Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Development of bone and muscle occurs within mesenchymal regions of the embryo after the tube-within-a-tube body plan is established during the fourth week of gestation. Bone formation occurs in two ways. During endochondral ossification , a cartilage model first forms and is eventually replaced with bone. This type of ossification underlies formation of the axial skeleton (vertebral column, ribs, and sternum), cranial base, and appendicular (limb) skeleton, with the exception of part of the clavicles. During intramembranous ossification , bone forms directly from mesenchymal cells without the prior formation of cartilage. This type of ossification underlies formation of the cranial vault and most of the bones of the face.
Three types of cells act in endochondral bone development: chondrocytes, osteoblasts , and osteoclasts . The former two function in secreting cartilage and bone matrix, respectively, whereas the latter is involved in bone resorption. Only the latter two cell types act in intramembranous ossification.
Three types of muscles form in the embryo: skeletal, smooth, and cardiac. Skeletal, or voluntary, muscle—the focus of this chapter—develops in association with bone as part of the musculoskeletal system. Smooth muscle develops in association with formation of the walls of the viscera, blood vessels, and glands. Cardiac muscle develops only in the heart. Development of smooth and cardiac muscle is discussed in relation to development of the gut tube, urinary system, and genital system (covered in Chapter 14, Chapter 15, Chapter 16 , respectively) and in relation to the heart (covered in Chapter 12 ).
Muscle development occurs in the embryo through the formation of myoblasts , which undergo extensive proliferation to form terminally differentiated, postmitotic myocytes . Myocytes express actin, myosin, and other contractile proteins and fuse to form contractile myofibers . Striated muscle development involves both prenatal and postnatal events: primary myogenesis (occurs during the stage of the embryo) and secondary myogenesis (occurs during the stage of the fetus) lay down the muscular system, and satellite cells act in muscle growth postnatally and in response to exercise or muscle damage.
