Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The endodermal gut tube created by body folding during the fourth week (covered in Chapter 4 ) consists of a blind-ended cranial foregut, a blind-ended caudal hindgut, and a midgut open to the yolk sac through the vitelline duct. The gut endoderm forms the lining (epithelium) of the gastrointestinal tract, and it recruits the adjacent splanchnic mesoderm to form the other layers and major components of the gut (smooth muscle, connective tissue, gut vasculature). As the process of body folding is completed, there remains a thin layer of splanchnic mesoderm called the dorsal mesentery, connecting the gut tube along its entire axial length to the dorsal portion of the abdominal body wall. Thus the primitive gut is a tube suspended from the dorsal abdominal body wall by the dorsal mesentery. The parts of the dorsal mesentery associated with the differentiating organs are named according to the region of the gut tube they suspend. Importantly, the dorsal mesentery is a vascular conduit and supplies all of the essential oxygen and nutrients to the developing gut. As covered in Chapter 13 , the arterial supply to the gut develops through consolidation and reduction of the ventral branches of the dorsal aortae that anastomose with the vessel plexuses originally supplying blood to the yolk sac. About five of these vitelline artery derivatives vascularize the thoracic foregut, and three—the celiac, superior mesenteric, and inferior mesenteric arteries —vascularize the abdominal gut. By anatomical convention, the boundaries of the foregut, midgut, and hindgut portions of the abdominal gut tube are determined by the respective territories of these three arteries. However, these regions and the site of some gastrointestinal organs are already demarcated by specific gene expression patterns before this vasculature is established and are refined by subsequent reciprocal endodermal-mesodermal interactions.
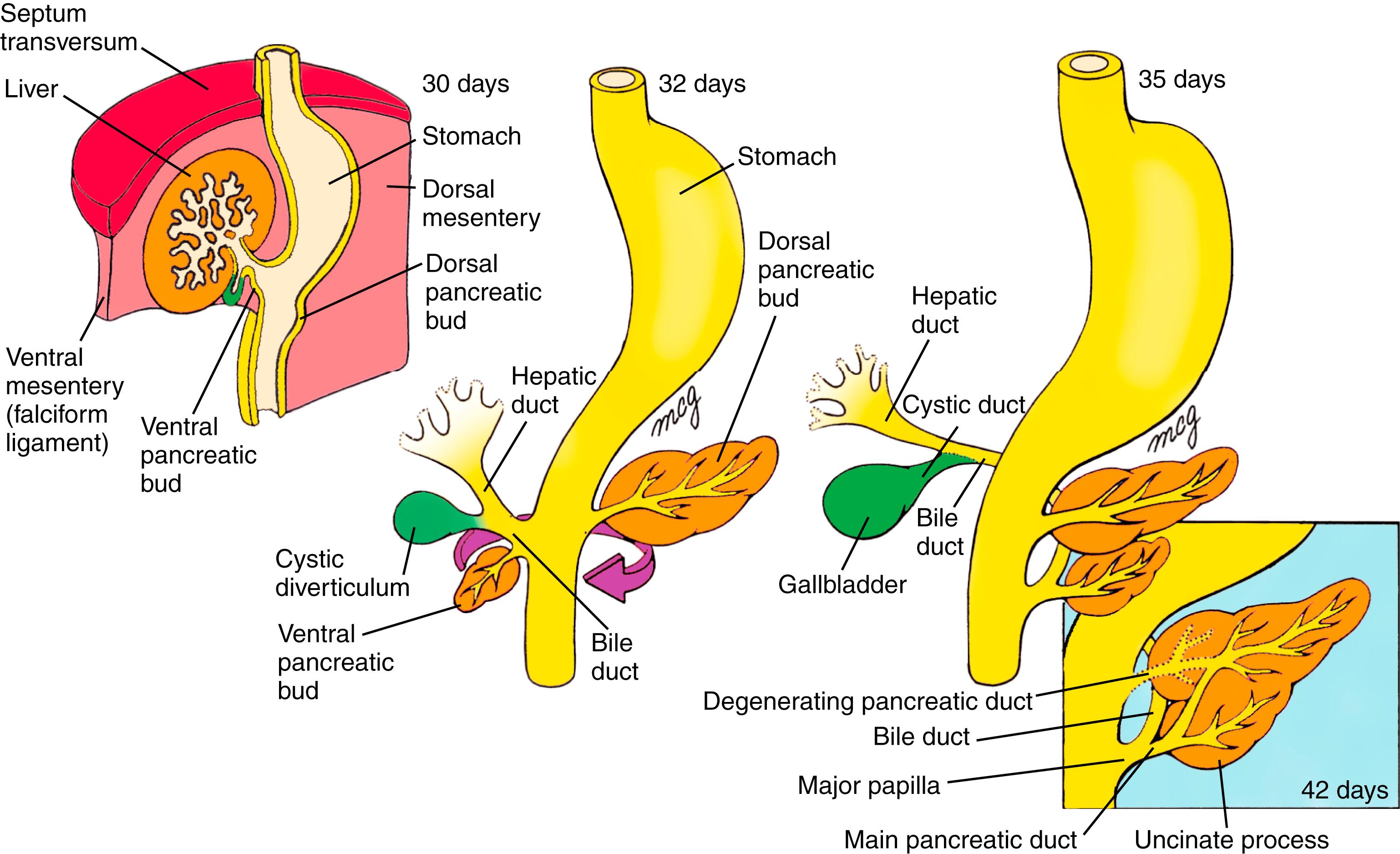
By the fifth week, the thoracic and abdominal portion of the foregut is visibly divided into the pharynx, esophagus, stomach, and proximal duodenum . The stomach is initially fusiform, and differential growth of its dorsal and ventral walls produces the greater and lesser curvatures. Meanwhile, hepatic, cystic, and dorsal and ventral pancreatic diverticula bud from the caudal duodenum into the ventral mesogastrium and give rise, respectively, to the liver, gallbladder and cystic duct, and pancreas . In addition, the spleen condenses from mesenchyme in the dorsal mesogastrium.
During the sixth and seventh weeks, the stomach rotates around longitudinal and dorsoventral axes so that the greater curvature is finally directed to the left and slightly caudal. This rotation shifts the liver to the right in the abdominal cavity and brings the duodenum and pancreas into contact with the posterior body wall, where they become fixed (i.e., secondarily retroperitoneal ). This event converts the space dorsal to the rotated stomach and dorsal mesogastrium into a recess called the lesser sac of the peritoneum . The pouch of dorsal mesogastrium forming the left lateral boundary of the lesser sac subsequently undergoes voluminous expansion, giving rise to the curtain-like greater omentum, which drapes over the inferior abdominal viscera.
The midgut forms the distal duodenum, jejunum, ileum, cecum, ascending colon, and proximal two-thirds of the transverse colon . The future ileum elongates more rapidly than can be accommodated by the early peritoneal cavity so that by the fifth week the midgut is thrown into an anteroposterior hairpin fold, the primary intestinal loop, which herniates into the umbilicus during the sixth week. As the primary intestinal loop herniates, it rotates around its long axis by 90 degrees counterclockwise (as viewed from the ventral side) so that the future ileum lies in the right abdomen and the future large intestine lies in the left abdomen. Meanwhile,
A 1-week-old infant male is seen in a community health clinic. His mother says that the boy has not been eating well for 2 days and has been irritable, especially after feeds. Then, beginning last night, he started vomiting a dark greenish liquid consistent with bile. On examination, the infant cries inconsolably and is weak. His heart rate is elevated and his extremities are cool: symptoms of dehydration. His abdomen is somewhat distended. Sips of hydration fluid are returned with bilious vomit.
