Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In response to inductive and permissive signals emanating from the endoderm, ectoderm, and midline mesoderm, cardiogenic precursors form a cardiac primordium within the splanchnic mesoderm at the cranial end of the embryonic disc called the cardiac crescent , or first heart field . In response to signals from the underlying endoderm, a subpopulation of cells within the first heart field form a pair of lateral endocardial tubes through the process of vasculogenesis . The cranial and lateral folding of the embryo during the fourth week results in the fusion of these tubes along the ventral midline in the future thoracic region, where they form a single primary heart tube . This tube consists of a single endocardial tube with adjacent mesoderm differentiating into cardiomyocytes.
The heartbeat is initiated around the 21st day, and its continual beating is required for normal heart development. Between weeks 4 and 8, the primary heart tube undergoes a series of events, including looping , remodeling , realignment , and septation , eventually leading to the transformation of a single heart tube into a four-chambered heart, thus laying down the basis for the separation of pulmonary and systemic circulations at birth.
Starting at the inflow end, the primary heart tube initially consists of the left and right horns of the sinus venosus , the primitive atrium , the atrioventricular canal , the primitive left ventricle , and a short outflow region. Lengthening of the primary heart tube and proper cardiac bending and looping are driven through the addition of cardiac precursor cells by the second heart field . At the outflow end, the main additions are the primitive right ventricle and the outflow tract that connects with the aortic sac at the arterial orifice. As the outflow tract lengthens, proximal (conus) and distal (truncus) components become distinguishable. Septation of the outflow tract leads to separate left and right ventricular outlets and to formation of the ascending aorta and pulmonary trunk. At the inflow end, the second heart field also contributes myocardium to the sinus venosus wall, the body of the right and left atrium , and the atrial septa .
Venous blood initially enters the sinus horns through paired, symmetrical common cardinal veins . However, as covered in Chapter 13 , changes in the venous system rapidly shift the entire systemic venous return to the right, so that all blood from the body and umbilicus enters the future right atrium through the developing superior and inferior caval veins . The left sinus horn becomes the coronary sinus , which collects blood from the coronary circulation. A process of intussusception incorporates the right sinus horn and the ostia of the caval veins into the posterior wall of the future right atrium. In this process, the pulmonary vein developing within the dorsal mesocardium shifts to the future left atrium because of the development of a dorsal mesenchymal protrusion . Subsequently, the walls of the pulmonary vein are partially incorporated into the atrial wall, forming the larger part of the dorsal left atrial wall. In the fifth and sixth weeks, the atrial septum starts to develop. This is a two-step process. It begins with the formation of the septum primum (primary atrial septum) , which is followed by formation of the septum secundum (secondary atrial septum) . The formation of this atrial septal complex results in separation of the right and left atria. However, the two septa do not fuse until after birth, allowing for right-to-left shunting of blood throughout gestation. The mitral (bicuspid) and tricuspid atrioventricular valves develop from atrioventricular cushion tissue during the fifth and sixth weeks. Meanwhile, the heart undergoes remodeling, bringing the future atria and ventricles into correct alignment with each other and aligning both ventricles with their respective future outflow vessels. During expansion of the primitive right and left ventricles, a muscular ventricular septum forms that partially separates the ventricles. During the seventh and eighth weeks, the outflow tract of the heart completes the process of septation and division. During this process, remodeling of the distal outflow tract cushion tissue (truncal cushions) results in the formation of the semilunar valves of the aorta and pulmonary artery. Fusion of the proximal outflow tract cushions (conal cushions) creates the outlet septum, resulting in the separation of left and right ventricular outlets. Complete ventricular septation depends on fusion of the outflow tract (conotruncal) septum, the muscular ventricular septum, and the atrioventricular cushion tissues.
The myocardium of the heart differentiates into working myocardium and myocardium of the conduction system . The epicardium grows out from the pro-epicardial organ covering the myocardium. It contributes to the formation of the coronary vasculature, which is necessary for oxygenation of the thickening myocardial wall and myocardial cell population.
A full-term baby boy is born to a primigravid (first gestation) mother after an uncomplicated pregnancy. The delivery goes smoothly, with healthy Apgar scores of 8/10 at 1 minute and 9/10 at 5 minutes. All growth parameters (length, weight, and head circumference) are normal, ranging between the 10th and 25th centiles. The newborn examination is also normal, and the infant is returned to his mother to begin breast feeding.
The boy initially feeds well, but he becomes sleepy and disinterested in feeding as the day progresses. At 20 hours after birth, he exhibits decreased peripheral perfusion, cyanosis, and lethargy. A pulse oximeter shows oxygen saturation in the low 80% range (normal equals >90%) with increasing

respiratory distress . Paradoxically, blood oxygen saturation worsens after administration of oxygen. The boy is emergently transferred to the neonatal intensive care unit in worsening shock. There, he is intubated, central intravascular catheters are placed, and he is started on prostaglandins .
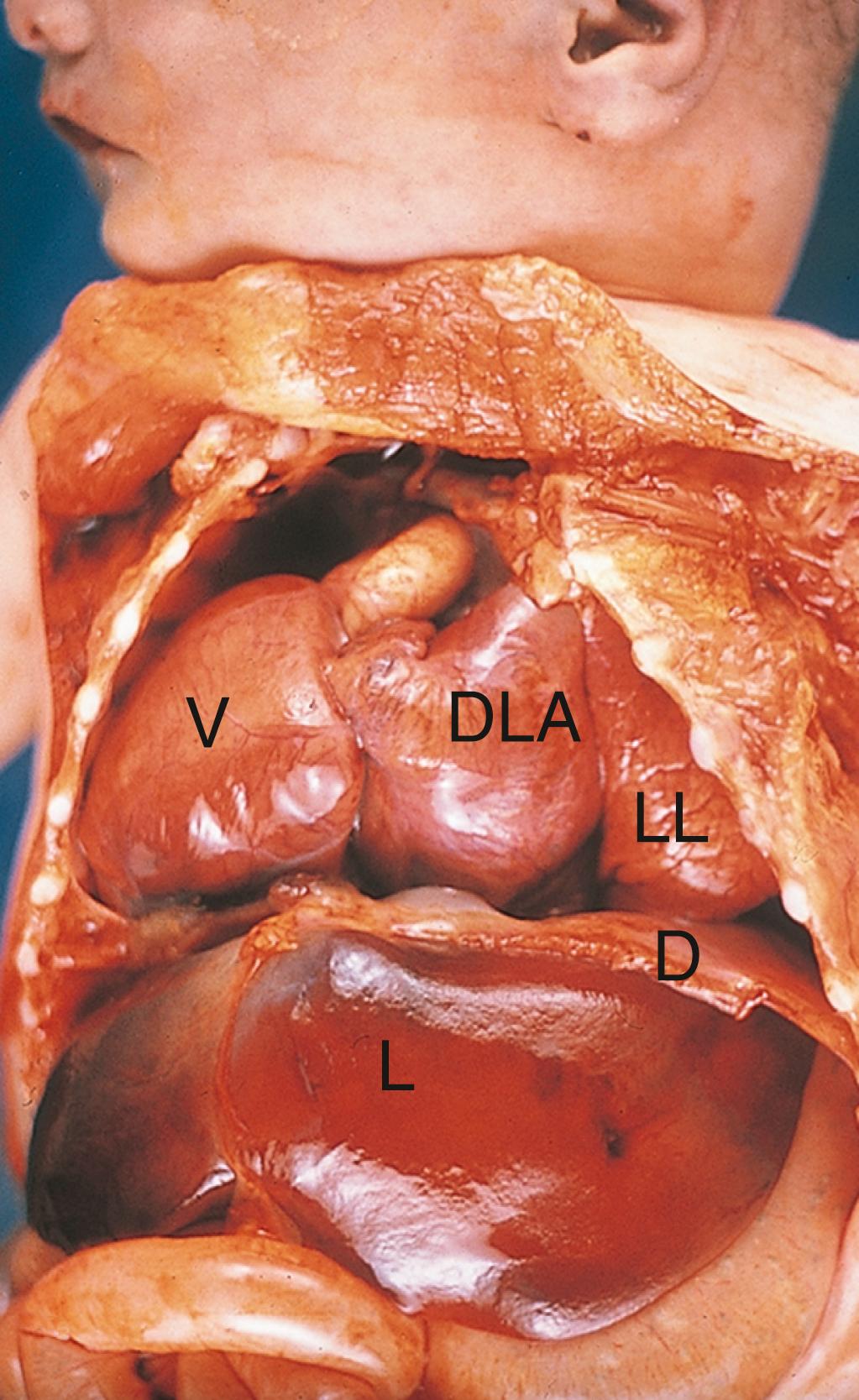
A chest X-ray shows cardiomegaly (enlarged heart) and increased pulmonary vascularity (indicative of increased blood flow). An echocardiogram shows a very small left ventricle with a small aortic outflow tract, leading to the diagnosis of hypoplastic left heart syndrome (HLHS).
HLHS is a shunt-dependent lesion: survival of these patients depends on maintaining a patent ductus arteriosus (PDA) to carry blood from the pulmonary artery to the aorta and out to perfuse the systemic circulation. Supplemental oxygen lowers resistance to pulmonary blood flow, causing blood to circulate to the lungs instead of crossing the PDA. Thus, administering supplemental oxygen actually decreases blood oxygen saturation. Administration of prostaglandins prevents the physiological closure of the ductus arteriosus, maintaining systemic perfusion until surgery can be performed. The first-stage surgery, called the Norwood procedure, connects the right ventricular outflow tract to the aorta, and a separate shunt is used to provide blood flow to the lungs. More surgeries follow at about 6 months and 2 to 3 years of age. Occasionally, heart transplantation is performed. The 5-year survival rate for HLHS is around 70%.
The heart is the first organ to function in human embryos. It begins beating as early as the 21st day, and starts pumping blood by the 24th to 25th day. Much of cardiac development, including remodeling and septation, occurs while the heart is pumping blood. This is necessary to provide nutrients and oxygen and to dispose of wastes during embryonic and fetal development, but this mechanical and electrical activity also plays an important role in the morphogenesis of the heart. The embryonic heart is first morphologically identifiable as a single tube composed of contractile myocardium surrounding an inner endocardial (endothelial) tube, with an intervening extracellular matrix. The heart is also an asymmetrical organ whose left-right patterning is established during gastrulation (left–right patterning is covered in Chapter 3 and later in this chapter).
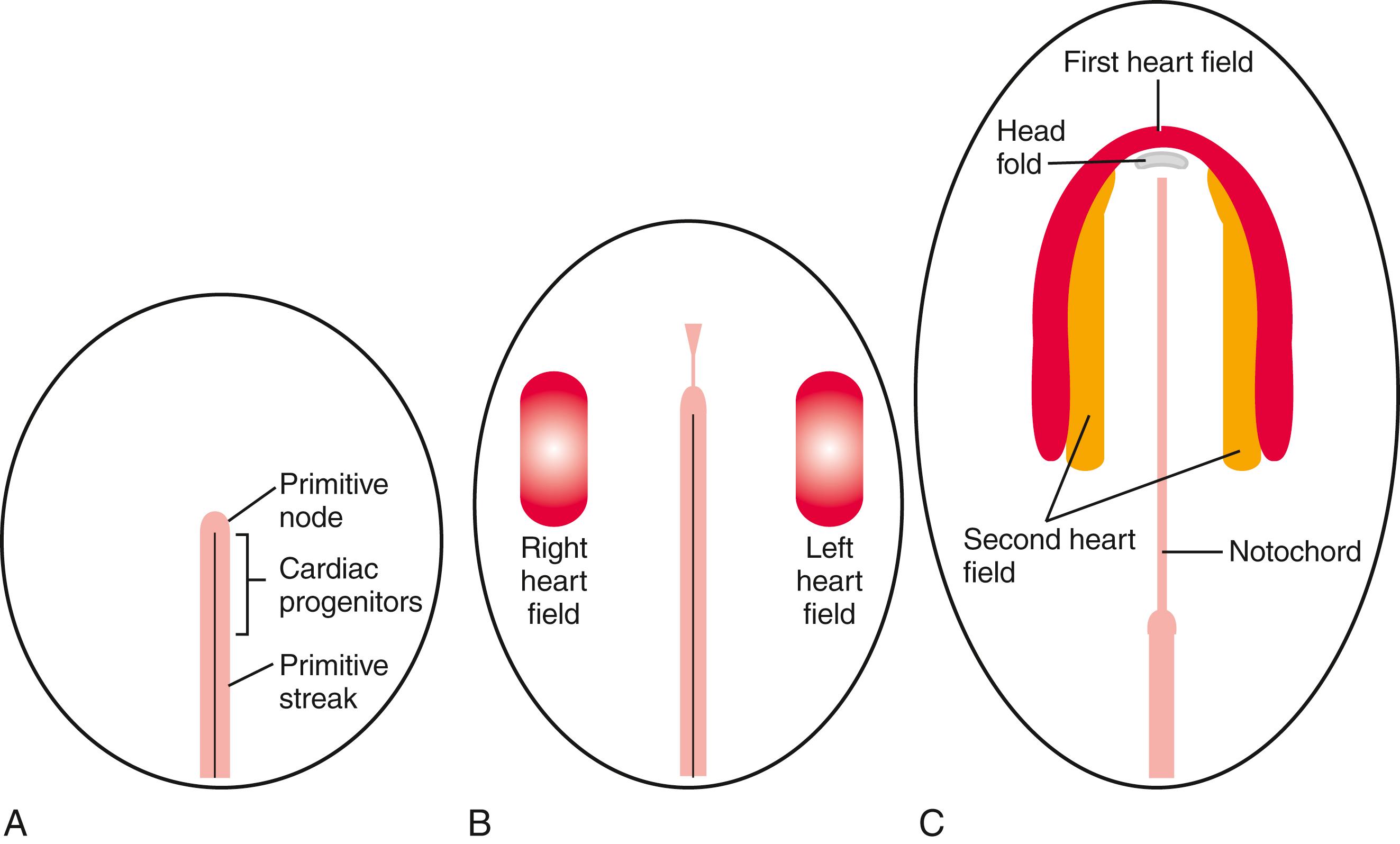
Cardiac progenitor cells are derived from intraembryonic mesoderm emerging from the cranial third of the primitive streak during early gastrulation. These progenitors leave the primitive streak, migrate in a cranial-lateral direction, and become localized on either side of the primitive streak ( Fig. 12.1A,B ). The cardiac progenitor cells eventually become localized within the cranial lateral plate mesoderm on both sides of the embryo, extending and arcing cranial to the developing head fold, forming a cardiac crescent (see Fig. 12.1C ). Cells in the cardiac crescent constitute the so-called first heart field . It is thought the cardiac cell lineage is specified from mesodermal cells within the first heart field. As discussed later, the first heart field is not the sole source of cardiogenic cells for the developing heart, as medial to the first heart field, there is already a population of second heart field cells (see Fig 12.1C ).