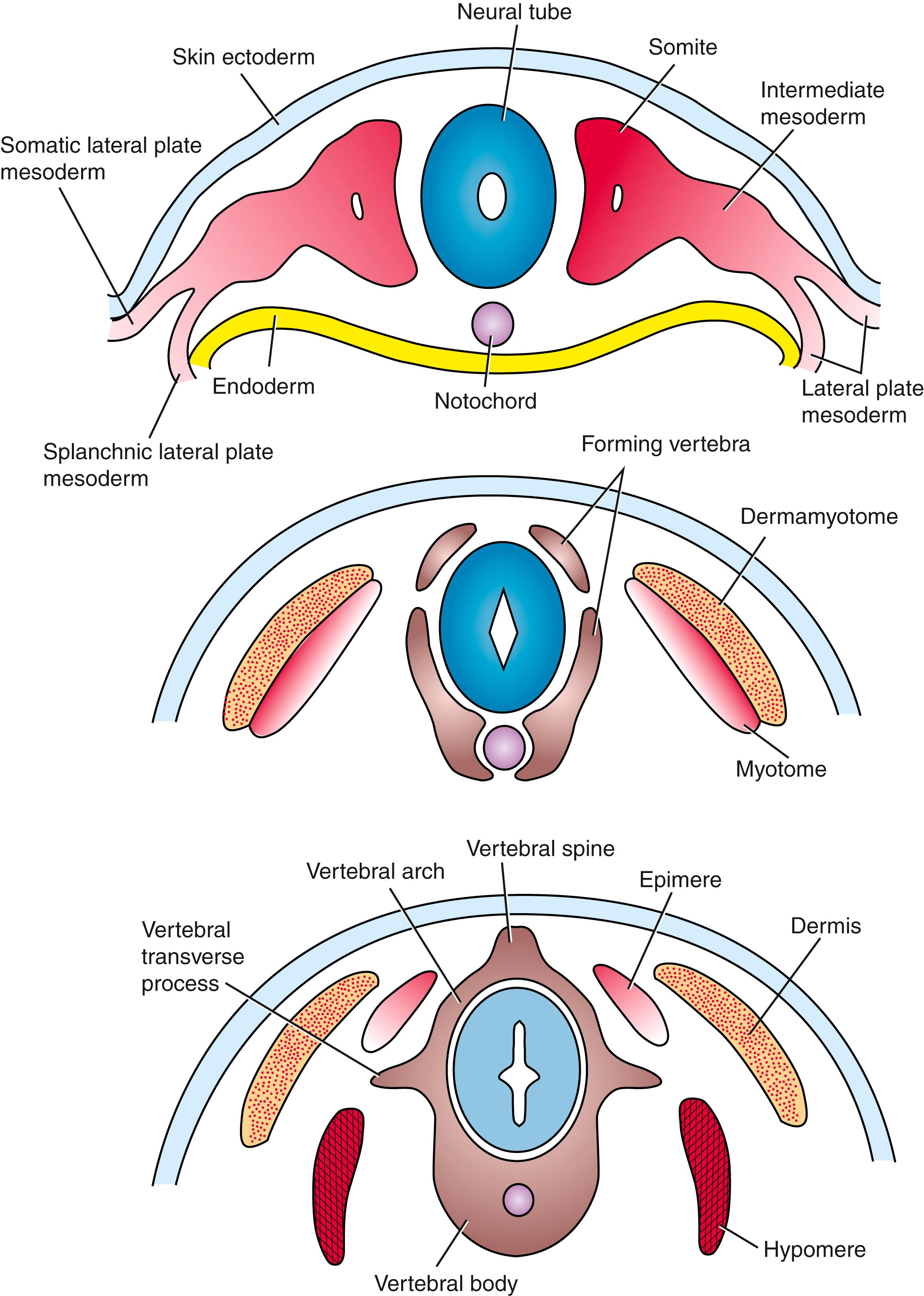
The muscles and bones of the trunk derive from the somites . Each somite initially forms two distinct zones: a sclerotome and a dermamyotome . The former gives rise to the bones of the axial skeleton. The latter forms the dermis of the back skin of the trunk and neck (with the remainder of the dermis of these regions forming from lateral plate mesoderm ), as well as the myotome , which forms the muscles of the trunk. The dermamyotome also gives rise to all the musculature of the limbs (covered in Chapter 20 ) and some of the tongue musculature (covered in Chapter 17 ). The syndetome , containing the progenitor of the tendons, develops between the myotome and the sclerotome. The lateral plate mesoderm forms the sternum and the bones of the pelvic girdle and limbs (covered in Chapter 20 ), and it contributes to the dermis of the trunk. As covered in Chapter 17 , the bones of the face and larynx arise from neural crest cells , as does most of the dermis of the head, whereas the facial, masticatory, and laryngeal muscles arise from unsegmented paraxial (called head or cranial ) mesoderm. The bones of the cranial vault and cranial base are formed from segmented paraxial mesoderm (e.g., exoccipital bone), unsegmented paraxial mesoderm (e.g., parietal bone), or neural crest (e.g., frontal bone).
Shortly after formation of the somitic myotome, the myotome splits into a dorsal epimere and a ventral hypomere . The epimere forms the deep epaxial muscles of the back, which are innervated by the dorsal ramus of the spinal nerve. In contrast, the hypomere forms the hypaxial muscles of the lateral and ventral body wall in the thorax and abdomen, which are innervated by the ventral ramus of the spinal nerve. Therefore, like all skeletal muscles, the innervation of these muscles (covered further in Chapter 10 ) reflects their embryonic origin.
