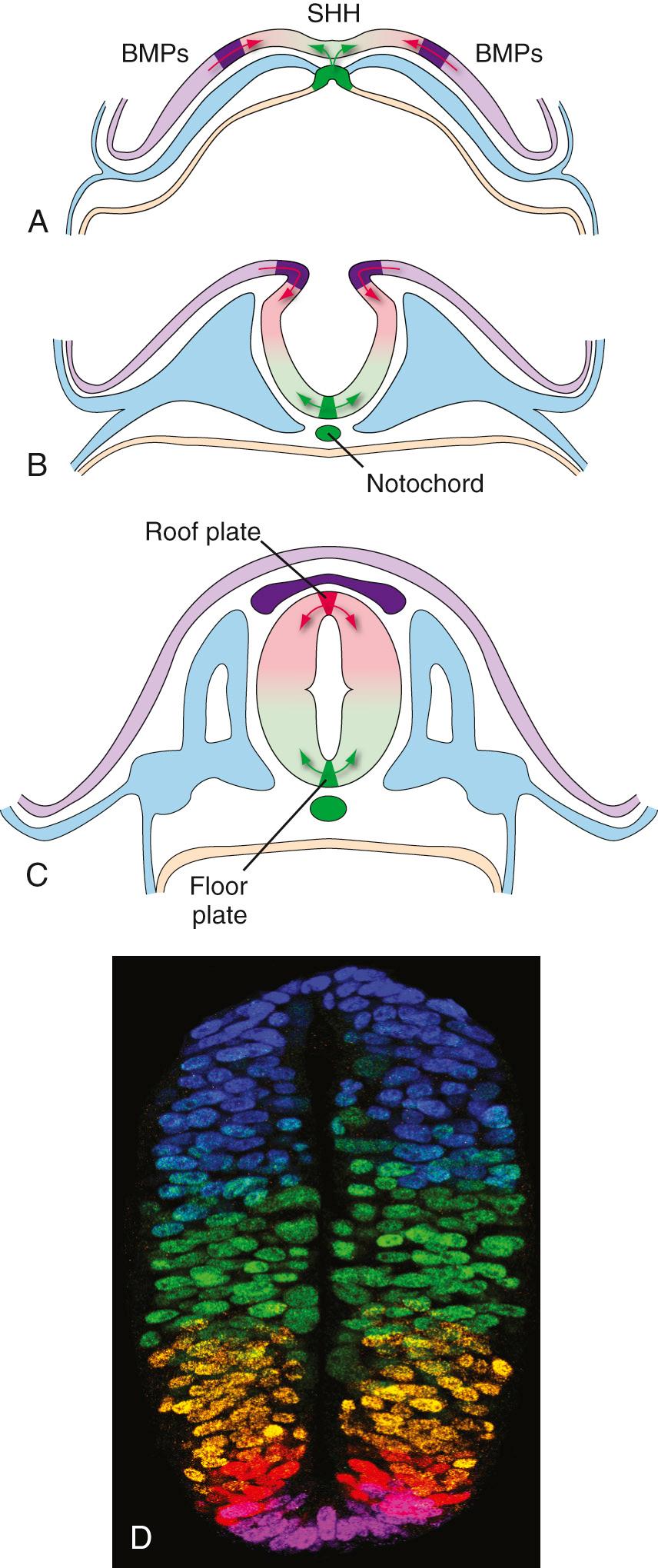
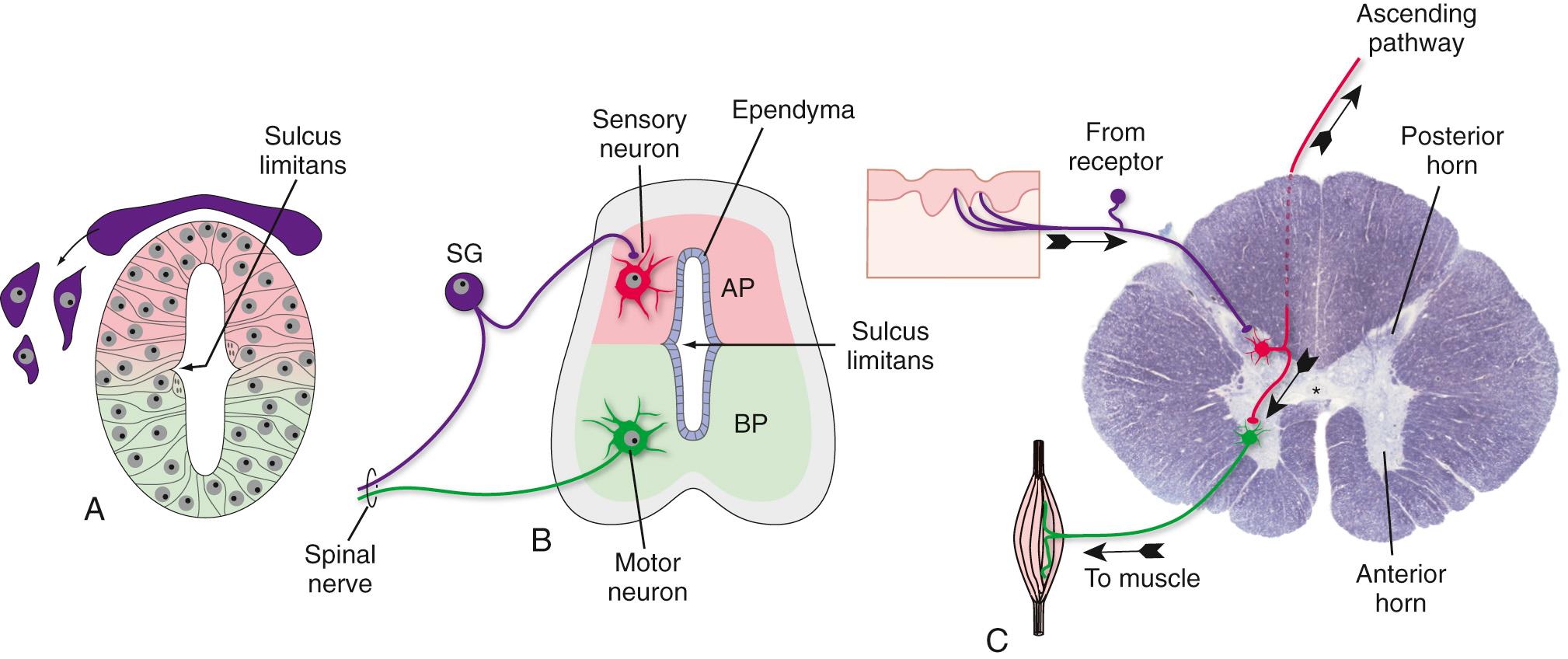
A recurring theme in neural development is the creation of concentration gradients of various signaling molecules, which in turn guide the development of different cell types or the growth of neuronal processes; one prominent example is dorsal-ventral patterning in the spinal cord and brainstem. The ectoderm near what will become the dorsal surface of the neural tube and the mesodermal notochord near the ventral surface produce different signaling molecules early in development. The opposing concentration gradients of these signaling molecules induce distinctive patterns of subsequent development in these two regions of the neural tube ( Fig. 2.5 ). This becomes morphologically apparent during the fourth week, when a longitudinal groove (the sulcus limitans ) appears in the lateral wall of the neural tube, separating it into a dorsal half and a ventral half throughout the future spinal cord and brainstem. The gray matter of the dorsal half forms an alar plate and that of the ventral half a basal plate ( Fig. 2.6 ; see also Fig. 2.2 ). This is a distinction of great functional importance because alar plate derivatives are primarily concerned with sensory processing, whereas motor neurons are located in basal plate derivatives. In the adult spinal cord, even though the sulcus limitans is no longer apparent, the central gray matter can be divided into a posterior (dorsal) horn and an anterior (ventral) horn on each side (see Fig. 2.6C ). The central processes of sensory neurons (derived from neural crest cells) end mainly in the posterior horn, which contains cells whose axons form ascending sensory pathways. In contrast, the anterior horn contains the cell bodies of somatic and autonomic motor neurons, whose axons leave the spinal cord and innervate skeletal muscles and autonomic ganglia. The same distinction between sensory alar plate derivatives and motor basal plate derivatives holds true in the brainstem, as discussed briefly in this chapter and in more detail in Chapter 12 . (The sulcus limitans probably does not extend beyond the brainstem in any meaningful way, although concentration gradients of various signaling molecules induce other distinctive dorsal/ventral patterns of development in the forebrain. Hence the alar/basal plate distinction is not useful for forebrain structures, even though some deal primarily with sensory processes and others with motor processes.)