To what degree cardiac progenitor cells within the epiblast and the primitive streak are specified remains unknown. Activin and Tgfβ produced by the hypoblast of the chick induce cardiogenic properties in some of the overlying epiblast cells ( Fig. 12.2A,B ). Other members of the Tgfβ superfamily, including nodal and Vg1, also play a role in inducing cardiogenic properties in the epiblast. During gastrulation, cardiac precursors residing in the primitive streak are uncommitted, but these progenitors become specified to become cardiogenic mesoderm soon after migrating into the lateral plate. Mesp1 (mesoderm posterior 1) and Mesp2 (mesoderm posterior 2), members of the basic HLH family of transcription factors, are expressed transiently during the primitive streak stage. Both are required for migration of the cardiac progenitor cells into the cranial region of the embryo, and both are implicated in the specification of the early cardiovascular lineage. Interaction of cranial lateral mesoderm with the endoderm is required for this cardiac specification. The endoderm secretes several signaling molecules—including Bmp, Fgf, activin, insulin-like growth factor 2, and Shh—that promote cell survival and proliferation of cardiogenic cells. One particularly important growth factor is Bmp2, which is essential for stimulating the expression of early cardiogenic transcription factors, such as Nkx2.5 (Nkx2 transcription factor related, locus 5) and Gata (proteins that bind to a DNA GATA sequence) within the lateral mesoderm. In the chick embryo, Bmp2 can induce expression of myocardial cell markers in ectopic regions (i.e., outside their proper position), whereas mouse embryos lacking Bmp2 fail to develop hearts. However, cardiac specification of the mesoderm still occurs in these embryos, likely as the result of overlapping functions of other Bmp family members with Bmp2.
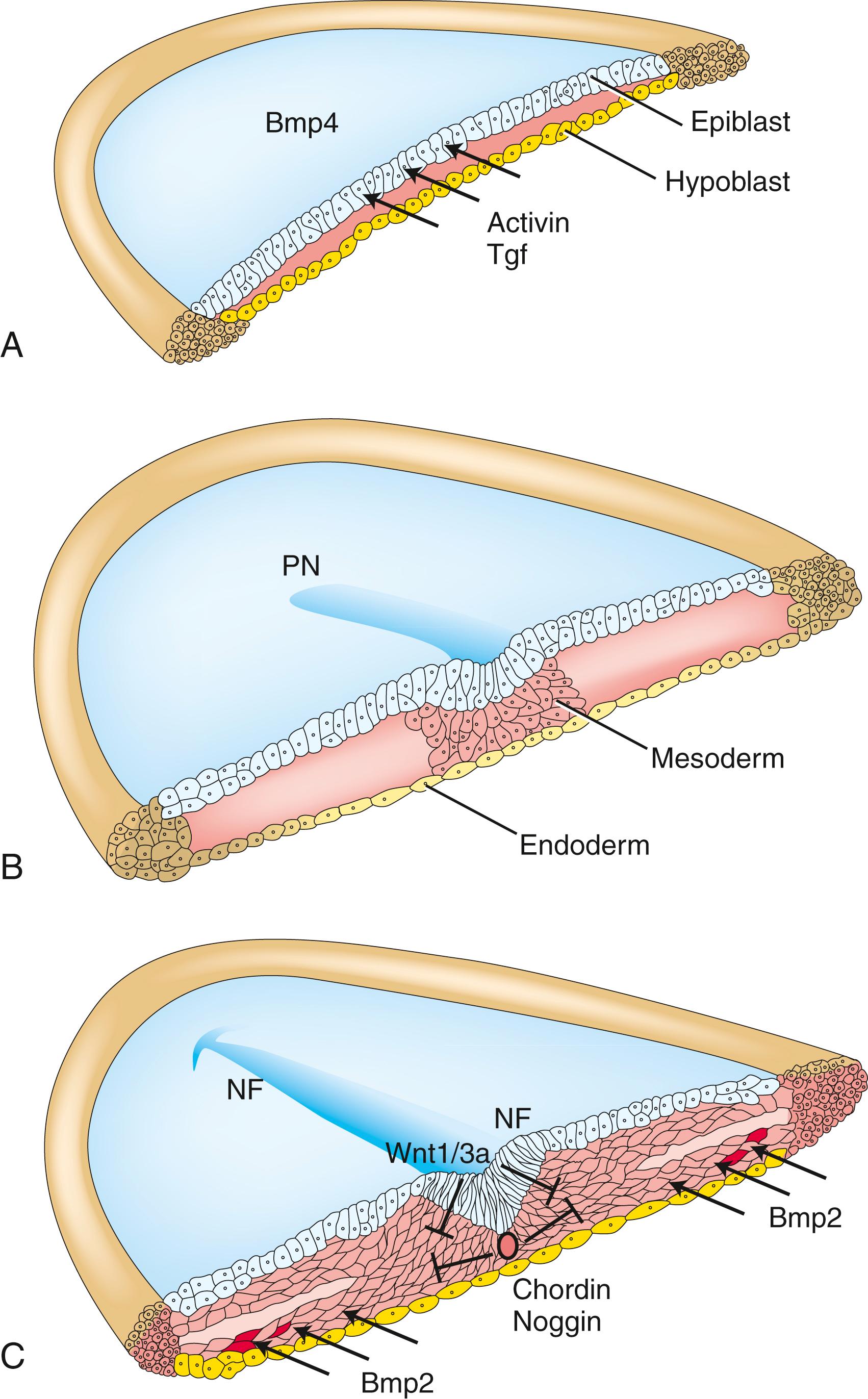
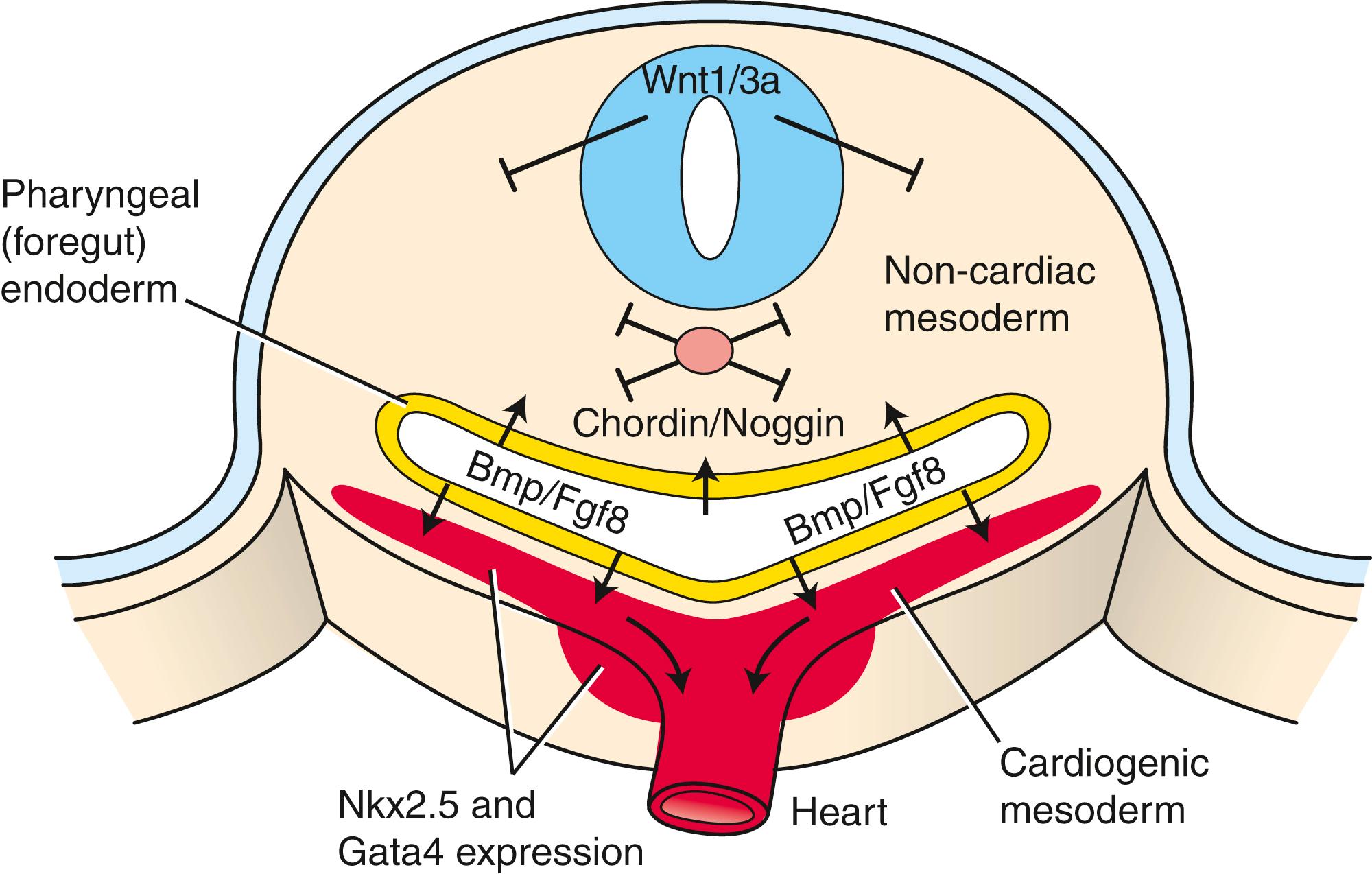
Bmp signaling specifies the cardiogenic lineage, but its effect on the mesoderm is limited to the lateral mesoderm. Why? The midline tissues release Bmp antagonists and inhibitors. The notochord synthesizes and releases chordin and noggin, two proteins that sequester Bmps and prevent binding to their receptors (see Fig. 12.2C ). If chordin activity is inhibited in cranial paraxial mesoderm, the medial mesoderm has the capacity to form cardiac cells. In addition, the developing neural plate ectoderm releases Wnt1 and Wnt3a, which also antagonize Bmp signaling. If Wnt signaling is abrogated in mouse embryos, multiple hearts are generated. Therefore, because of the antagonizing effects of chordin/noggin and Wnt signaling on Bmp signaling, the influence of Bmp on mesoderm is limited to lateral regions.