Intravenous hydration is begun and a nasogastric tube is placed to decompress the abdomen. The infant is transferred by ambulance to the children’s hospital, where an upper gastrointestinal tract (upper GI) series is ordered (i.e., sequential X-rays done after ingestion of barium, a radio-opaque liquid used to coat the inside of the digestive system). This study shows markedly delayed gastric emptying and dilation of the duodenum with delayed filling of the jejunum. As the jejunum fills with barium, it takes on an “apple peel” appearance ( Fig. 14.1 ). A subsequent X-ray shows partial filling of the small intestine, which lies predominantly in the right abdomen. The diagnosis of intestinal malrotation with intestinal obstruction is made, and emergency surgery is performed. In the operating room, the surgeons find a midgut volvulus (torsion of the small intestine) with an ischemic bowel twisted around a narrow mesenteric pedicle. They untwist the bowel and return it to the abdomen. One day later, they re-examine the bowel and remove a 20-cm necrotic portion. Later, the small intestines are reconnected and the bowel fixed in place so that twisting cannot recur.
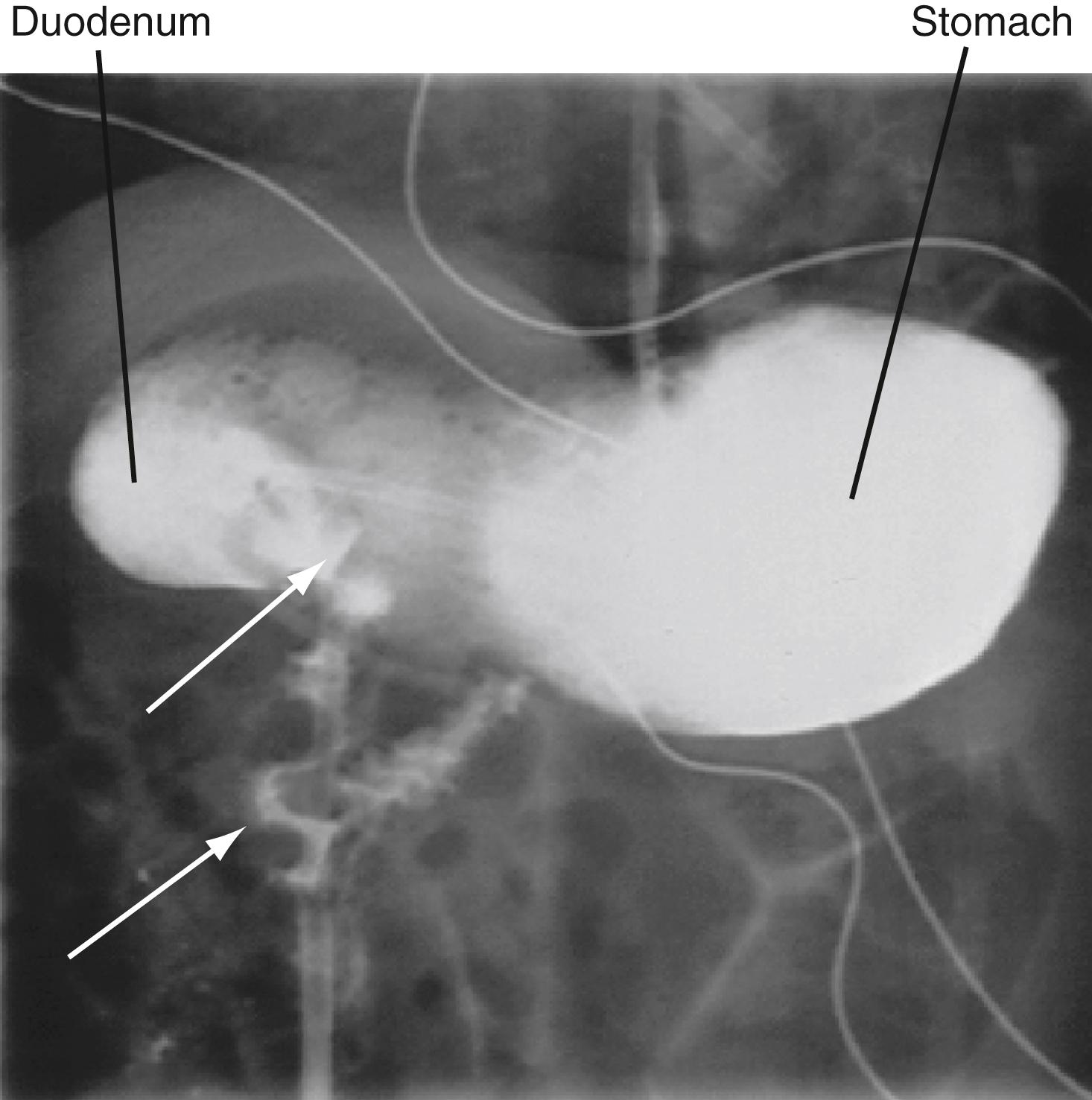
Intestinal malrotation occurs when the midgut fails to complete its rotation during the 10th through 12th week of development as it returns to the peritoneal cavity from the umbilical herniation. This leaves the small intestine in the right side of the abdomen tethered to the mesenteric vasculature by a narrowed mesentery. The small bowel can twist around this narrow tether, causing intestinal obstruction and cutting off its circulation, resulting in necrosis. This usually presents in infancy, but cases presenting as late as young adulthood have been reported. The cause of intestinal malrotation is unknown, but recent studies in chicks and mice link midgut malrotation to perturbed production of the extracellular matrix molecule hyaluronan within the midgut dorsal mesentery.