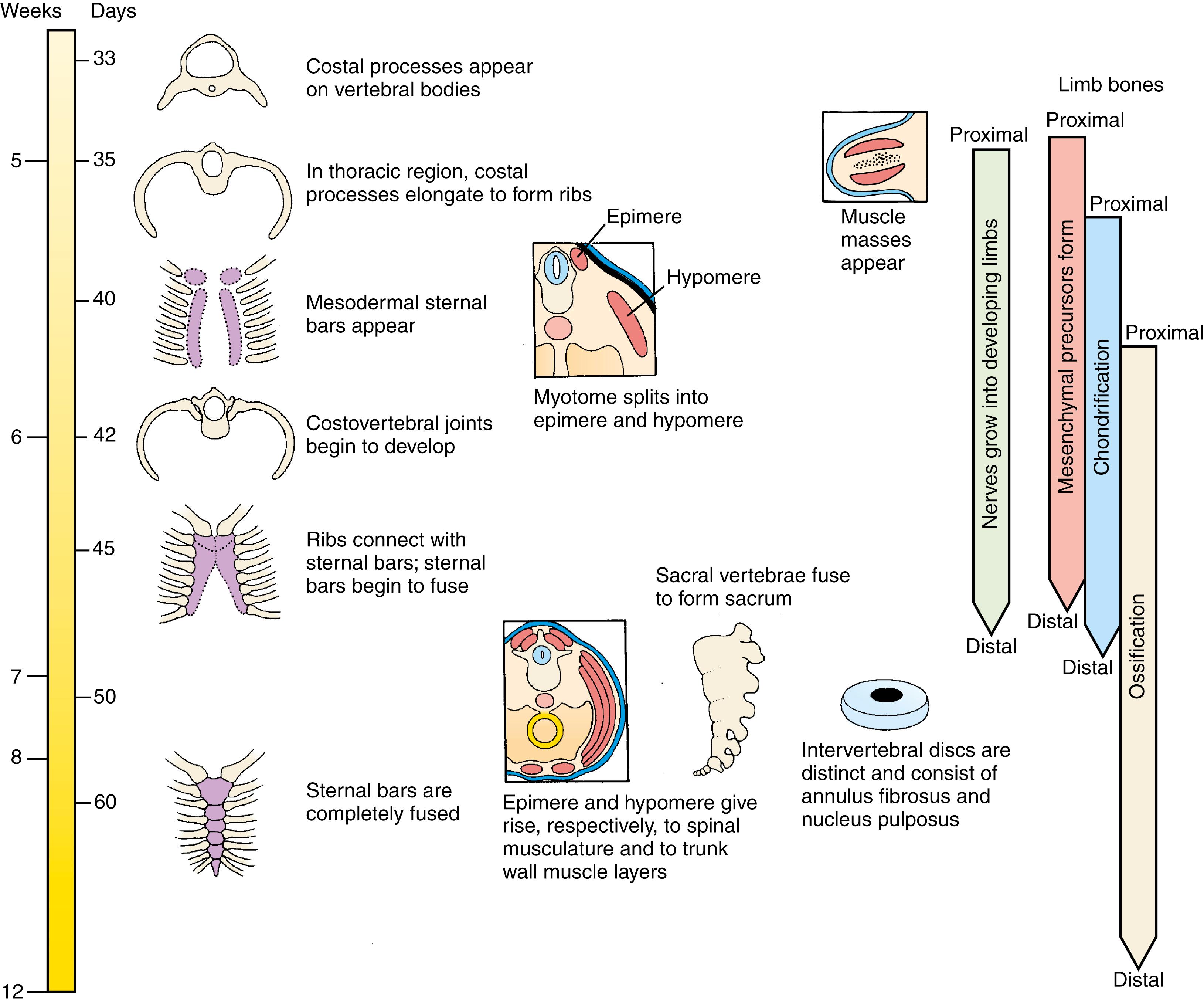
Formation of the vertebral column involves the process of resegmentation of the sclerotomes of the somites. During resegmentation, the sclerotome of each somite (except for occipital somites one to four) subdivides into cranial and caudal segments, each of which fuses, respectively, with the adjacent caudal or cranial segment. Resegmentation allows motor axons and dorsal root ganglia to lie between vertebrae, rather than running through them. In contrast, skeletal muscles keep their original segmental arrangement, connecting the two adjacent vertebrae and allowing movement. In contrast, occipital somites one to four do not undergo resegmentation and fuse (together with the cranial part of cervical somite one) to generate the exoccipital bone.