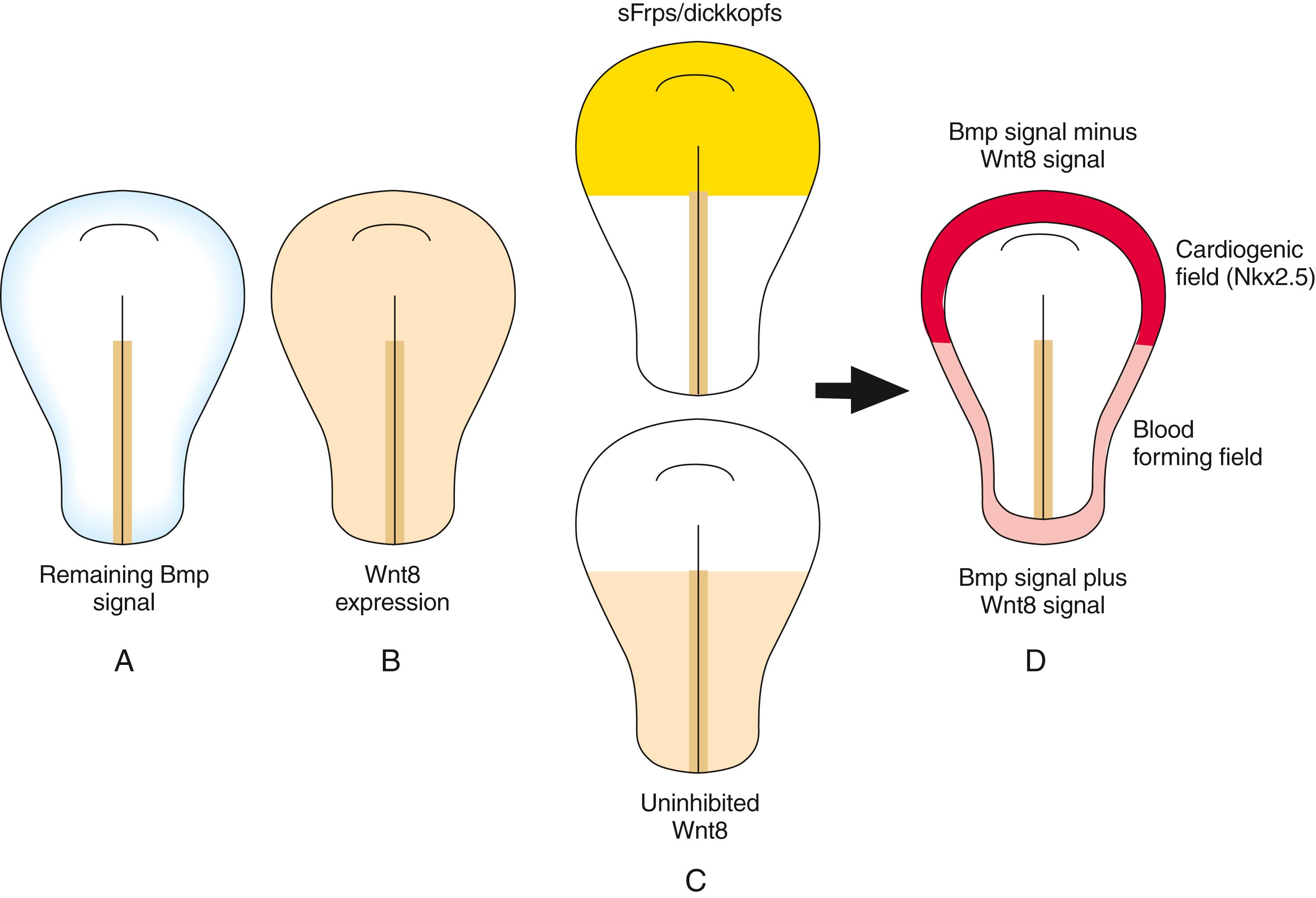
But why is the cardiogenic region limited to the cranial portion of the lateral mesoderm? We know from transplantation experiments that the caudal lateral plate mesoderm can respond to cardiac specification signals: if grafted into the cranial region, it transforms into cardiogenic cells. As covered above, Wnt1/Wnt3a and chordin/noggin inhibit the effects of Bmps on mesoderm. However, other Wnts (e.g., Wnt8) expressed in the cranial and caudal mesoderm also inhibit Bmp effects on the mesoderm. Knowing that Bmp signaling is required for cardiac mesoderm formation, how can Bmp still exert its influence on the cranial lateral mesoderm in the presence of these Wnts but not on the caudal lateral plate? The answer is that other molecules secreted by the cranial endoderm antagonize the negative effects of Wnts on Bmp-driven heart formation. These include secreted frizzled-like proteins (sFrps) that sequester Wnts and dickkopfs that bind to and inhibit the Wnt co-receptors of the Lrp (low-density lipoprotein receptor–related protein) class ( Fig. 12.3 ). Hence, in the absence of Wnt signaling, the effect of Bmp is to promote the cardiac lineage in the cranial portion of the lateral mesoderm, whereas in the presence of Wnt signaling, Bmp initiates a blood vessel–forming capacity in the caudal portion of the lateral plate mesoderm. However, studies suggest that canonical Wnt signaling has biphasic effects on cardiogenesis depending on the time of action, promoting cardiac specification during gastrulation but later obstructing it. Non-canonical Wnt signaling (Wnt5a and Wnt11) also promotes cardiogenesis.
Several cardiac transcription factors are activated within the first heart field. The earliest transcription factors with limited expression within the cardiac lineage include Nkx2.5, Tbx5, and members of the Gata family. Nkx2.5 is expressed in cardiac progenitor cells soon after the onset of gastrulation under the influence of endodermal derived Bmp. Downstream targets of Nkx2.5 include several other cardiac genes, such as Mef2c, ventricular myosin, and Hand1. A human ortholog of Nkx2.5, designated NKX2.5, maps to chromosome 5q35.2, and mutations in this gene are associated with human congenital heart disease, including atrial septal defects, ventricular septal defects, and defects in the conduction system. Nkx2.5 knockout mice die in utero but still form a heart, albeit one without left ventricular markers, with incorrect looping, and with a defective cranial-caudal identity. Therefore, Nkx2.5 expression is not solely responsible for dictating the cardiac cell lineage. Mice null for Gata4 have fewer cardiomyocytes. Mice lacking Gata5 are normal but exhibit elevated Gata4 levels, suggesting a compensatory effect for the loss of Gata5. Gata5 null mice also lacking one of the Gata4 genes exhibit profound cardiac defects. This suggests that Gata4 and Gata5 act cooperatively in directing early cardiac lineage. Nkx2.5 and Gatas may mutually reinforce each other’s cardiac expression, as each contains promoter regions for the other.
In summary, the program of early cardiac specification is quite flexible, but it requires the presence of particular morphogens to provide a permissive environment for lineage specification. Moreover, no single transcription factor or signaling molecule has been identified that is solely responsible for encoding myocardial specification and differentiation. Rather, it seems that a combination of factors working together act to stably specify the cardiac cell lineage.
![]()
Animations are available online at StudentConsult.
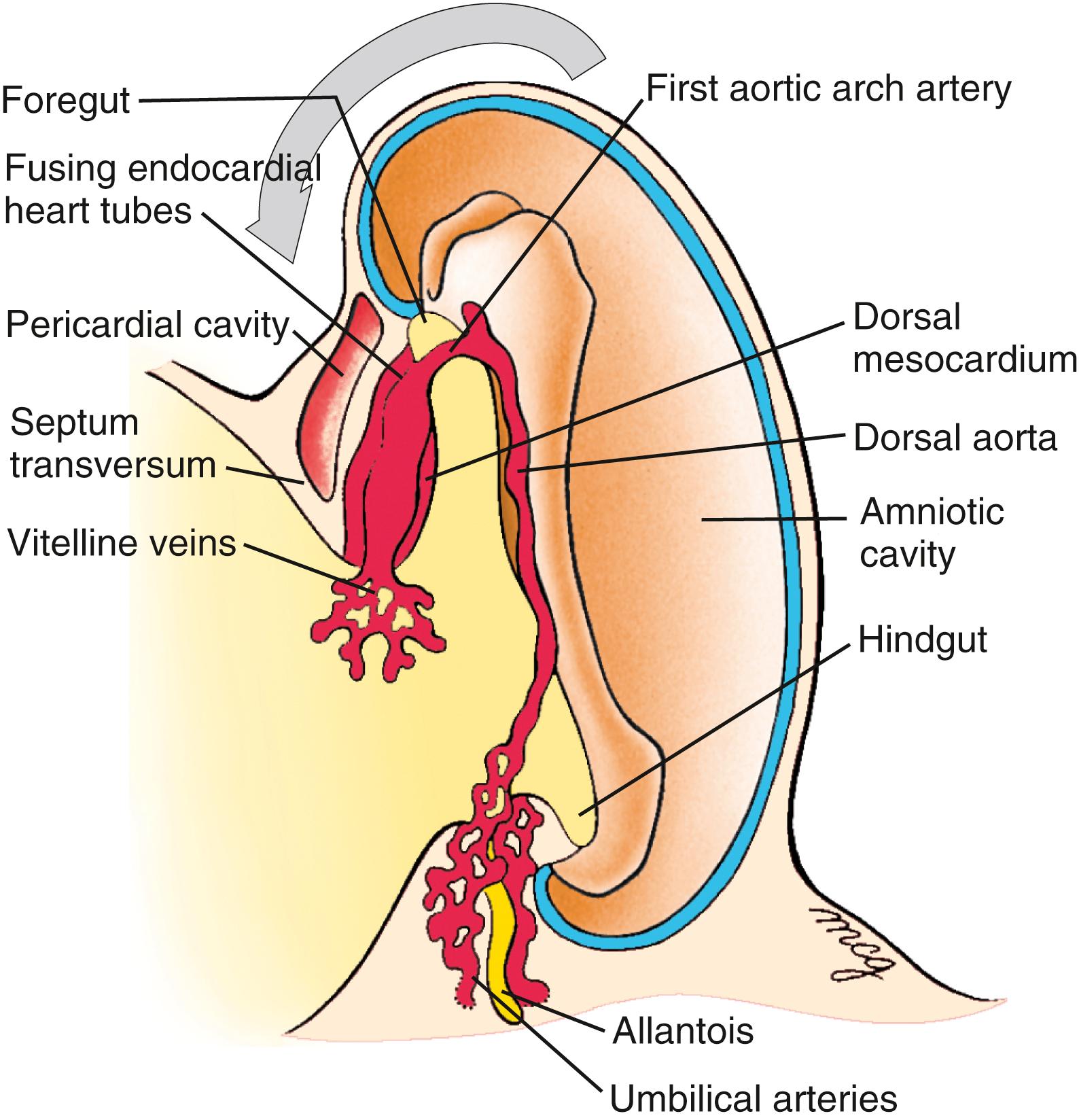
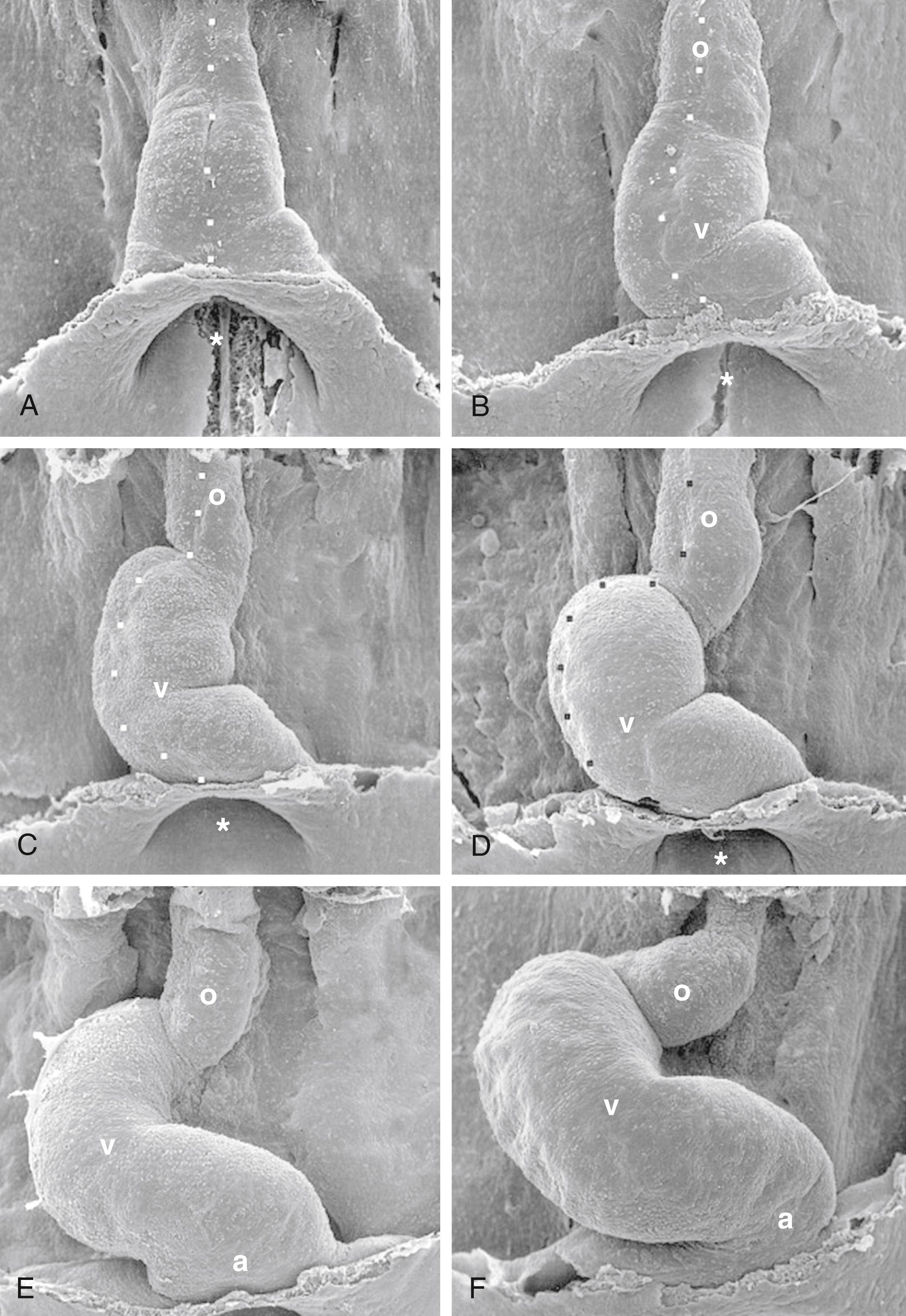
With formation of the intraembryonic coelom, the lateral plate mesoderm subdivides into somatic and splanchnic layers; the first heart field forms within the splanchnic mesodermal subdivision. During the process of body folding (covered in Chapter 4 ), the cranialmost portion of the first heart field is pulled ventrally and caudally to lie ventral to the newly forming foregut endoderm ( Fig. 12.4 ). As the lateral body folds move medially, they bring the right and left sides of the first heart field together, and the two limbs of the first heart field fuse at the midline, caudal to the head fold and ventral to the foregut ( Fig. 12.5A–D ). This fusion occurs at the site of the anterior intestinal portal and progresses in a cranial-to-caudal direction as the foregut tube lengthens. As the two limbs of the first heart field fuse, a recognizable pair of vascular elements called the endocardial tubes develops within each limb of the first heart field (see Fig. 12.5B,C ). These vessels form within the first heart field from a seemingly distinct progenitor population from other endothelial subtypes through mechanisms that are still not understood. The cells of the endocardial tubes coalesce into a single tube as the limbs of the first heart field join to make the primary heart tube (see Fig. 12.5C,D ). If fusion of the first heart field limbs fails, two tube-like structures form rather than a single primary heart tube, leading to cardia bifida (however, both tubes persist, contract, and continue to undergo cardiogenesis). The primary heart tube harbors progenitors for the atria and left ventricle, as well as endocardium. As the fusion process continues, cell proliferation in the first heart field continues to add the more caudal segments of the heart, including the atrioventricular canal, the primitive atria, and a portion of the sinus venosus (covered later in the chapter). Late in the third week, cranial body folding brings the developing heart tube into the thoracic region ( Figs. 12.4, 12.6 ; also covered in Chapter 11, Chapter 4 ).