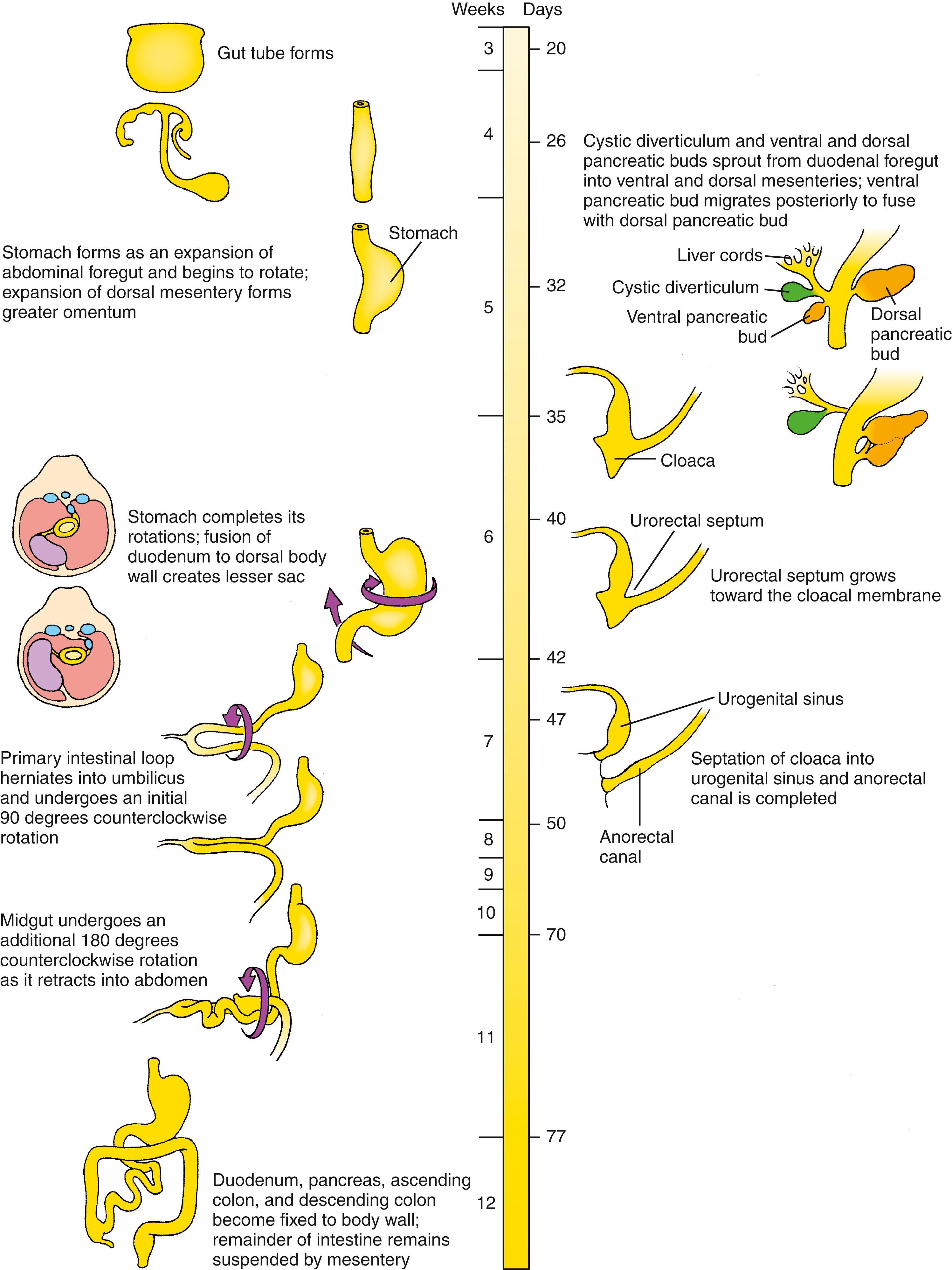
the cecum and appendix differentiate, and the jejunum and ileum continue to elongate. During the 10th through 12th weeks, the intestinal loop is retracted into the abdominal cavity and rotates through an additional 180 ° counterclockwise to produce the definitive configuration of the small and large intestines. Asymmetric cell behavior across the left-right axis of the dorsal mesentery initiates the counterclockwise gut rotation, and failure to correctly rotate leads to intestinal malrotation and midgut volvulus .
The hindgut forms the distal third of the transverse colon, the descending and sigmoid colon , and the upper two-thirds of the anorectal canal . Just superior to the cloacal membrane, the primitive hindgut forms an expansion called the cloaca . During the fourth to sixth weeks, a coronal urorectal septum partitions the cloaca into the urogenital sinus , which will give rise to urogenital structures, and a dorsal anorectal canal .
Between the sixth and eighth weeks, intestinal villi develop as the gut mesoderm and its associated intestinal epithelium form multiple projections into the intestinal lumen. Cytodifferentiation of the gut epithelium depends on interactions with the underlying mesoderm and is regionally specified based on the cranial-caudal axis and the radial axis (lumen to outer tunic) of the gut. Migrating neural crest cells form the enteric nervous system .
![]()
Animations are available online at StudentConsult.
As covered in Chapter 4 , the longitudinal and transverse folding of the embryo in the third and fourth weeks converts the flat trilaminar embryonic disc into a trilaminar, elongated cylinder ( Fig. 14.2 ). Because of cranial and caudal body folding, cranial and caudal endodermal pockets form (see Fig. 14.2A–F ). As the cranial and caudal pockets elongate with lengthening of the embryo, the lateral body folds meet in the ventral midline and fuse to generate the elongated body cylinder (see Fig. 14.2G,H ). The outer layer is the ectoderm (the future epidermis), which now covers the entire outer surface of the embryo except in the umbilical region, where the yolk sac and the connecting stalk emerge. The innermost layer is the endodermal primary gut tube . Separating these two layers is a layer of mesoderm that contains the coelom. Thus the three germ layers bear the same fundamental topologic relation to each other after folding as they did in the flat embryonic disc.
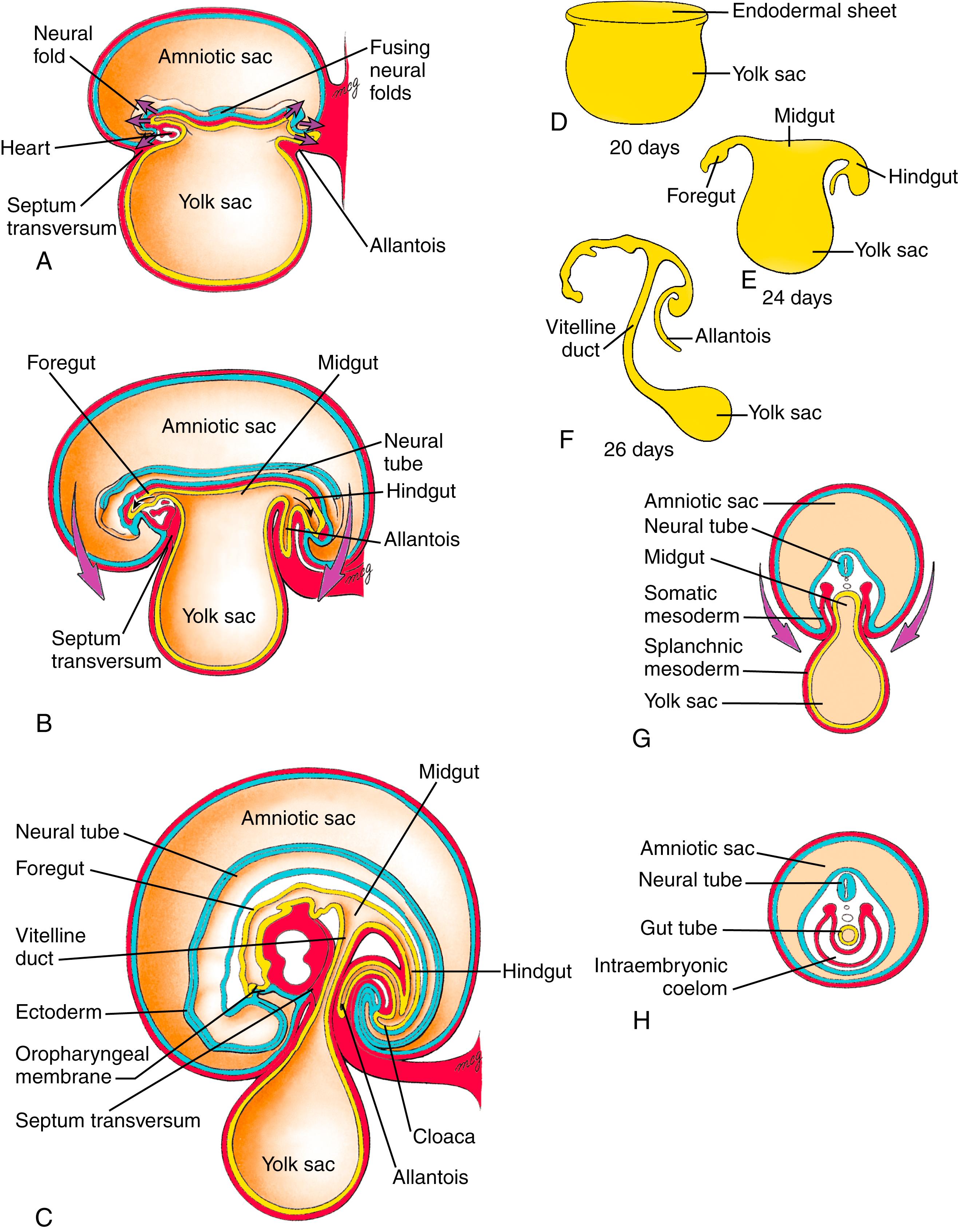
Body folding plays an essential role in internalizing the endoderm, as mutations in genes involved in body folding exhibit not only body folding defects but also endodermal tube defects. By the time body folding is nearly complete, the gut tube consists of cranial and caudal blind-ending tubes, the presumptive foregut and hindgut , and a central midgut , which still opens ventrally to the yolk sac. Cranially, the foregut terminates at the oropharyngeal membrane (or buccopharyngeal membrane); caudally, the hindgut terminates at the cloacal membrane . Because the embryo and gut tube lengthen relative to the yolk sac, and folding continues to convert the open midgut into a tube, the neck of the yolk sac narrows until it becomes the slender vitelline duct. The vitelline duct (or yolk stalk ) and yolk sac are eventually incorporated into the umbilical cord. Table 14.1 lists the organs and structures that are ultimately derived from the three portions of the gut tube.