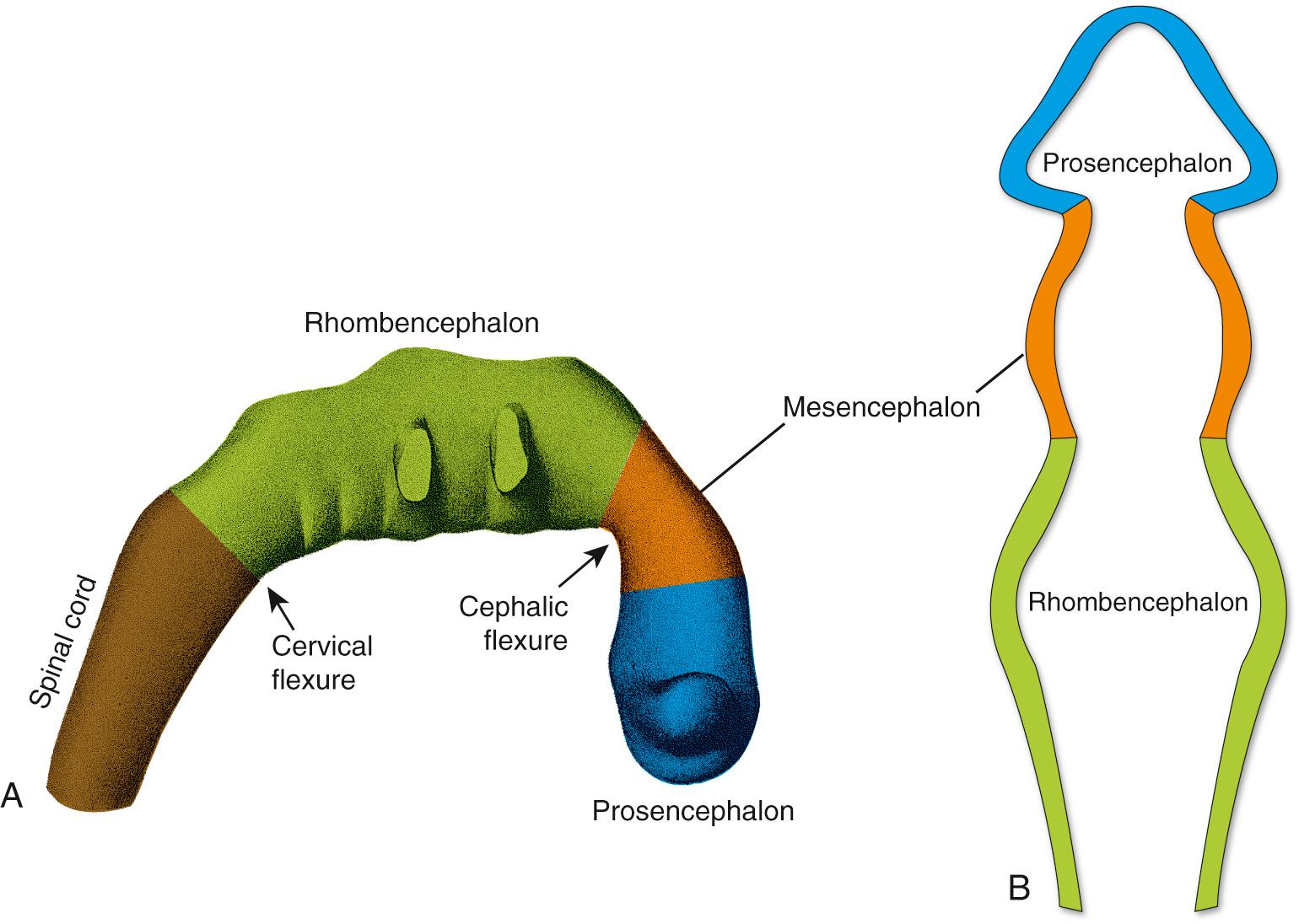
Similar strategies, involving concentration gradients of signaling molecules, come into play in specifying the longitudinal development of the neural tube. The neural tube is never a simple, straight cylinder. Even before it has completely closed, bulges begin to appear in the rostral end of the neural tube in the region of the future brain, and bends begin to appear as well (see Fig. 2.3C ).