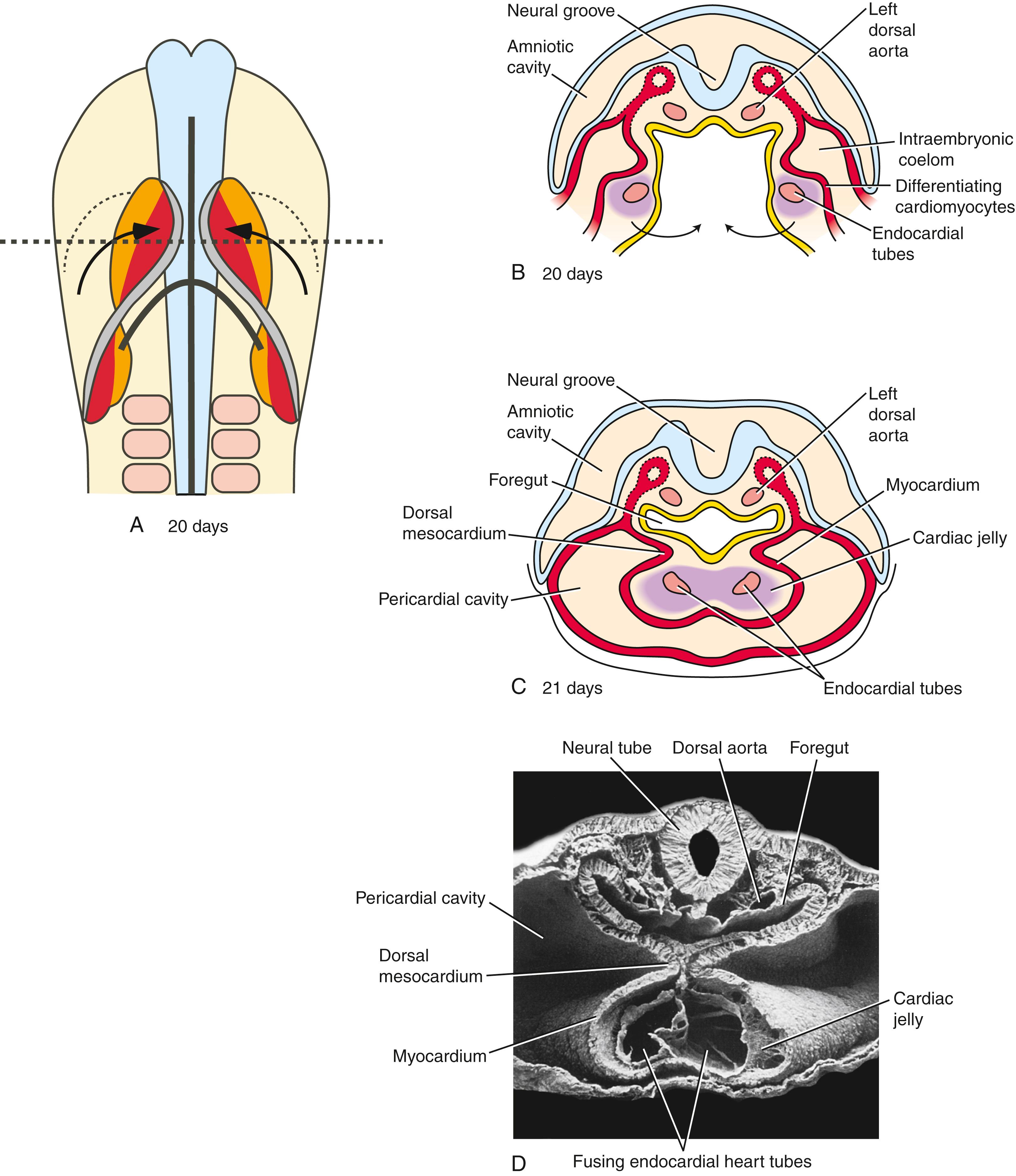
By the 21st to 22nd day, the primitive endocardial tube is surrounded by a mass of splanchnic mesoderm containing myocardial progenitors that aggregate around fused endocardial tubes to form the myocardium . A thick layer of acellular extracellular matrix, the cardiac jelly , is deposited mainly by the developing myocardium, separating it from the endocardial tube ( Fig. 12.7 ). The epicardium (visceral lining of the pericardial cavity covering the heart) is formed later by a population of mesodermal cells that are independently derived from splanchnic mesoderm migrating onto the outer surface of the myocardium (covered later in the chapter).
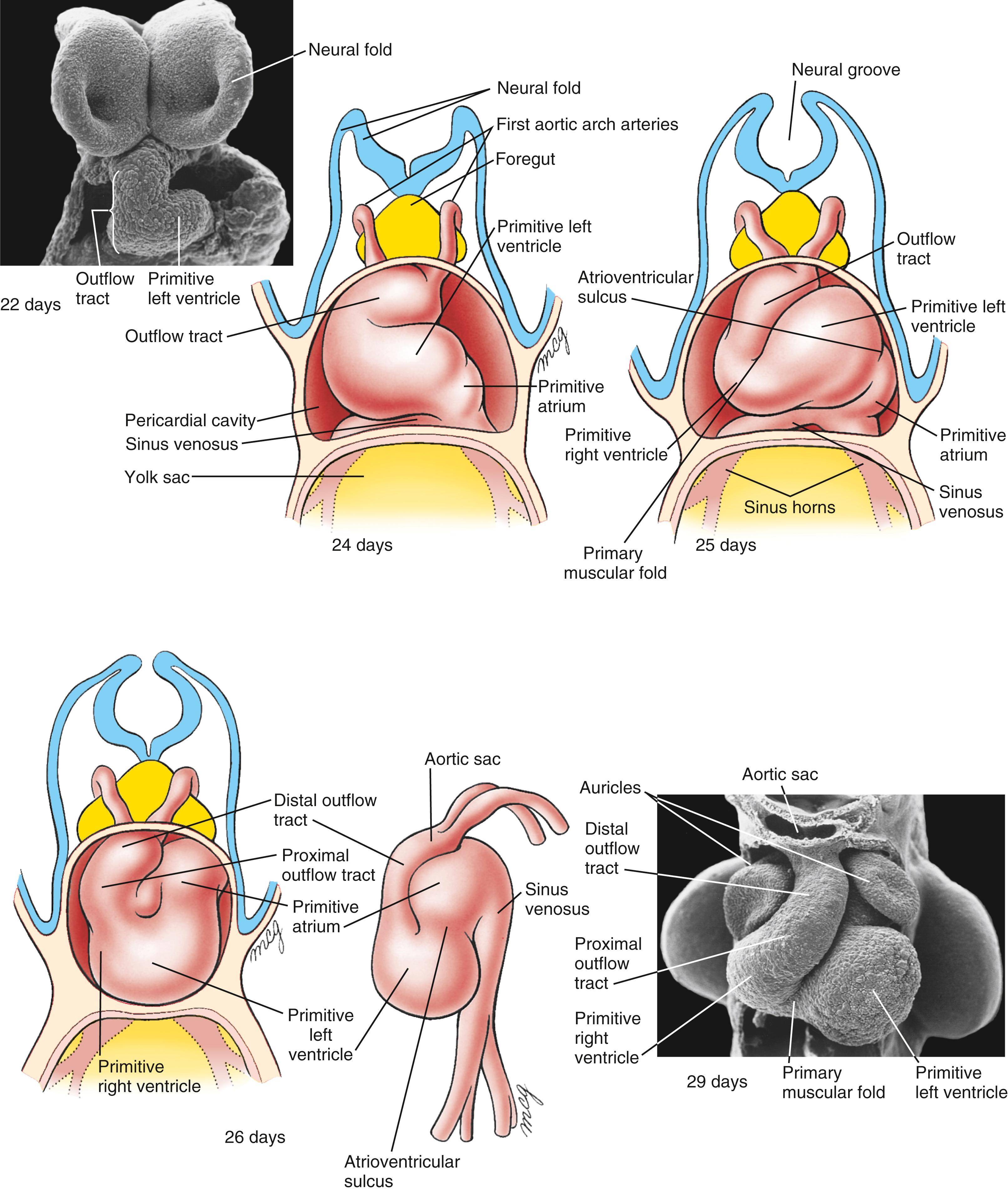
A series of constrictions and expansions develop in the primary heart tube ( Fig. 12.8 ). Over the next 5 weeks, as the tubular heart lengthens, these expansions contribute to the various heart chambers. Starting at the caudal (inflow) end, the sinus venosus consists of the partially confluent left and right sinus horns , into which the common cardinal veins (covered later in the chapter) drain. Cranial to the sinus venosus, the next chamber is the primitive (or common ) atrium , which, as a result of the subsequent formation of the atrial septal complex, eventually becomes divided into the right and left atria. Connected in series with the atrium are the atrioventricular canal , the primitive left ventricle , and the developing primitive right ventricle and outflow tract . The primitive left ventricle is separated from the primitive right ventricle by a primary muscular fold (formerly referred to as the bulboventricular fold ), the latter contributing to the muscular ventricular septum . Whereas the atria, atrioventricular canal, and left ventricle are largely derived from the first heart field, the right ventricle and outflow tract are not. Rather, they come from an additional source of cardiac precursor cells, referred to as the second heart field . The outflow tract forms the outflow region for both the left and right ventricles. The outflow tract can be subdivided into a proximal outflow tract (conus arteriosus), which eventually becomes incorporated into the left and right ventricles, and the distal outflow tract (truncus arteriosus), which eventually splits to form the ascending aorta and pulmonary trunk. The distal outflow tract is connected at its cranial end to a dilated expansion called the aortic sac . The aortic sac is continuous with the first aortic arch artery and, eventually, is continuous with the other four aortic arch arteries as they develop. The aortic arch arteries form major arteries transporting blood to the head and trunk (covered in Chapter 13 ).