| Regions of Differentiated Gut Tube | Accessory Organs Derived from Gut Tube Endoderm |
|---|---|
| Foregut | |
| Pharynx | Pharyngeal pouch derivatives (see Chapter 17 ) |
| Thoracic esophagus | Lungs (see Chapter 11 ) |
| Abdominal esophagus | |
| Stomach | |
| Proximal half of duodenum (superior to ampulla of pancreatic duct) | Liver parenchyma and hepatic duct epithelium Gallbladder, cystic duct, and common bile duct Dorsal and ventral pancreas |
| Midgut | |
| Distal half of duodenum | |
| Jejunum | |
| Ileum | |
| Cecum | |
| Appendix | |
| Ascending colon | |
| Right two-thirds of transverse colon | |
| Hindgut | |
| Left one-third of transverse colon | |
| Descending colon | |
| Sigmoid colon | |
| Anorectal canal | Urogenital sinus and derivatives (see Chapter 15 , Chapter 16 ) |
During the process of lateral body folding, the endodermal lining of the gut tube remains covered by a wall of lateral plate splanchnic mesoderm (see Fig. 14.2G,H ). This mesoderm condenses and differentiates into the lamina propria, submucosa, muscular walls, vascular elements, and connective tissue of the gastrointestinal tract and organs. Once the basic gut tube regions are formed, various organs develop within specific regions that are delineated by restricted gene expression and tissue-tissue interactions.
When the coelom first forms, the gut is broadly attached to the dorsal body wall by the mesoderm ( Fig. 14.3A ). However, in the region of the future abdominal viscera (from the abdominal esophagus to the most proximal part of the future rectum), the mesenchyme within this region of attachment gradually disperses during the fourth week, resulting in formation of a thin, bilayered dorsal mesentery that suspends the abdominal viscera in the coelomic cavity (see Fig. 14.3B ). Because the abdominal gut tube and its derivatives are suspended within this part of the intraembryonic coelom that later becomes the peritoneal cavity , they are referred to as intraperitoneal viscera.
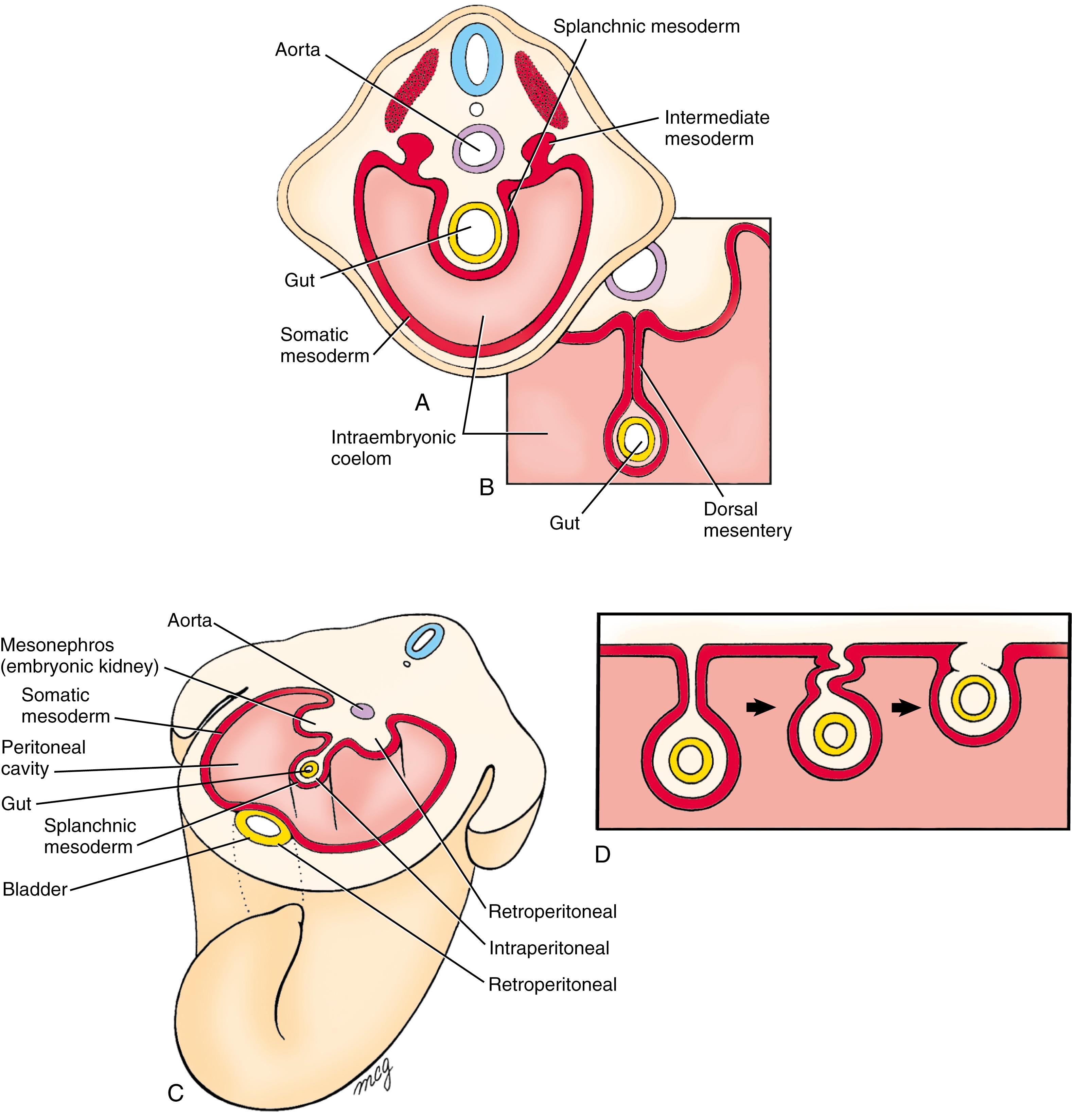
In contrast to the intraperitoneal location of most of the gut tube and its derivatives, some of the visceral organs develop within the body wall and are separated from the coelom by a covering of serous membrane (see Fig. 14.3C ). These organs are said to be retroperitoneal . It is important to realize that the designation retroperitoneal means that an organ is located behind the peritoneum from a viewpoint inside the peritoneal cavity—not that it is necessarily located in the posterior body wall. Thus the kidneys are retroperitoneal, but so is the bladder, which develops in the anterior body wall (see Fig. 14.3C ).