A newlywed couple, both divorcees with previous children, decides to have a child together. The woman becomes pregnant within a few months of trying and, because of her “advanced maternal age” of 38, her obstetrician recommends non-invasive prenatal screening (NIPS) to rule out trisomy. This screen returns normal and the pregnancy progressed normally except for an ultrasound at 20 weeks that shows that the length of the long bones is less than normal. Otherwise, the pregnancy progresses normally.
The couple delivers a healthy girl at 39 weeks of gestational age without complications. Over the next few months, the family becomes increasingly concerned that their daughter seems to have short arms and legs and bears little resemblance to either parent. The girl is referred to the genetics clinic and is noted to have rhizomelia (shortening of the proximal limbs), short fingers, a large head, and a flat nasal bridge ( Fig. 8.1 ). X-rays confirm the diagnosis of achondroplasia . The parents are told that their daughter’s adult height will be around 4 feet. They are reassured somewhat when they learn that she should have normal intelligence and a normal life expectancy.

Achondroplasia, a Greek word that means “without cartilage formation,” is the most common and most recognizable form of dwarfism. It is caused by mutations in the FIBROBLAST GROWTH FACTOR RECEPTOR 3 (FGFR3) gene. In contrast to aneuploidy syndromes, like trisomy 18, in which advanced maternal age increases incidence, achondroplasia is associated with advanced paternal age, with 80% of cases resulting from new mutations in the FGFR3 gene. See later “In the Clinic” entitled “Defects in Skeletal Development,” and “In the Research Lab” entitled “Molecular Regulation of Bone and Joint Development,” for further coverage.
There are two types of bones in the body: those that develop via endochondral ossification and those that develop via intramembranous ossification . During endochondral bone development, formation of a cartilaginous template precedes ossification. This pathway of differentiation is used by all axial (vertebral column, sternum, and ribs) and appendicular (limb) bones of the body, with the exception of part of the clavicle. The cranial base, sensory capsules, and pharyngeal arch cartilages also form via endochondral ossification (covered in Chapter 17 ). Cartilage can grow rapidly in the embryo and postnatally in the growth plates. In the adult, cartilage persists in regions of load (e.g., articular cartilage) or flexibility (e.g., laryngeal cartilages). Rather than forming from a cartilaginous template, bones may develop by intramembranous ossification, that is, directly from the mesenchyme. Intramembranous bones typify most bones of the face and cranial vault and are called dermal or membrane bones .
Endochondral bones are formed by three cell types: chondrocytes (cartilage cells), osteoblasts (bone-forming cells), and osteoclasts (bone-resorbing cells). Chondrocytes have three tissue origins: the paraxial mesoderm forms the axial skeleton, including the exoccipital portion of the cranial base; the lateral plate mesoderm forms the appendicular skeleton, pelvic girdle, and sternum; and the neural crest cells (i.e., ectodermal cells) give rise to the cartilaginous elements in the face and larynx. Osteoblasts arise directly from the hypertrophic chondrocytes and from mesenchymal stem cells brought in during vasculogenesis. Osteoclasts arise from the hematopoietic system.
Dermal bones develop from neural crest cells (facial bones, rostral laryngeal bones, and the frontal bone of the skull) or from unsegmented paraxial (head/cranial) mesoderm (e.g., parietal bone of the skull and caudal laryngeal bones; covered in Chapter 17 ). In dermal bones, the osteoblasts directly differentiate within the mesenchyme.
The striated muscles of the trunk and limb are derived from the segmented paraxial mesoderm , that is, the somites . Most of the tongue musculature also arises from the somites (the so-called occipital somites; covered in Chapter 17 ), whereas all other craniofacial muscles arise from the unsegmented cranial paraxial mesoderm and the prechordal plate mesoderm (i.e., lateral and cranial midline head mesoderm, respectively; covered in Chapter 3 ). The tongue and limb myoblast precursors undergo extensive migration to reach their final destination. Initially the myogenic cells, the myoblasts , proliferate. However, they soon exit the cell cycle and terminally differentiate to form myocytes . The myocytes express contractile proteins, such as actin and myosin and fuse to form myofibers , each of which consists of a multinucleated syncytium (i.e., a cellular mass with multiple nuclei) containing the contractile myofibrils . The tongue and extraocular muscles express unique myosin heavy chains needed for the function of mastication and eye movement, respectively.