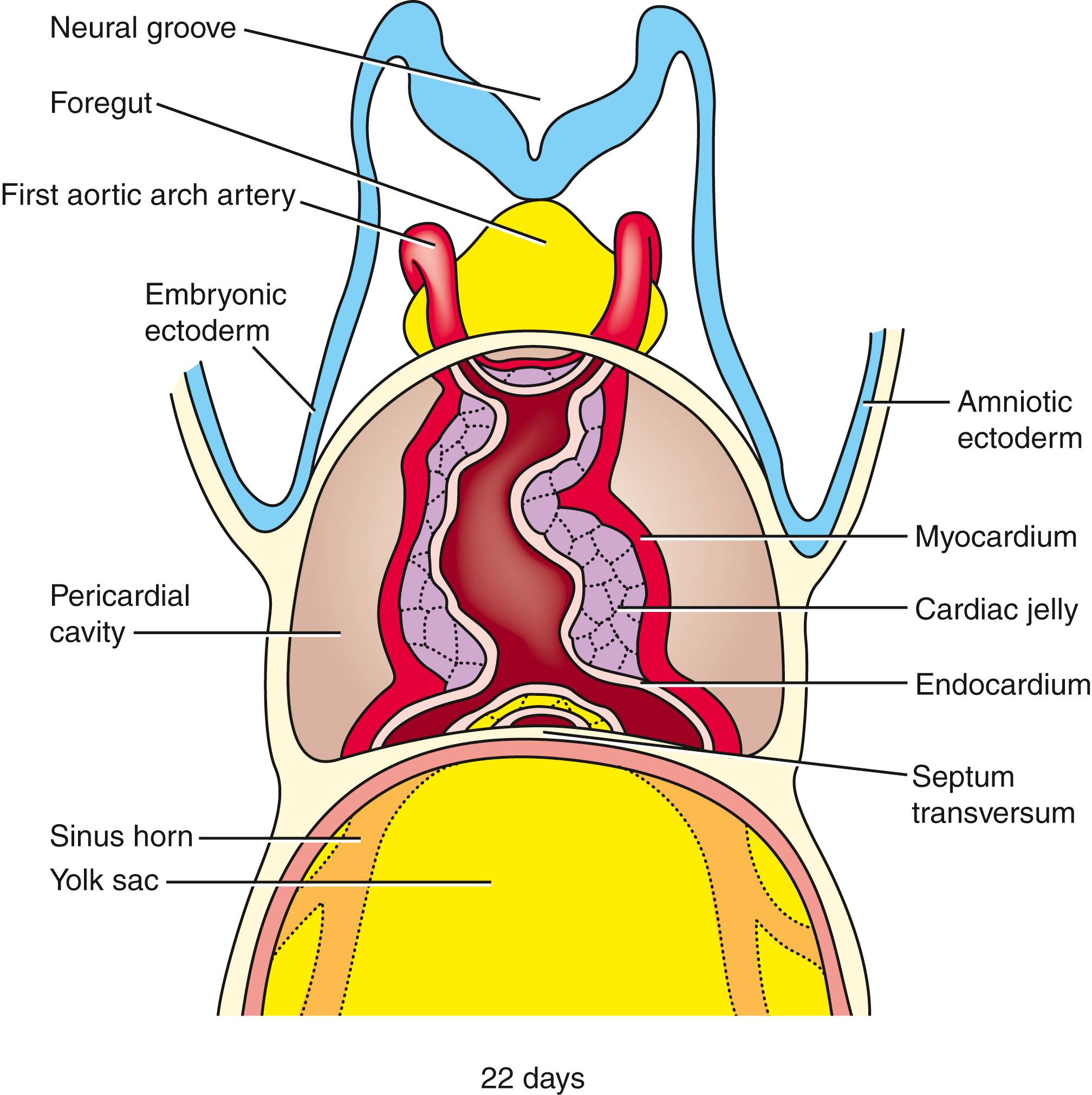
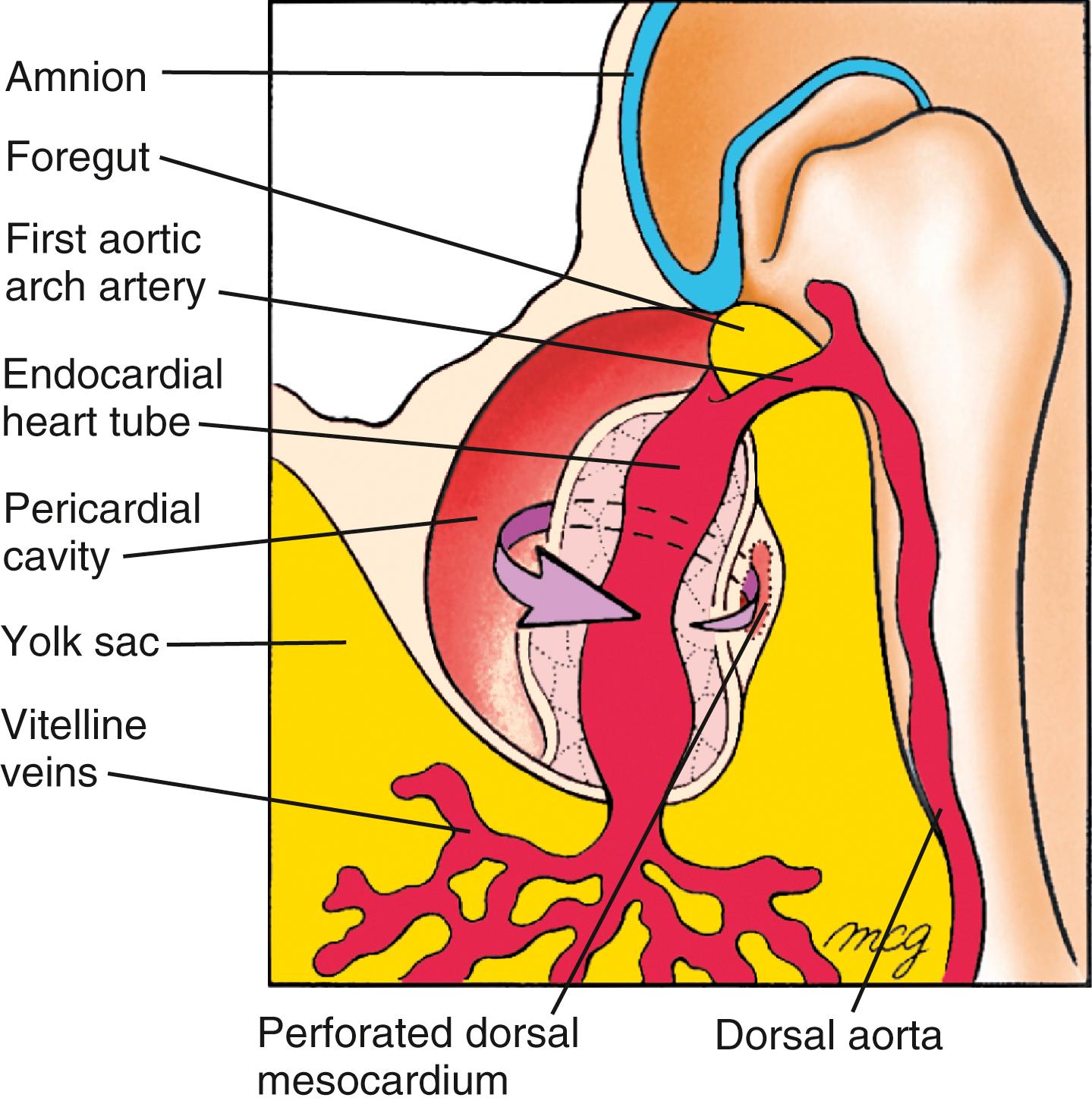
The primary heart tube is initially suspended in the developing pericardial cavity by a dorsal mesocardium (dorsal mesentery of the heart) formed by splanchnic mesoderm located beneath the foregut. Subsequently, this dorsal mesocardium ruptures over almost the entire length of the heart tube, with the exception of the caudalmost aspect, where a small but very important component of the dorsal mesocardium persists. As a result, the heart is suspended in the pericardial cavity by its developing arterial and venous poles, with the region of the ruptured dorsal mesocardium becoming the transverse pericardial sinus within the pericardial sac of the definitive heart ( Fig. 12.9 ). Ligatures sometimes are passed through this space and around the vessels at either pole to control blood flow in children or adults undergoing surgery.
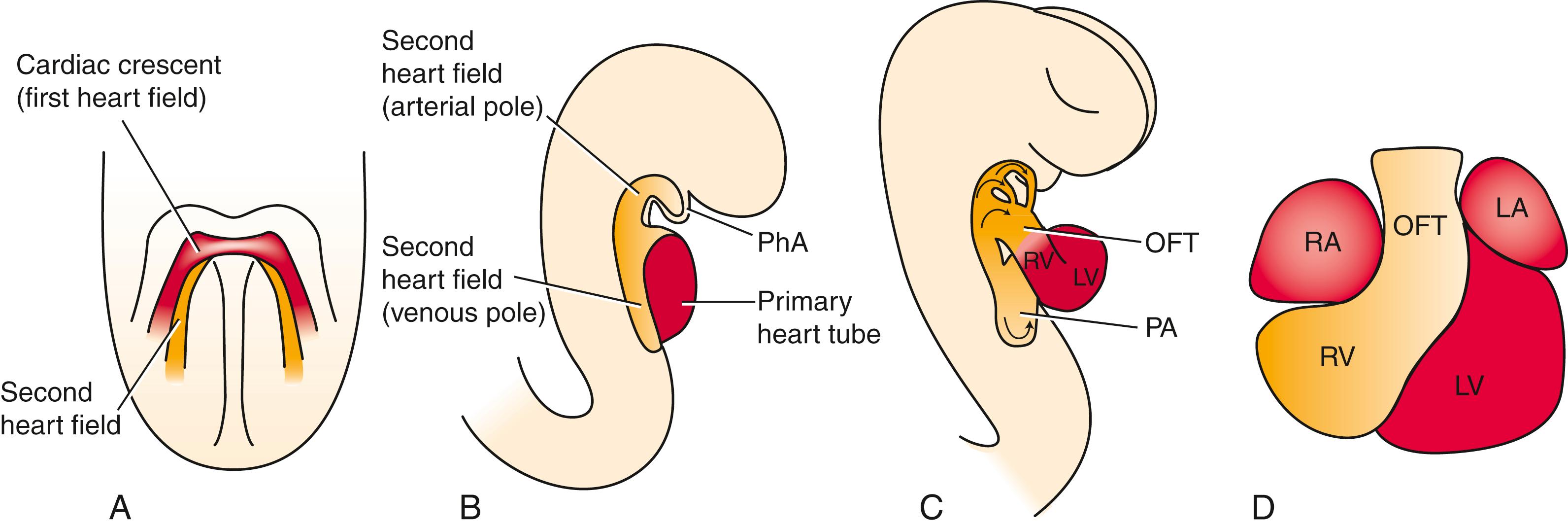
As noted earlier, not all cardiac cells found in the mature heart come from the first heart field. Rather, additional sources of cardiogenic precursors are recruited from the mesoderm immediately adjacent and medial to the initial cardiac crescent ( Fig. 12.10 ). While the developing primary heart tube continues to expand, there is progressive recruitment of cardiac progenitor cells from outside the original first heart field at both the arterial (cranial) and venous (caudal) poles. The source of these cells is referred to as the second heart field . The primitive heart tube lengthens at both ends, particularly the outflow (arterial) end, through the addition of cardiac progenitors from the second heart field mesoderm. Lineage-tracing studies suggest that in mammals, the proximal and distal outflow tract, the right ventricle, and a portion of the venous pole and atria are derived from the second heart field mesoderm (see Fig. 12.10D ).
![]()
Animations are available online at StudentConsult.
Cells of the first and second heart fields arise from a common precursor in the early embryo and diverge at or before the time of gastrulation. Recent studies suggest progenitors for the second heart field lie just medial and slightly caudal to the first heart field within the lateral plate mesoderm (see Figs. 12.1, 12.10 ). Like the first heart field, the second heart field is subjected to the influences of Bmps and Fgfs released by the foregut (pharyngeal) endoderm that activate cardiogenic transcription factors. However, the medial location of the second heart field at the cardiac crescent stage also positions these cells closer to the negative influence of Wnts and chordin/noggin emanating from the developing notochord and neural plate (see Figs. 12.2, 12.10 ). Manifestation of the cardiac cell lineage within the second heart field is likely suspended until the primary heart tube is formed and the intervening distance between the second heart field and the midline neural tube/notochord is increased. Cells of the second heart field are contiguous with the first heart field, and characterized by elevated proliferation and delayed differentiation.
As the two limbs of the cardiac crescent move toward the midline during fusion, the second heart field cells contact the dorsal surface of the primary heart tube (future inner curvature of the heart) and end up at both the cranial and caudal ends of the developing dorsal mesocardium (see Fig. 12.10B,C ). It is after the second heart field cells come to lie ventral to the foregut that expression of Nkx2.5 and Gata4 increases in the second heart field ( Fig. 12.11 ). Second heart field cells lying just cranial to the arterial outflow of the heart tube and ventral to the developing pharyngeal endoderm assume a right ventricular identity, whereas cells more caudal to the arterial outflow contribute to the wall of the proximal and distal outflow tracts. Those at the inflow end of the heart tube contribute myocardial cells to the wall of the atria, atrial septum, and sinus venosus. The bulk of heart tube lengthening comes from proliferation within the second heart field at the arterial pole.