Further complicating the intraperitoneal/retroperitoneal distinction is that some parts of the gut tube that are initially suspended by mesentery later become fused to the body wall, thus taking on the appearance of retroperitoneal organs (see Fig. 14.3D ). These organs, which include the ascending and descending colon, duodenum, and pancreas, are said to be secondarily retroperitoneal .
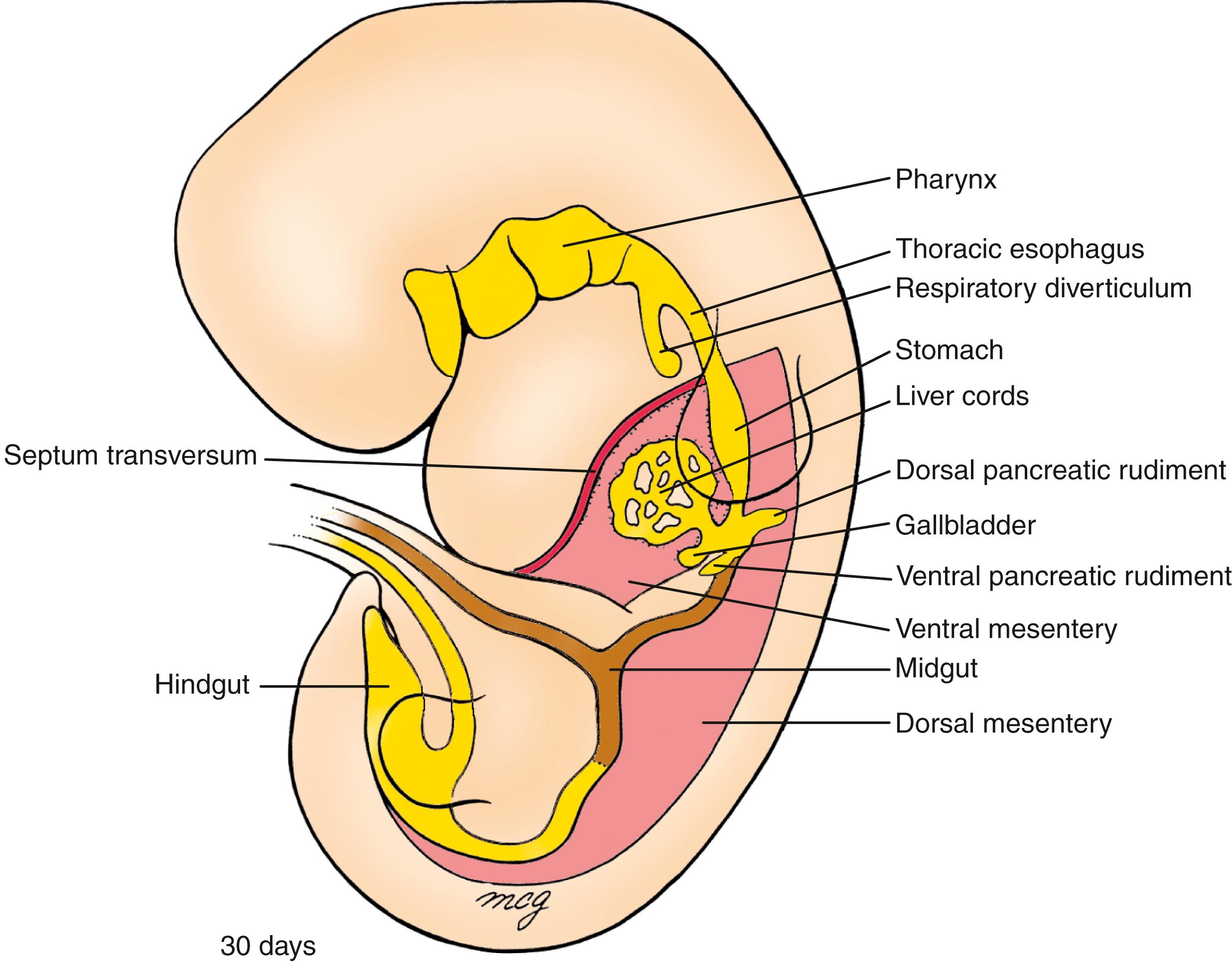
At the end of the fourth week, almost the entire abdominal gut tube—the portion within the peritoneal cavity from the abdominal esophagus to the superior end of the developing cloaca—hangs suspended by the dorsal mesentery. Except in the region of the developing stomach, the coelomic cavities in the lateral plate mesoderm on either side of the embryonic disc coalesce during folding to form a single, continuous peritoneal cavity. In the stomach region, the gut tube remains connected to the ventral body wall by the thick septum transversum. By the fifth week, the caudal portion of the septum transversum thins to form the ventral mesentery connecting the stomach and developing liver to the ventral body wall ( Fig. 14.4 ).