Striated muscle development occurs in three waves. First, there is primary myogenesis , which occurs in the embryo. This is followed by secondary myogenesis , which occurs in the fetus and gives rise to the bulk of fetal muscle. Finally, postnatal muscle growth involves satellite cells , small quiescent cells underlying the basal lamina of the muscle fiber. During postnatal growth and in response to exercise or muscle damage, satellite cells form myocytes, which permit further muscle growth. Satellite cells in the trunk and limb arise from the somites , whereas satellite cells in the head arise from the unsegmented paraxial mesoderm.
The smooth muscle of the gut and cardiac muscle form from splanchnic mesoderm , whereas the smooth muscle contributing to blood vessels and hair follicles arises locally within the mesoderm. Smooth muscle can also form from neural crest cells. For example, the iris and ciliary muscles (covered in Chapter 19 ) are derived from cranial neural crest cells, as is the smooth muscle of the dermis of the head and neck.
Commitment to the chondrogenic, osteoblastic, and myogenic lineages is determined by distinct transcription factors. Commitment to the chondrogenic lineage requires the transcription factor Sox9 , which regulates collagen type II expression—a key constituent of the early cartilaginous matrix. Commitment to the osteoblastic lineage requires Runx2 (runt-related transcription factor 2, also known as Cbfa1 or core binding factor 1), a transcription factor. Misexpression of Runx2 in primary fibroblasts can induce the expression of bone markers, such as collagen type I, osteocalcin, bone sialoprotein, and alkaline phosphatase, required for mineralization. Gene inactivation of the transcription factor Sox9 in mice affects the early development of all cartilaginous bones. In contrast, loss of function of Runx2 in mice results in ossification defects caused by lack of osteoblasts; however, the cartilaginous templates of the endochondral bone still form ( Fig. 8.2 ). Osterix, a zinc finger transcription factor that is downstream of Runx2, is essential for osteoblast development; in the absence of osterix, osteoblasts also do not differentiate.

Mutations in SOX9 and RUNX2 genes also occur in humans. Mutations in the SOX9 gene result in campomelic dysplasia , typified by bowing of the long bones and defects in all endochondral bones. Campomelic dysplasia is also associated with XY sex reversal in males (covered in Chapter 16 ). Mutations in the RUNX2 gene cause cleidocranial dysplasia , characterized by clavicular hypoplasia (which allows juxtaposition of the shoulders), large open sutures in the skull, a wide pubic symphysis, and dental abnormalities, such as delayed erupting or supernumerary teeth (covered in Chapter 17 ).
Striated muscle development is determined by expression of the myogenic (or muscle) regulatory factors (MRFs), the basic helix-loop-helix transcription factors Myf5 , Myf6 (previously called Mrf4 ), MyoD , and myogenin (MyoG) . A combination of Myf5, Myf6, and MyoD induces commitment of cells to the myoblast lineage, whereas myogenin, MyoD, and Myf6 are required for terminal differentiation to form myocytes . Myocytes are characterized by the expression of contractile proteins, such as the myosin heavy chains (MyHC), and they fuse to form multinucleated myofibers. The role of MRFs in muscle determination and in the molecular network regulating their expression varies in different regions of the body (see later “In the Research Lab” entitled “Regional Differences in Development of Muscles”). Satellite cells require the paired-box transcription factor Pax7 ; in its absence, satellite cells initiate development, but fail to survive.