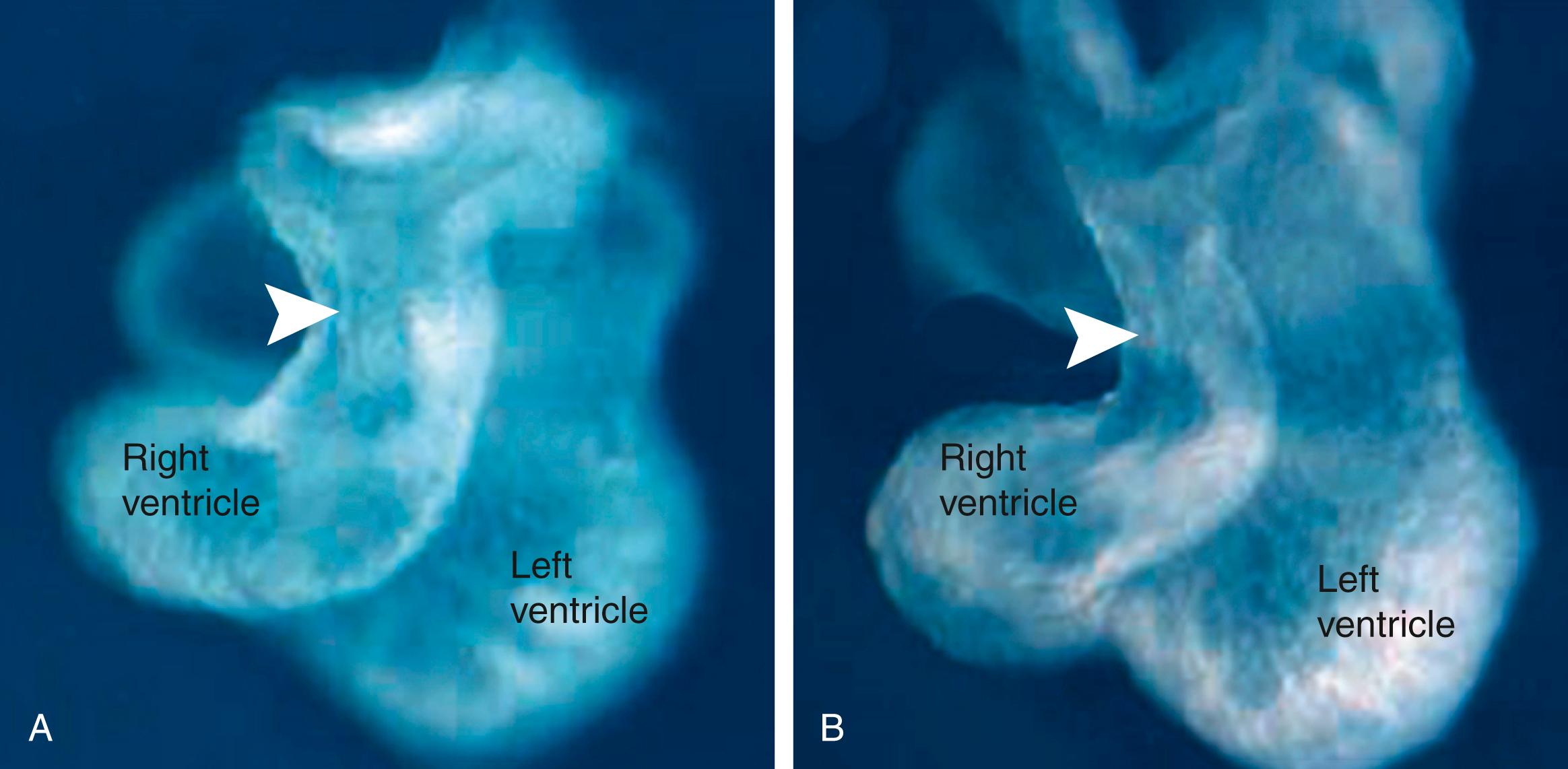
Mutations in particular genes reveal regional sensitivities of the myocardium that reflect the origin of their cardiomyocyte progenitors. For instance, in Tbx5-deficient mice (Tbx5 is a T-box transcription family member that is expressed in the primary heart tube), the atrium is abnormal and the left ventricle is hypoplastic. Yet, the right ventricle and outflow tract seem normal, suggesting that this mutation mainly targets proliferation and development of cells of the first heart field. The second heart field expresses a Lim-homeodomain transcription factor, Islet-1 (Isl1). Mice null for Isl1 typically develop only two heart chambers: the atria and the left ventricle. The outflow tract is missing, right ventricular markers are not expressed, and the posterior atrial myocardium is hypoplastic. Fgf8 is expressed in the ectoderm and pharyngeal endoderm near the arterial pole of the heart tube. Fgf8 is also expressed in the second heart field cells where it is required for their continued proliferation at the arterial pole. Fgf8 hypomorphs (an embryo with a partial loss-of-function mutation; i.e., Fgf8 expression in the hypomorph is knocked down but is not eliminated) die as a result of abnormal outflow tract development. Tbx1 (haplosufficient in 22q11.2 deletion syndrome), a transcription factor expressed in the second heart field, interacts genetically with Fgf8. Loss of Tbx1 expression in the second heart field reduces myocardial cell number in the outflow tract and right ventricle ( Fig. 12.12 ), whereas forced Tbx1 overexpression in the second heart field causes an expansion of the outflow tract. From these studies, it is clear that in addition to a first heart field, a large portion of the definitive heart tube arises from the second heart field. Several other signaling molecules and transcription factors play important roles in mediating continued proliferation or survival of second heart field cells, including Shh, canonical Wnts, Pdgf, retinoic acid and retinoic acid receptors, Mef2c, Msx1, Msx2, Hand2, Tbx18, Shox2, Foxa2, Foxc1, and Foxc2. Lengthening of the heart tube by the second heart field plays an important role in proper cardiac looping and septation of the heart. The second heart field is also a part of a larger field of cardiopharyngeal mesoderm , activating the branchiomeric skeletal muscle program (covered in Chapter 17 ).
![]()
Animations are available online at StudentConsult.
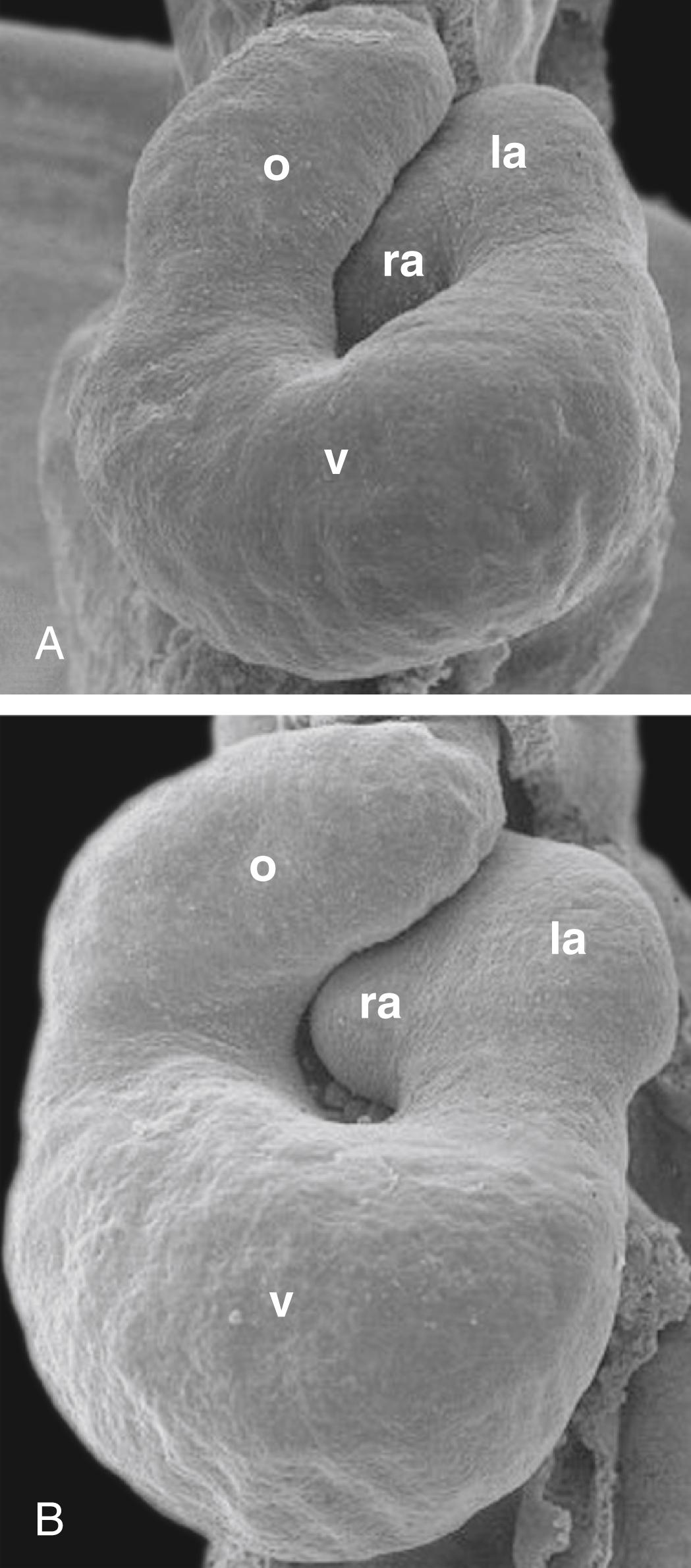
On day 23, the primary heart tube begins to elongate and simultaneously bend into a C-shaped structure, with the bend extending toward the right side. Formation of this bend is not simply a matter of forming a kink in the tube, with the right side of the tubular heart becoming the outer curvature and the left side forming the inner curvature. Rather, it seems that the ventral surface of the primary heart tube forms the right outer curvature of the C-shaped heart, because the ventral surface is displaced toward the right by torsional forces working along the craniocaudal axis ( Fig. 12.13 ). With the rupture of the dorsal mesocardium, much of the dorsal side of the straight primary heart tube becomes situated on the inner curvature of the C-shaped heart. As the heart tube continues to elongate at both arterial and venous poles, it takes on an S-shaped configuration. In the process, the primitive right ventricle is displaced caudally, ventrally, and to the right; the developing primitive left ventricle is displaced to the left. The primitive atrium acquires a more dorsal and cranial position ( Fig. 12.14 ; see also Fig. 12.8 ). By day 28, the elongation of the heart tube is complete, but there continues to be additional remodeling such that the outflow tract comes to lie between the presumptive future atria, and the atrioventricular canal aligns with both ventricles (see Fig. 12.8 ). The end result of cardiac looping is to bring the four presumptive chambers of the future heart into their correct spatial relationship to each other. The remainder of heart development consists mostly of remodeling these chambers, developing the appropriate septa and valves between them, and forming the epicardium, coronary vasculature, and cardiac innervation and conducting system.
As covered in Chapter 3 , abnormal left-right axis determination can lead to development of heterotaxy (with an estimated incidence of 3 out of 20,000 live births). This term describes any defect ascribed to abnormal left-right axis formation, be it a reversal of some organs (partial situs ambiguus) or a reversal of all viscera (situs inversus totalis) . With regard to the heart, this may include abnormal looping, resulting in ventricular inversion ( Fig. 12.15 ). Proper looping toward the right is a prerequisite for proper cardiac septation, as it is required to bring the primitive left ventricle toward the left, the primitive right ventricle toward the right, and the outflow tract region to the middle. Because individuals with situs inversus totalis exhibit a reversal in handedness of all organs, they exhibit few problems. In contrast, visceroatrial heterotaxy syndrome in humans (where the abdominal viscera and the atrial pole are oriented on opposing sides) is associated with structural defects, including a common atrium, malalignment of the atrioventricular canal and outflow tract, and abnormal venous and arterial vascular connections.
Besides inverted situs, indeterminate left-right axis formation can lead to bilateral left-sidedness or right-sidedness, so-called isomerism. For example, in the condition called right atrial isomerism, both atria have right atrial morphology. Similarly, in left pulmonary isomerism, both lungs have the lobar and hilar anatomy of the left lung.
The chambers of the heart are developmentally, electrophysiologically, and pharmacologically distinct. How does this regionalization develop within a single heart tube? Fate mapping studies show that cardiac progenitor cells within the epiblast are topologically organized such that the cardiac inflow progenitors are located more lateral, and the outflow progenitors more medial. Subsequently, during the process of gastrulation, this orientation converts to a craniocaudal (arterial/venous) topography by the time of the cardiac crescent stage. Cells within the first heart field are still plastic with regard to chamber specification: if caudal cardiac progenitor tissue substitutes for cranial cardiogenic tissue, proper hearts are generated. However, soon afterward, commitment to particular chambers is evident by the expression of chamber-specific regulators.