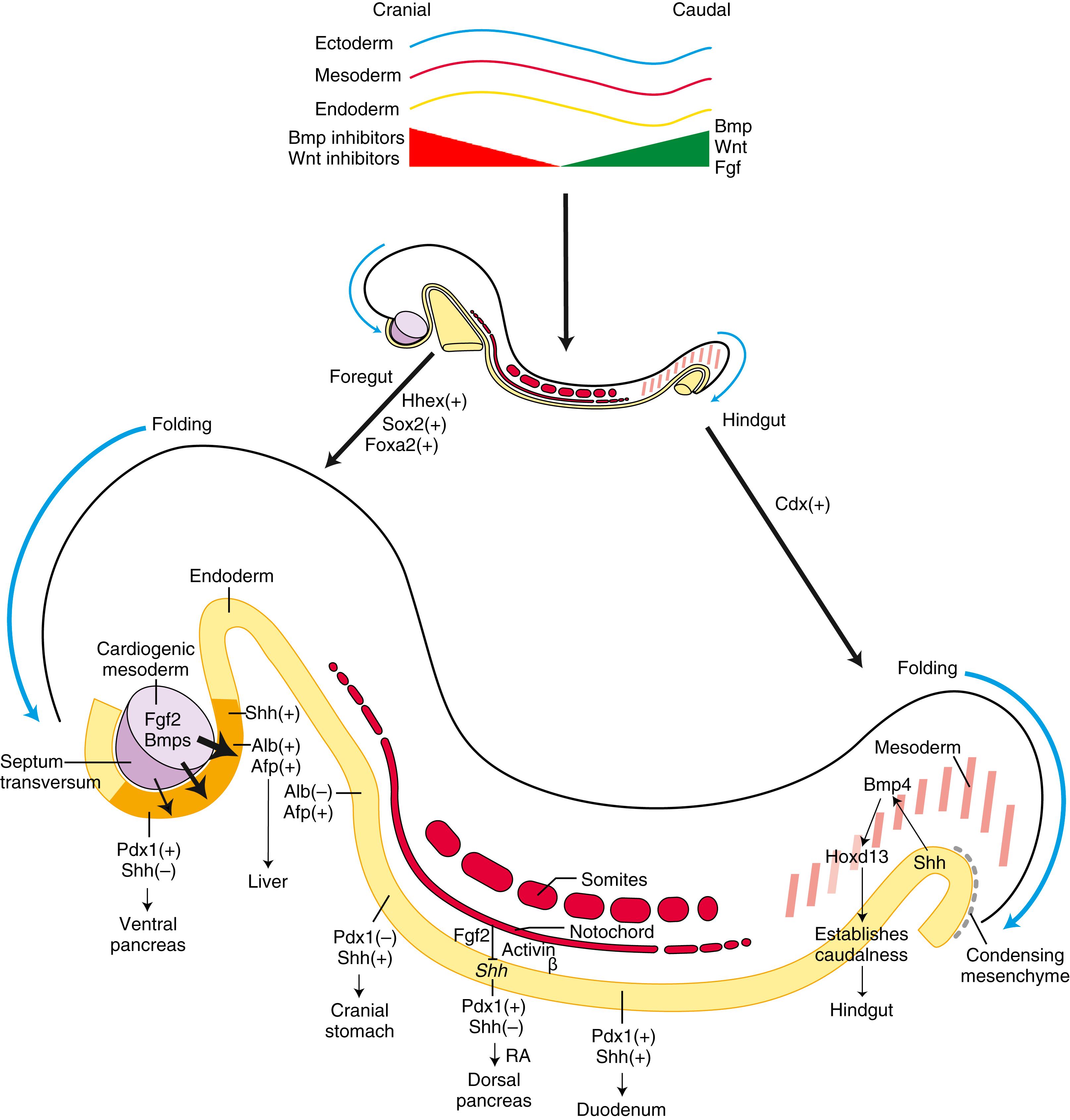
By convention, the terms foregut , midgut , and hindgut correspond to the territories of the three arteries supplying the abdominal gut tube. In addition, the terms foregut and hindgut are used to describe the endodermal regions cranial and caudal to the anterior and posterior intestinal portals, respectively. As covered in Chapter 13 , the gut tube and its derivatives are vascularized by unpaired ventral branches of the descending aorta. These branches develop by a process of consolidation and reduction from the left and right vitelline artery plexuses that arise on the yolk sac, spread to vascularize the gut tube, and anastomose with the dorsal aortae (see Fig. 13.15 ). About five definitive aortic branches supply the thoracic part of the foregut (the pharynx and thoracic esophagus; development of the pharyngeal part of the foregut is covered in Chapter 17 ). Three arteries serve the remainder of the gut tube: the celiac trunk , which supplies the abdominal foregut (the abdominal esophagus, stomach, and cranial half of the duodenum and its derivatives); the superior mesenteric trunk , which supplies the midgut; and the inferior mesenteric artery , which supplies the hindgut. To avoid strangulation of gut vessels, their development must be coordinated with the complex looping characterizing gut morphogenesis. Indeed, arteriogenesis in the midgut is commensurate with the onset of gut rotation and proceeds strictly on the left side of the dorsal mesentery.
Different endodermal segments are marked by specific patterns of segmental and homeotic gene expression within the developing gut well before formation of the three arteries that supply the gut and are used by convention to subdivide it into foregut, midgut, and hindgut regions ( Fig. 14.5 ). For instance, during late-stage mouse gastrulation, Lim homeobox-1 (Lhx1), homeobox expressed in embryonic stem (ES) cells-1 (Hesx1), and cerberus-like (Cerl) are expressed within the cranial definitive endoderm, whereas caudal-type homeobox (Cdx) expression demarcates the caudal endoderm.
![]()
Animations are available online at StudentConsult.
Regionalization of the gut plays an important role in demarcating sites of organ formation. Regional specification of endoderm and its interaction with mesoderm, neural crest cells, and ectoderm play important roles in mediating the development of the pharyngeal arches and pharyngeal vasculature, as well as in organ formation in the cranial region (covered in Chapter 13, Chapter 17 ). The following text is limited to gastrointestinal development caudal to the pharyngeal arches.
How does the early endoderm obtain its cranial-caudal identity? Less is known regarding the regionalization of the early endoderm than is known regarding that of the ectoderm and mesoderm. The ectoderm and mesoderm acquire much of their regional identity during gastrulation through a variety of signaling molecules derived from the primitive streak and organizer (primitive node in humans) (covered in Chapter 3 ). These same signaling molecules are involved in the regionalization of the endoderm. One of the earliest known markers delineating regional differences of the definitive endoderm was discovered from work in Drosophila, where the gene named “caudal” was identified and found to be required for gut formation (see Fig. 14.5 ). Caudal homologs in vertebrates include Cdx1, Cdx2, and Cdx4. In vertebrates, Cdx2 is expressed in the caudal endoderm, mesoderm, and ectoderm of primitive streak–stage embryos before the expression of most Hox genes (see Fig. 14.5 ). Loss of Cdx2 expression within the endoderm of mice transforms the distal hindgut endoderm into a foregut esophageal-like epithelium. Nodal expression in the primitive streak promotes anterior endodermal fate and expression of Hhex, an anterior endodermal gene. Mice null for Hhex fail to form a liver and die during embryogenesis. Caudal Wnt signaling is also required for early hindgut specification, as mice null for the Wnt downstream targets, Tcf4 and Tcf1, exhibit severe caudal truncations. Other genes important in early gut regionalization include Shh, Fgfs, Bmps, and Wnt antagonists so that by the end of gastrulation, the endoderm is partitioned into broad regions along the cranial-caudal axis.
As indicated earlier, the process of regionalizing the gut tube into foregut, midgut, and hindgut is likely initiated by events that occur during gastrulation. However, regionalization of the endoderm is further refined by tissue-tissue interactions between the germ layers. The overlying ectoderm and mesoderm provide not only permissive influences but also inductive influences on the endoderm. For instance, in vitro, mouse cranial endoderm in presomitic embryos can be respecified to express caudal endodermal markers through interactions with caudal mesoderm. However, by the early somitic stage, the endoderm is more restricted in its developmental potential. Several transcription factors and morphogens involved in refining the regionalization of the gut have been identified, some of which are illustrated in Figs. 14.5 and 14.6 and are covered briefly later.