As covered in Chapter 4 , the somites are transient segmented structures derived from paraxial mesoderm . They contain the progenitors of the axial skeleton, trunk musculature and associated tendons, trunk dermis, endothelial cells, smooth muscle cells, brown adipose tissue, and meninges of the spinal cord. Somites are initially epithelial balls with a central cavity that contains a population of loose core cells —the somitocoele (or arthrotome ) cells ( Fig. 8.3A ,B). Shortly after forming, each somite separates into subdivisions that give rise to specific mesodermal components. The ventromedial part of the somite undergoes an epithelial-to-mesenchymal transition , and these cells form the sclerotome ; after formation of the sclerotome, the remainder of the somite consists of a dorsal epithelial layer called the dermamyotome ( Fig. 8.3A,C ). The sclerotome will develop into the vertebrae and ribs . As shown in Figs. 8.3A, 8.4 , cells in the ventral portion of the sclerotome migrate to surround the notochord and form the rudiment of the vertebral body ; those cells in the dorsal portion of the sclerotome surround the neural tube and form the rudiment of the vertebral arch and vertebral spine ; cells located more laterally in the sclerotome form the vertebral transverse process and ribs .
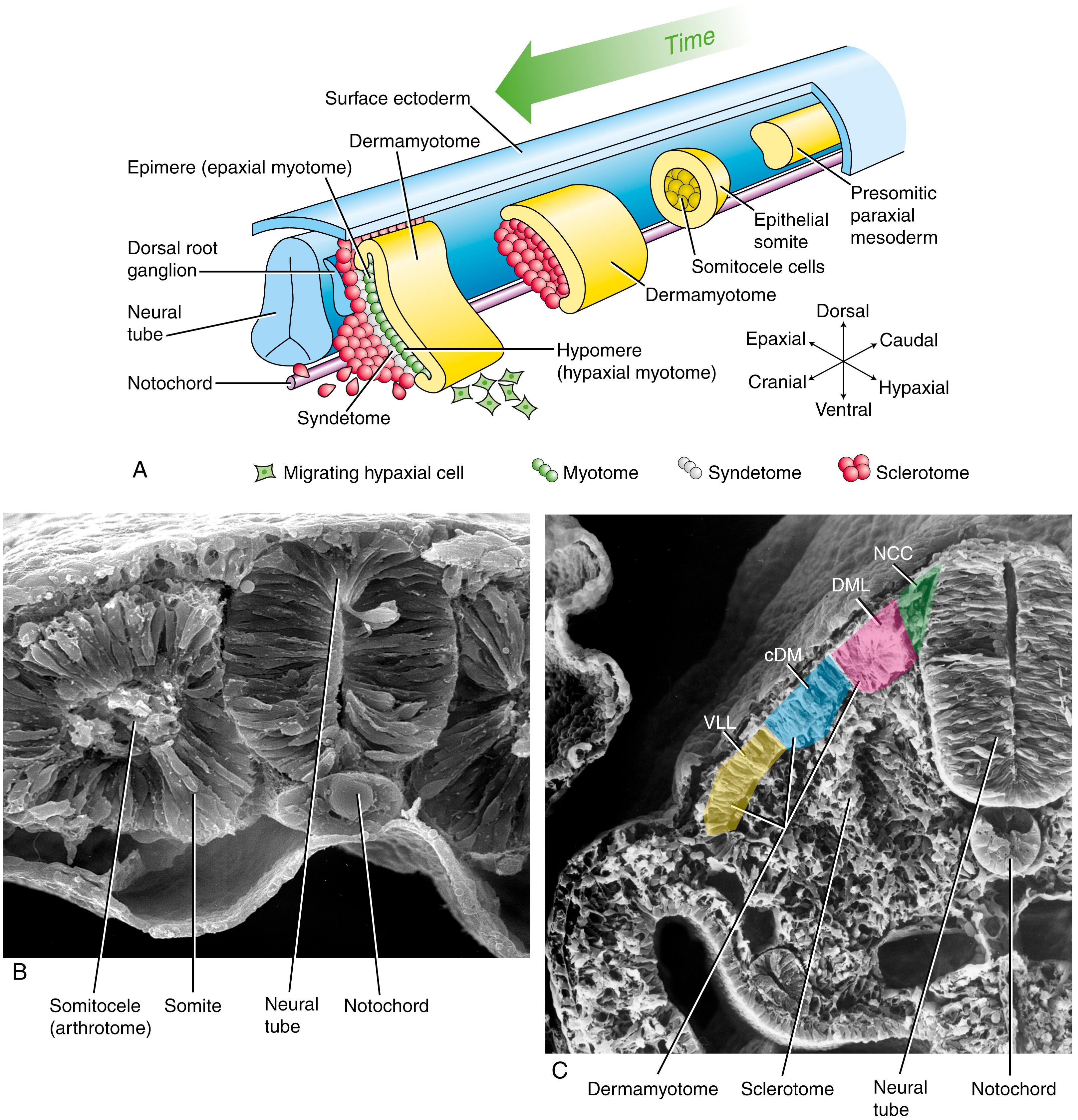
The dermamyotome initially retains its epithelial structure (see Fig. 8.3C ) and contains the presumptive myogenic and dermal cells. By undergoing a second epithelial-to-mesenchymal transition, the dermamyotome gives rise to the myotome, containing committed muscle cells (see Fig. 8.3A ). The factors involved in specification and patterning of the sclerotome and dermamyotome are covered in Chapter 4 (also see Fig. 4.26 ) and in the following “In the Research Lab” entitled “Subdivision of Somite.” The arthrotome gives rise to the intervertebral joints and the annulus fibrosis of the intervertebral disc.