Regionalization of the heart is likely an outcome of having at least two separate heart areas within the first heart field. In mice, clonal analysis suggests that the atrial region becomes clonally distinct (i.e., clones of progenitor cells become restricted to a single compartment) before the rest of the heart. Tbx5 is linked to atrial lineage determination. Initially expressed in the entire first heart field, Tbx5 expression becomes limited to the sinus venosus and atria, with some expression in the left ventricle (i.e., first heart field derivatives; Fig. 12.16 ). Tbx5 knockout mice exhibit severe hypoplasia of these chambers, whereas forced expression of Tbx5 throughout the heart leads to loss of ventricular-specific gene expression, essentially “atrializing” the heart. Mutations in human TBX5 have been identified in families with Holt-Oram syndrome , which includes heart chamber malformations, atrial septal defects, and cardiac conduction system anomalies. Irx4, an Iroquois homeoprotein, is expressed only in the cranial portion of the first heart field (see Fig. 12.16 ); later, it is restricted to ventricular cells, where it stimulates the expression of ventricular myosin heavy chain-1 (Mhc1v) and suppresses atrial myosin heavy chain-1 (Mhc1a). Irx4 may maintain the cranial-caudal phenotype of the heart by suppressing atrial commitment because loss of Irx4 expression in mice leads to ectopic expression of atrial markers in the ventricles. Once the initial heart tube begins to lengthen and cardiac bending and looping begin, major changes occur in the expression of several chamber/region-restricted transcription factors, with the expression of a number of genes becoming increasingly restricted to atrial, atrioventricular, ventricular, and outflow tract regions. For example, Tbx20 encodes a transcription factor with heart chamber–promoting characteristics. Tbx20 negatively regulates Tbx2, a transcription factor normally expressed in non-chamber myocardium, such as the wall of the atrioventricular canal and outflow tract, by sequestering receptor-mediated Smad signaling. Hence, Tbx20 and Tbx2 work in concert to delineate chamber from non-chamber myocardium along the cardiac tube.
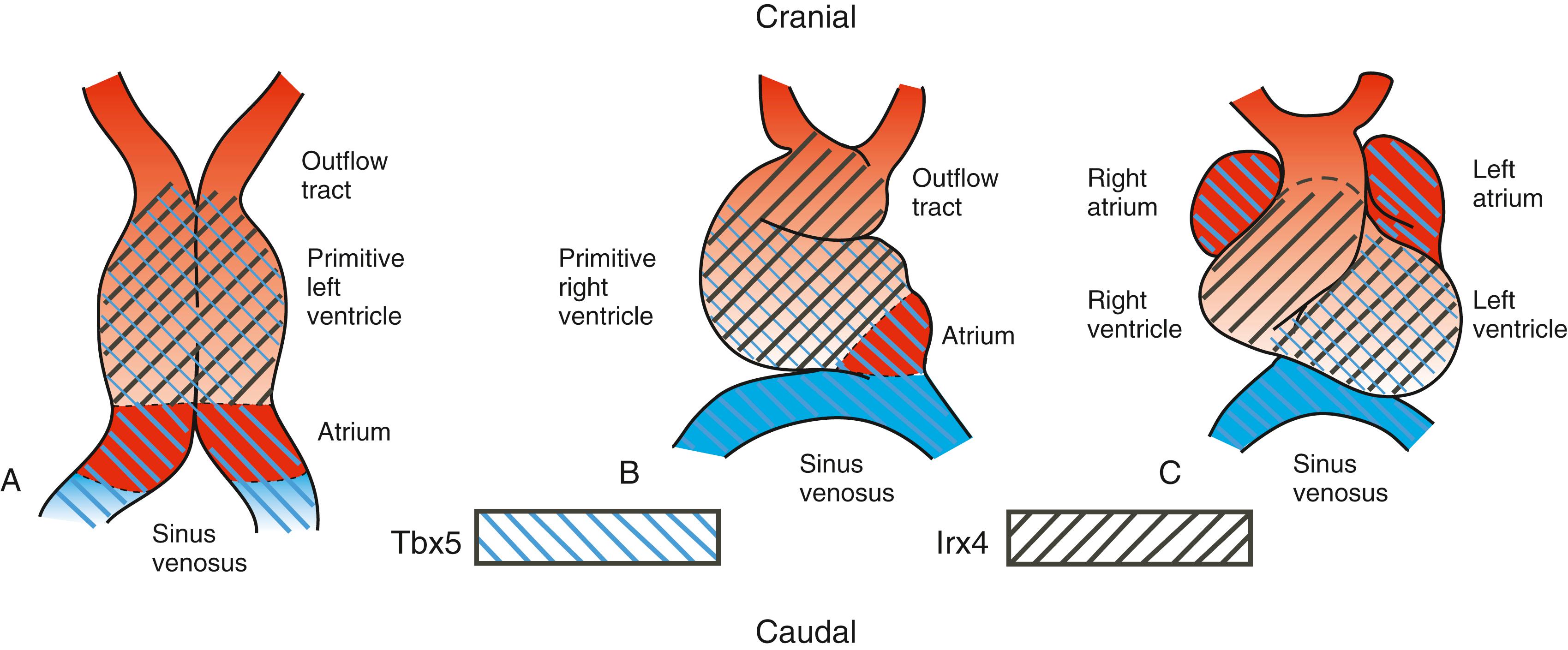
Expression of several of the chamber-specific properties depends on many of the same cranial/caudal-patterning influences driving regionalization of the neural ectoderm and paraxial mesoderm. Application of excess retinoic acid during early chick embryo cardiogenesis causes “atrialization” or “caudalization” of the primitive heart tube, as indicated by ubiquitous expression of Tbx5 throughout the heart tube, whereas treatment with retinoic acid antagonists leads to “ventricularization.” Atrial gene expression in mice expands with retinoic acid treatments in utero. A potential mechanism for localized retinoid signaling in embryos is the restricted expression of retinaldehyde dehydrogenenase2 (Raldh2), a limiting enzyme in retinoic acid biosynthesis. Restricted expression of Raldh2 to the caudal border of the cardiogenic field is correlated with the caudal limit of atrial gene expression in both chick and mouse embryos. Mouse embryos deficient in Raldh2 have reduced Tbx5 expression in the caudal heart field, lack atria and limbs, and die in utero. Recent studies support the hypothesis that retinoic acid plays an essential role in establishing the caudal boundary of the cardiogenic field.
Many of the major vessels of the embryo, including the paired dorsal aortae, develop at the same time as the endocardial tube. The inflow and outflow vessels of the future heart make connections with the endocardium of the primary heart tube even before this tube translocates into the thorax. The paired dorsal aortae , which form the primary outflow vessels of the heart, develop in the dorsal mesenchyme of the embryonic disc on either side of the notochord. As the flexion and growth of the head fold carry the heart tube into the cervical and then thoracic region, the cranial ends of the dorsal aortae are pulled ventrally until they form a dorsoventral loop—the first pair of aortic arch arteries ( Fig. 12.17 ; see also Figs. 12.4, 12.7, 12.8, 12.9 ). A series of four more aortic arch arteries develop during the fourth and fifth weeks in connection with the mesenchymal pharyngeal arches (covered in Chapter 13, Chapter 17 ). In addition, the craniocaudal flexure facilitates cardiac looping by bringing the venous (sinus venosus) and arterial (distal outflow tract and aortic sac) poles closer to one another in a process called convergence .
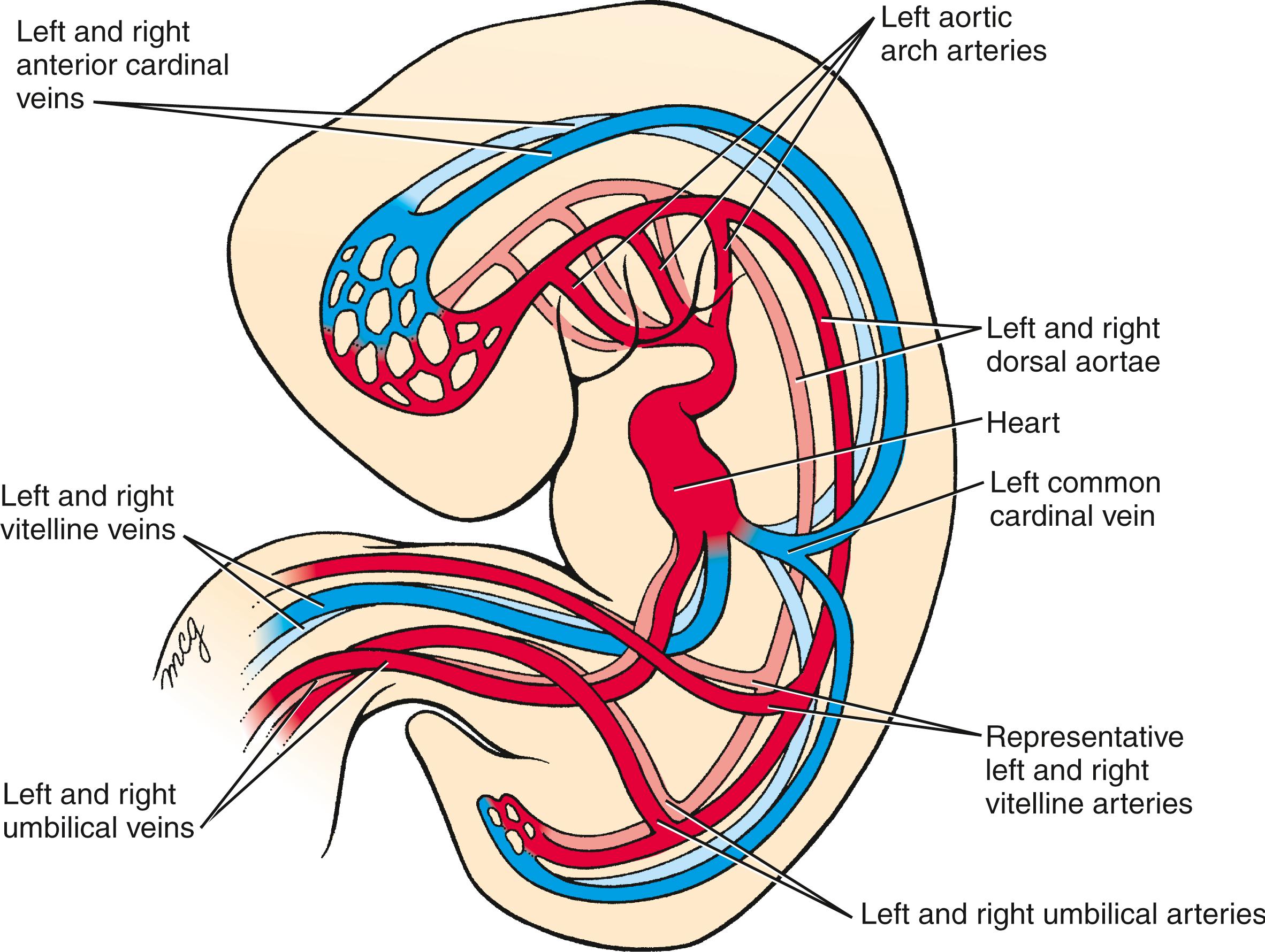
Six vessels, three on each side (see Fig. 12.17 ), initially provide the inflow to the heart. Venous blood from the body of the embryo enters the heart through a pair of short trunks, the common cardinal veins . These form from the confluence of the paired posterior cardinal veins draining the trunk and the paired anterior cardinal veins draining the head region (see Fig. 12.17 ). A pair of vitelline veins drains the yolk sac, and a pair of umbilical veins delivers oxygenated blood to the heart from the placenta. The embryonic venous system is discussed in Chapter 13 .
At day 22, the primitive circulatory system is bilaterally symmetrical: right and left cardinal veins (common, anterior, and posterior) drain the two sides of the body, and blood from the heart is pumped into the right and left aortic arches and dorsal aortae. The paired dorsal aortae fuse at axial levels T4 to L4 during the fourth week to form a single midline dorsal aorta. The venous system undergoes a complicated remodeling (detailed in Chapter 13 ), with the result that all systemic venous blood drains into the right atrium through the newly formed superior and inferior caval veins.
The heart starts to beat on day 21, and by day 24 to 25, blood begins to circulate throughout the embryo. Venous return initially enters the right and left sinus horns via the common cardinal, umbilical, and vitelline veins ( Fig. 12.18 ). Within the next few weeks, the venous system is remodeled so that all systemic venous blood enters the right sinus horn via the superior and inferior caval veins (see Fig. 12.18 ). As venous inflow shifts to the right, the left sinus horn ceases to grow and transforms into a small venous sac on the posterior wall of the heart (see Fig. 12.18 ). This structure gives rise to the coronary sinus and the small oblique vein of the left atrium . The coronary sinus will receive most of the blood draining from the coronary circulation of the heart.