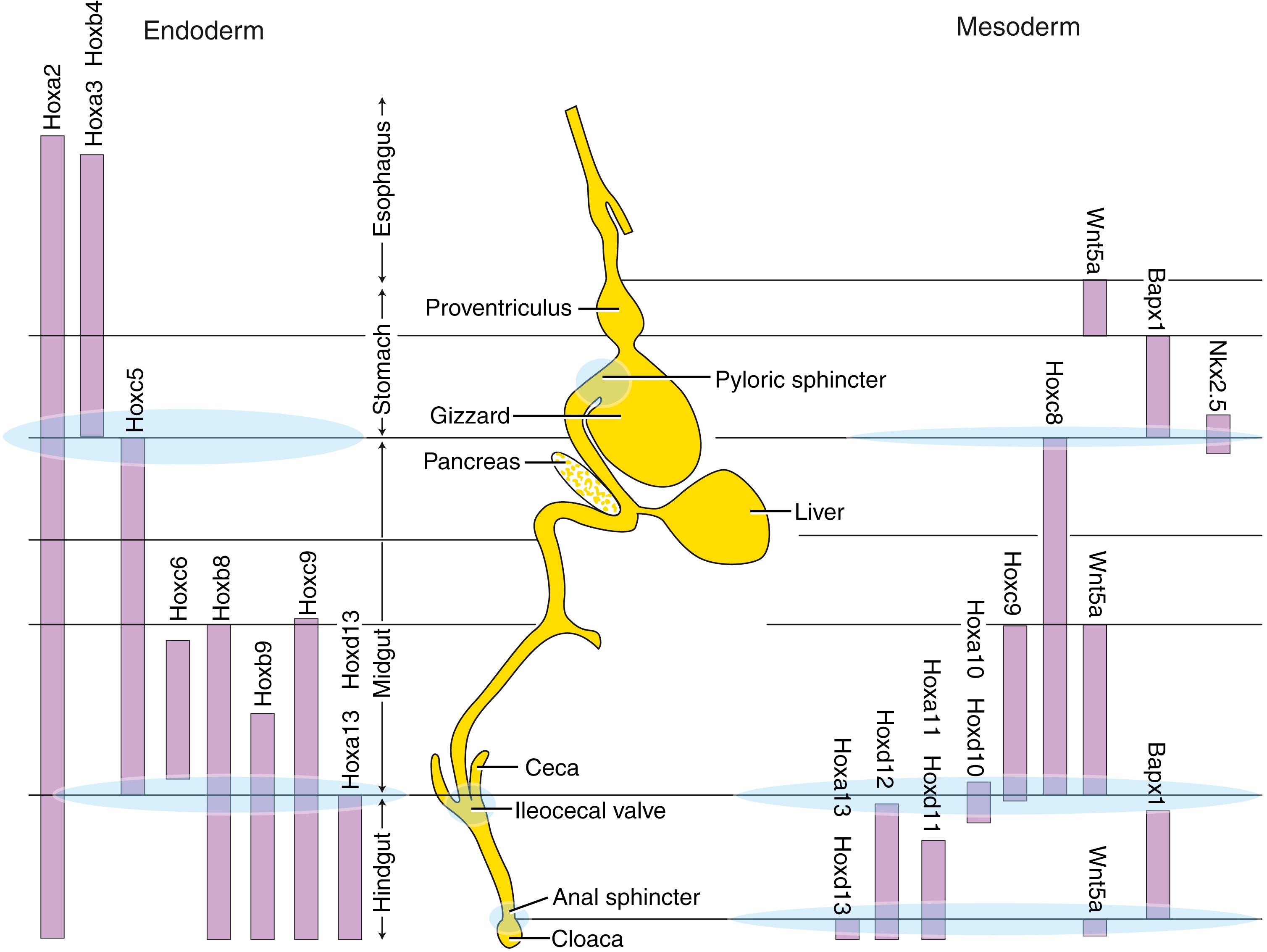
After initiation of cranial body folding, the endoderm of the ventral foregut is situated adjacent to the caudal cardiogenic mesoderm. In fish, chick, and mouse embryos, cardiogenic mesoderm, by releasing Bmps and Fgfs, stimulates Shh expression in the ventral foregut endoderm, thereby promoting hepatic development (expression of hepatic markers, albumin, and alpha-fetoprotein) and repressing pancreatic development (see Fig 14.5 ). In regions where the ventral foregut endoderm is exposed to low levels of Fgf2, the endoderm begins expressing Pdx1 (pancreas and duodenal homeobox gene), which, in turn, represses Shh expression and supports ventral pancreatic development. In the non-hepatic dorsal foregut endoderm, the expression of hepatic markers in the dorsal endoderm is repressed by the overlying dorsal axial mesoderm (possibly through Wnt and Fgf4 signaling). Thus interactions between mesoderm and endoderm play crucial roles in dictating regional organ development.
The endoderm of the foregut forms many sections of the gastrointestinal tract including the esophagus, stomach, pancreas, and proximal duodenum. Pdx1 is expressed in the early stomach and in the dorsal and ventral prepancreatic and preduodenal endoderm (see Fig. 14.5 ). In mice, Shh is expressed along the entire length of these endodermal regions, except at the site of pancreatic bud formation. Perturbation of Shh signaling within the Pdx1-expressing endoderm results in the ectopic expression of insulin in the stomach and duodenum, suggesting that Shh normally represses expression of pancreatic cell fate in all Pdx1-positive endoderm.
The dorsal endoderm of the gut tube is in contact with the notochord, and this interaction is required for proper dorsal pancreatic development. Studies in several species show that the notochord specifically represses Shh expression in the prepancreatic endoderm by releasing Fgf2 and activin β, thereby removing its repressive effect on Pdx1 expression and promoting dorsal pancreas development (see Fig. 14.5 ). Induction of the dorsal pancreatic bud also requires retinoic acid signaling, which seems to regulate the expression boundaries of Pdx1 and Cdx and hence the position of the dorsal pancreas.
The notochord is eventually displaced from the pancreatic endoderm by the fusing dorsal aortae, and this places the pancreatic endoderm under the influence of endothelial cells. In vivo and in vitro studies in Xenopus and mice show that Vegf released by the dorsal aortic endothelial cells promotes pancreatic endocrine cell fate specification.
Hox genes are also expressed within gut endoderm and play important roles in regional specification and development of the gut (see Fig. 14.5 and Fig. 14.6 ). In the chick, Hoxa3 gene expression is regionally restricted, demarcating the boundary between foregut and midgut, with Hoxd13 expression limited to the caudalmost hindgut mesoderm. Interactions between the hindgut endoderm and mesoderm seem to be responsible for restricting Hoxd13 expression. Hoxd13 instills caudal identity to the hindgut because when misexpressed in more cranial mesoderm of chick embryos, the stomach endoderm can be transformed into intestinal endoderm. Hox genes also play a role in demarcating regions of the gastrointestinal tract, particularly specifying sites of sphincter formation that separate the gut segments (see Fig. 14.6 ). For instance, mice null for Hoxd12 or Hoxd13 have severe muscular defects in the anal sphincter, and mice null for the full Hoxd cluster between Hoxd4 and Hoxd13 lack ileocecal sphincters and form abnormal pyloric and anal sphincters.
The stomach first becomes apparent during the early part of the fourth week, as the foregut just caudal to the septum transversum expands slightly. On about day 26, the thoracic foregut begins to elongate rapidly. Over the next 2 days, the presumptive stomach, now much farther removed from the lung buds, expands further into a fusiform (Latin for “spindle-shaped”) structure that is readily distinguished from adjacent regions of the gut tube ( Fig. 14.7 ). During the fifth week, the dorsal wall of the stomach grows faster than the ventral wall, resulting in the formation of the greater curvature of the stomach . Concurrently, deformation of the ventral stomach wall forms the lesser curvature of the stomach . By the end of the seventh week, the continual differential expansion of the superior part of the greater curvature results in the formation of the fundus and cardiac incisura .