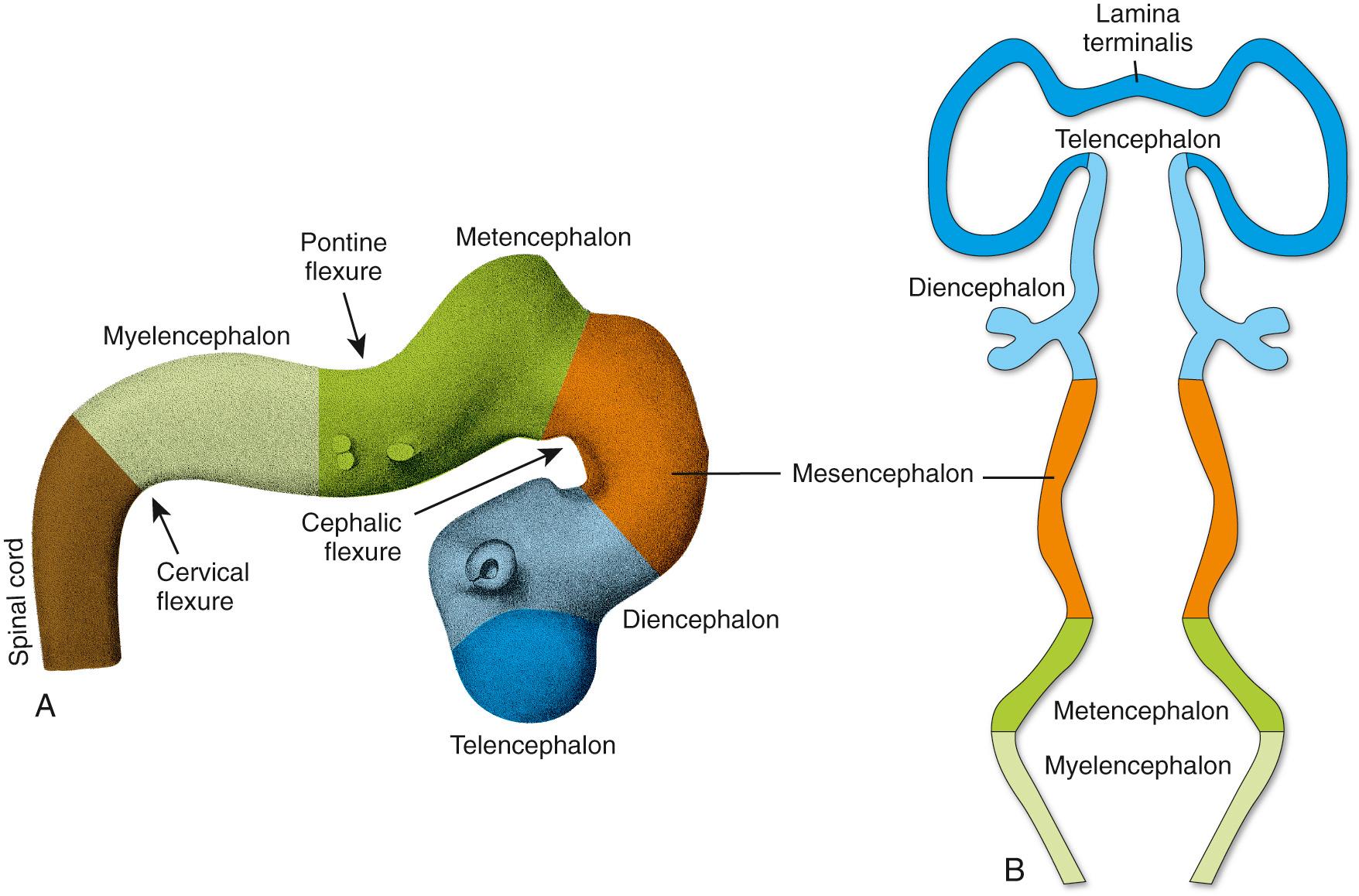
During the fourth week, three bulges, or vesicles, are apparent and are referred to as the primary vesicles ( Fig. 2.7 ). From rostral to caudal, these are the prosencephalon (Greek for “front brain,” or forebrain), the mesencephalon (Greek for “midbrain”), and the rhombencephalon (named for its rhomboidal shape—see Figs. 2.7B and 2.8B ), which merges smoothly with the caudal (spinal) portion of the neural tube. The prosencephalon develops into the forebrain. The mesencephalon becomes the midbrain of the adult brainstem, and the rhombencephalon (sometimes referred to as the hindbrain ) becomes the rest of the brainstem and the cerebellum ( Table 2.1 ).
| Primary Vesicle | Secondary Vesicle | Neural Derivatives | Cavity |
|---|---|---|---|
| Prosencephalon (forebrain) | Telencephalon Diencephalon |
Cerebral hemispheres Thalamus, hypothalamus, retina, other structures |
Lateral ventricles Third ventricle |
| Mesencephalon | Mesencephalon | Midbrain | Cerebral aqueduct |
| Rhombencephalon (hindbrain) | Metencephalon Myelencephalon |
Pons, cerebellum Medulla |
Part of fourth ventricle Part of fourth ventricle, part of central canal |
The three primary vesicles are not arranged in a straight line but rather are associated with two bends or flexures in the neural tube (see Fig. 2.7A ). One of these, the cervical flexure, occurs between the rhombencephalon and the spinal cord but straightens out later in development, thus rendering it of little significance in the adult. The second, the cephalic (or mesencephalic ) flexure, occurs at the level of the future midbrain and persists in the adult as an 80- to 90-degree bend between the axes of the brainstem and the forebrain (see Fig. 3.1 ). The cephalic flexure is not present in most vertebrates but exists in humans and other animals that walk on two legs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here