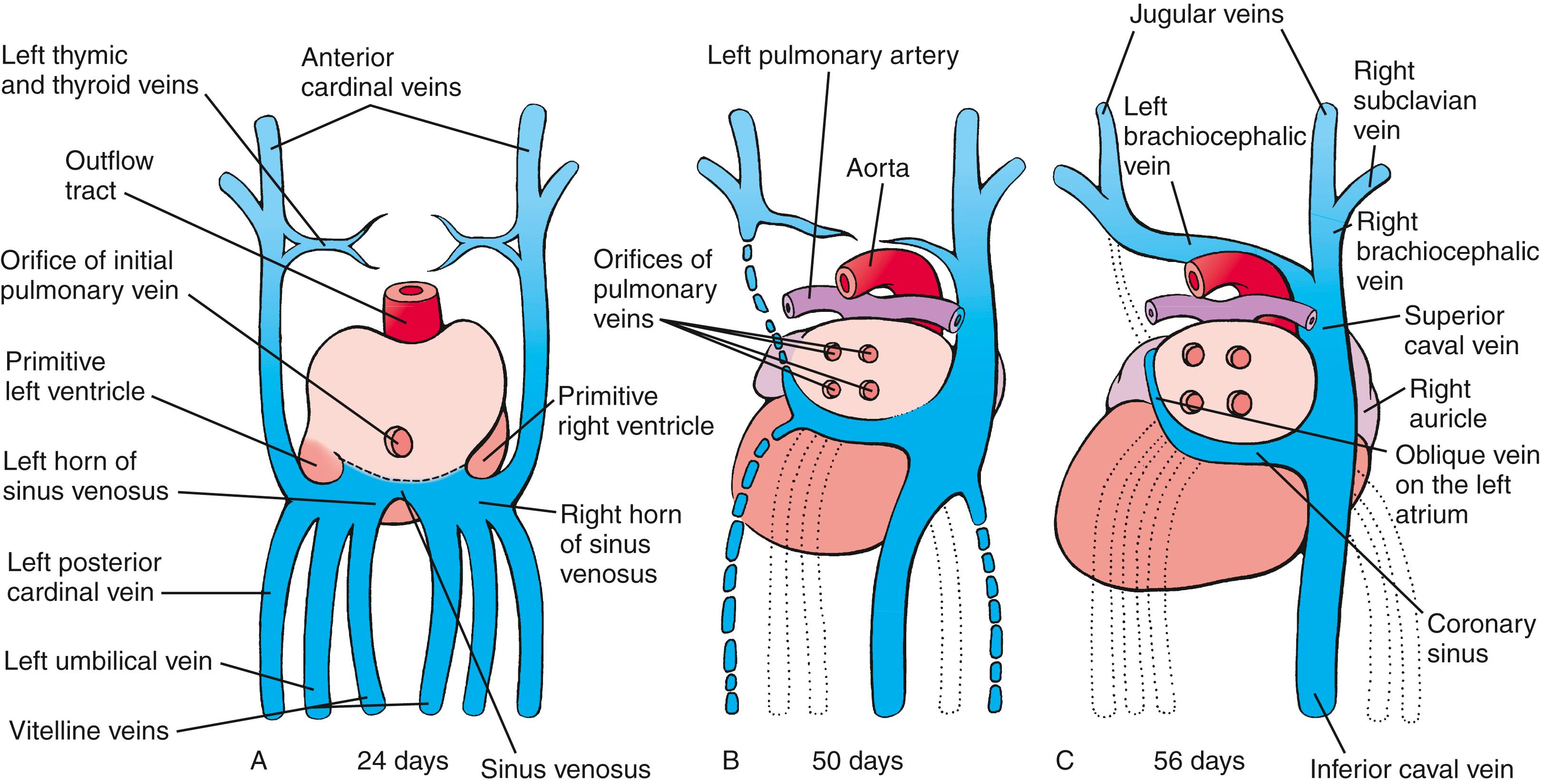
As the right sinus horn and the caval veins enlarge to keep pace with the rapid growth of the rest of the heart, the right side of the sinus venosus is gradually incorporated into the right caudal/dorsal wall of the developing atrium, displacing the original right half of the primitive atrial wall farther to the right ( Figs. 12.18, 12.19 ). The differential growth of the right sinus venosus also repositions the opening of the vestigial left sinus horn (the future coronary sinus) to the right. The portion of the atrium consisting of the incorporated sinus venosus is now called the sinus venarum . The original right side of the primitive atrium is distinguished in the adult heart by the pectinate (comb-like) trabeculation of its wall, which contrasts with the smooth wall of the sinus venarum.
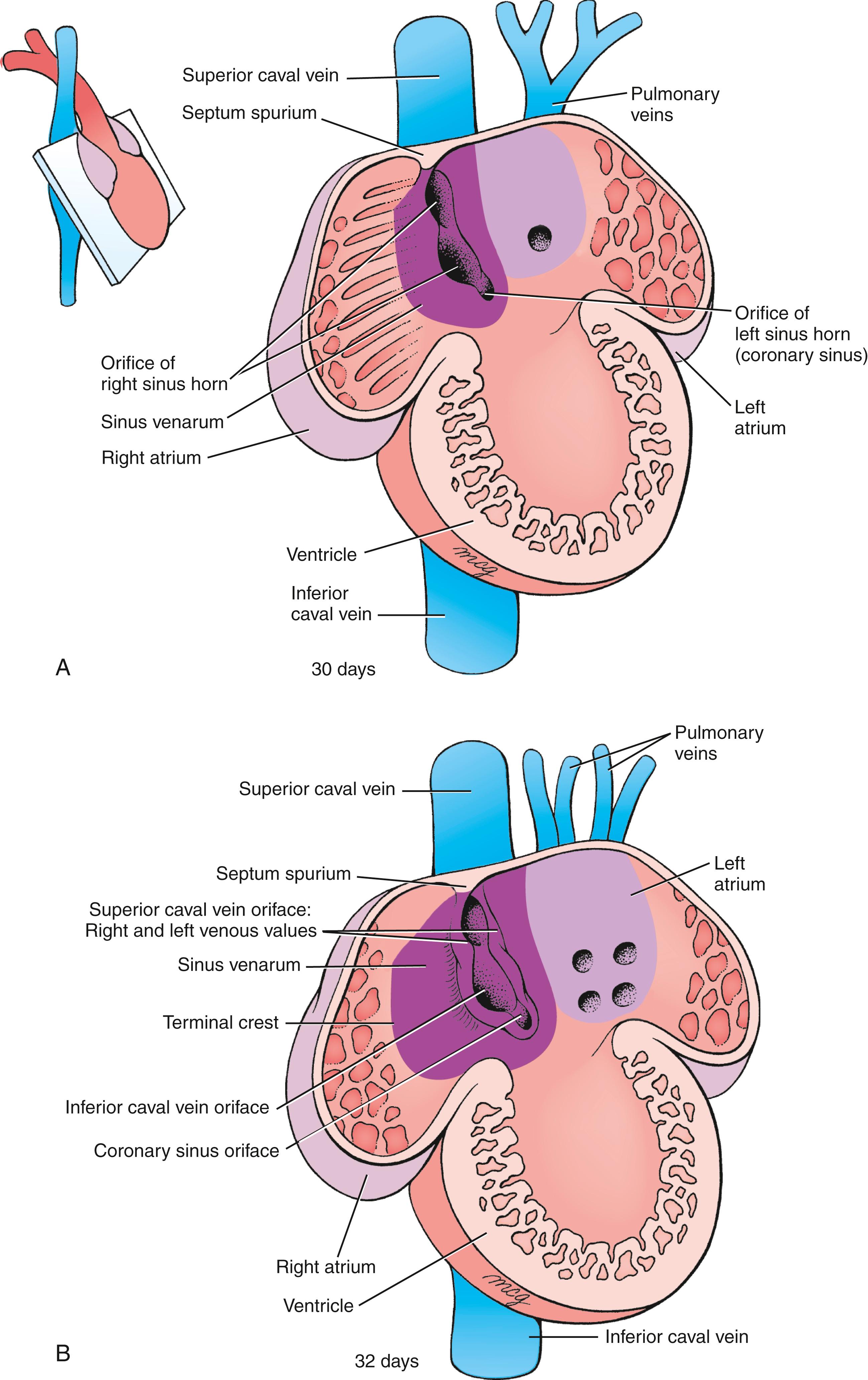
Through a process of intussusception (folding in of an outer layer) of the right sinus venosus, the openings, or ostia , of the superior and inferior caval veins and future coronary sinus are incorporated into the dorsal wall of the definitive right atrium, where they form the orifices of the superior and inferior caval veins and the orifice of the coronary sinus (see Fig. 12.19B ). As this occurs, a pair of tissue flaps, the left and right venous valves , develops on either side of the three ostia (see Fig. 12.19B ). Cranial to the sinuatrial orifices, the left and right valves join to form a transient septum called the septum spurium , which, along with the left venous valve, becomes part of the septum secundum, one of the septa contributing to the separation of the definitive right and left atria (covered later in the chapter). The right venous valve persists and contributes to the formation of the valve of the inferior caval vein and valves of the coronary sinus . Incorporation of the sinus venosus tissue into the dorsal wall of the right atrium results in remodeling of the right atrial chamber and the formation of the right atrial appendage (the right auricle ).
The terminal crest now delimits the trabeculated right atrium from the smooth-walled sinus venarum (see Fig. 12.19B ). The sinoatrial node , the cardiac pacemaker, is an important element of the cardiac conducting system and is located at the junction of the superior caval vein and the terminal crest. The cardiac impulse generated in the sinoatrial node reaches the atrioventricular (AV) node using several preferred pathways.
While the right atrium is remodeled during the fourth and fifth weeks, the left atrium undergoes a somewhat similar process. During the fourth week, the pulmonary vein originates as a midline structure within the caudal dorsal mesocardium , which connects the lung rudiments to the dorsal wall of the developing common atrium. From its initial midline position, the pulmonary vein shifts to the left (see Figs. 12.18, 12.19A ) as a result of asymmetrical growth of a projection of second heart field mesenchymal cells called the dorsal mesenchymal protrusion or spina vestibuli . The pulmonary vein promptly splits into right and left pulmonary branches, which bifurcate again to produce a total of four pulmonary veins. These veins then grow toward the lungs, where they anastomose with veins developing within the mesoderm investing the bronchial buds (covered in Chapter 11 ). As a result of intussusception, the pulmonary venous system opens into the left atrium initially through a single orifice and eventually through four orifices forming the definitive pulmonary veins (see Fig. 12.19A,B ), where they form the smooth wall of the definitive left atrium. The trabeculated left side of the primitive atrium is also displaced ventrally and to the left, where it forms a left atrial appendage (the left auricle ).
![]()
Animations are available online at StudentConsult.
Structural and functional partitioning of the heart into four chambers occurs through a process called valvuloseptal morphogenesis , which encompasses septation (formation of septal structures) and valvulogenesis (formation of valves). Major events for cardiac septation occur between days 28 and 37 of gestation. Two basic processes play key roles in generating septa. Differential growth and remodeling are mainly responsible for generating the muscular ventricular and atrial septa, but these processes alone never fully partition the heart chambers. For that, endocardium-derived and neural crest cell–derived cushion tissue is required. In the atrioventricular and outflow tract regions, while cardiac looping continues, extracellular matrix is secreted between endocardium and myocardium, chiefly by the myocardial layer ( Fig. 12.20A ). This essentially causes the endocardial layer to balloon into the lumen of these two regions. Near the completion of cardiac looping, some of the endocardial cells in the atrioventricular and outflow tract regions undergo an epithelial-to-mesenchymal transition (EMT), generating endocardium-derived mesenchyme that invades this extracellular matrix, proliferates, and differentiates into connective tissue. These mesenchyme-filled bulges (in the atrioventricular region) and ridges (along the length of the outflow tract) are often referred to as cushion tissues (see Figs. 12.20B,C, 12.21 ). After the initial formation of the endocardium-derived atrioventricular cushions, some epicardium-derived mesenchymal cells also populate the atrioventricular cushions. As covered later in the chapter, not only does the cushion tissue of the outflow tract contain endocardium-derived cells, but neural crest cells also invade these ridges. Thus, the cushion tissue of the outflow tract consists of both mesoderm-derived mesenchymal cells (endocardium-derived cushion tissue) and ectoderm-derived mesenchymal cells (neural crest cell–derived cushion tissue) (see Fig. 12.20B ). Proper development of atrioventricular and outflow tract cushion tissues is essential for completing septation. Two major atrioventricular cushions fuse and contribute to the separation of the atria and ventricles, generate the fibrous (or membranous) portion of the ventricular and atrial septa, and, together with lateral cushions, are involved in the formation of atrioventricular valves (see Fig. 12.20B ). The outflow tract cushions are involved in separation of the aorta from the pulmonary artery, ventricular septation, and in formation of the semilunar valves.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here