The sclerotome and dermamyotome of the somite are specified by opposing Shh and Wnt signals from the notochord/floor plate and the dorsal neural tube/ectoderm/migrating neural crest, respectively (see Fig. 8.15A ). This is similar to the opposing roles of Shh and Wnts during specification of the dorsal-ventral axis of the neural tube (covered in Chapter 4, Chapter 9 ) and otic vesicle (covered in Chapter 18 ). Bmp antagonists are required for the early development of sclerotome, suggesting that Bmp signaling inhibits sclerotomal development. Overexpression of Shh promotes sclerotome formation and the expression of the homeobox genes, Pax1, Pax9, and Bapx1, all of which are necessary for sclerotome formation. In contrast, overexpression of Wnts induces the dermamyotome.
![Fig. 8.15, Development of the Myotome (A) Myogenic cells in the myotome first form from the edges of the dermamyotome (i.e., the epaxial or dorsomedial lip [DML] , the hypaxial or ventrolateral lip [VLL] , and the cranial and caudal borders) in response to Wnt signals from the dorsal neural tube and ectoderm, Shh from the notochord and floor plate, and notch from migrating neural crest cells (not shown), together with noggin antagonism of Bmp signaling. Arrows indicate the directions of cell movements from the dermamyotome into the myotome during primary myogenesis. (B) Myogenic precursors populate the myotome from the central region of the dermamyotome, which expresses Pax3 and Pax7. These contribute to later phases of growth. The dermamyotome also gives rise to cells in the dermis. Arrows indicate cell movements from the dermamyotome into the myotome ( curved and straight arrows ) during primary myogenesis (A), from the dermamyotome into the myotome ( dashed arrows giving rise to cells colored black), and from the dermamyotome into the dermis (solid arrows) during later development (B). Fig. 8.15, Development of the Myotome (A) Myogenic cells in the myotome first form from the edges of the dermamyotome (i.e., the epaxial or dorsomedial lip [DML] , the hypaxial or ventrolateral lip [VLL] , and the cranial and caudal borders) in response to Wnt signals from the dorsal neural tube and ectoderm, Shh from the notochord and floor plate, and notch from migrating neural crest cells (not shown), together with noggin antagonism of Bmp signaling. Arrows indicate the directions of cell movements from the dermamyotome into the myotome during primary myogenesis. (B) Myogenic precursors populate the myotome from the central region of the dermamyotome, which expresses Pax3 and Pax7. These contribute to later phases of growth. The dermamyotome also gives rise to cells in the dermis. Arrows indicate cell movements from the dermamyotome into the myotome ( curved and straight arrows ) during primary myogenesis (A), from the dermamyotome into the myotome ( dashed arrows giving rise to cells colored black), and from the dermamyotome into the dermis (solid arrows) during later development (B).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentoftheMusculoskeletalSystem/5_3s20B9780323696043000088.jpg)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here