During the seventh and eighth weeks, the developing stomach undergoes a 90 ° -rotation around its craniocaudal axis, so that the greater curvature lies to the left and the lesser curvature lies to the right (see Fig. 14.7D ). The right and left vagus plexuses, which originally run through the mesoderm on either side of the gut tube, thus rotate to become posterior (dorsal) and anterior (ventral) vagal trunks in the region of the stomach. The stomach also rotates slightly around a ventrodorsal axis, so that the greater curvature faces slightly caudal and the lesser curvature slightly cranial (see Fig. 14.7D ).
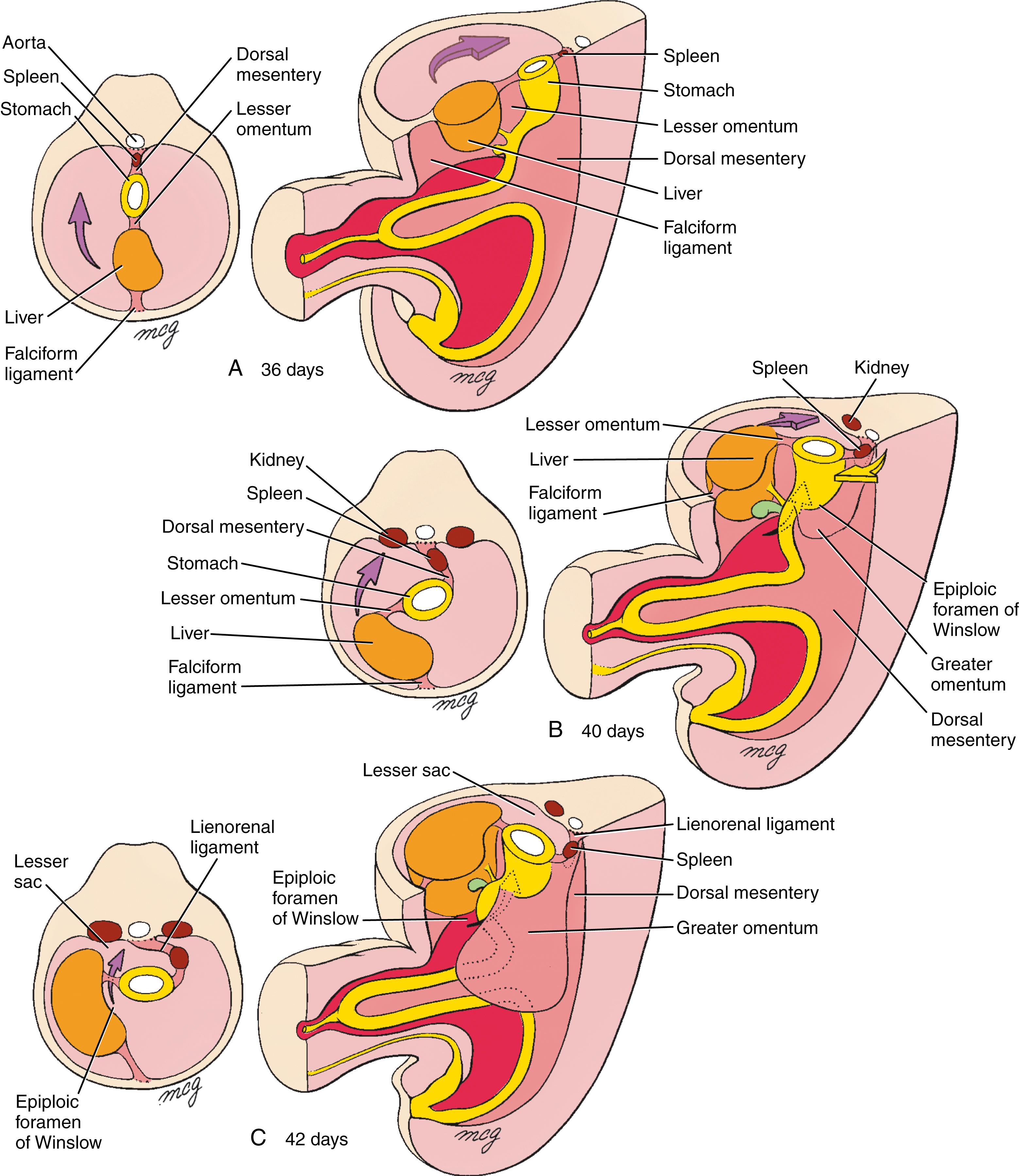
The rotations of the stomach bend the presumptive duodenum into a “C” shape and displace it to the right until it lies against the dorsal body wall, to which it adheres, thus becoming secondarily retroperitoneal. The rotation of the stomach and the fusion of the duodenum create an alcove dorsal to the stomach called the lesser sac of the peritoneal cavity ( Fig. 14.8 ). The rest of the peritoneal cavity is now called the greater sac . The lesser sac enlarges because of progressive expansion of the dorsal mesogastrium connecting the stomach to the posterior body wall. The resulting large, suspended fold of mesogastrium, called the greater omentum , hangs from the dorsal body wall and the greater curvature of the stomach and drapes over more inferior organs of the abdominal cavity (see Fig. 14.8C ). The portion of the lesser sac directly dorsal to the stomach is now called the upper recess of the lesser sac , and the cavity within the greater omentum is called the lower recess of the lesser sac . The lower recess is obliterated during fetal life as the anterior and posterior folds of the greater omentum fuse together.
On about day 22, a small endodermal thickening, the hepatic plate , forms on the ventral side of the duodenum. Over the next few days, cells in this plate proliferate and form the hepatic diverticulum , which grows into the mesenchymal cells of the inferior region of the septum transversum ( Fig. 14.9 ). The hepatic diverticulum gives rise to ramifying cords of hepatoblasts (the liver primordial cells). Under the influence of notch signaling and other regulatory proteins (covered in the following “In the Research Lab” entitled “Hepatoblast Specification and Fate”), hepatoblasts become hepatocytes (parenchyma), bile canaliculi of the liver, or hepatic ducts . In contrast, the mesoblastic supporting stroma of the liver develops from the septum transversum and splanchnic mesoderm originating near the stomach, as well as from endothelial precursor cells (that develop into the sinusoidal endothelium of the liver). Recent evidence suggests that up to one-third of the endothelial cells of the sinusoidal, portal, and central veins are actually derived from the sinus venosus, which flanks the liver during its development. Cardiogenic mesoderm, endothelium, and the septum transversum mesenchymal cells emit growth factor signals (including Vegfs, Bmps, and Fgfs) that are required for liver parenchymal development (covered in the following “In the Research Lab” entitled “Hepatoblast Specification and Fate”).
As covered in Chapter 13 , the liver is a major early hematopoietic organ of the embryo. Hematopoietic stem cells originating from the yolk sac wall (and later from the aortic, gonad, and mesonephric region) colonize the embryonic liver, expand their numbers, and diversify before populating other hematopoietic organs. Hepatic progenitors along with the hepatic stromal cells generate a hematopoietic microenvironment necessary for adult-type hematopoiesis. As the hematopoietic function is shifted to the peripheral organs, hepatocytes begin upregulating the expression of numerous genes related to mature liver function (e.g., those associated with amino acid metabolism and detoxification). Throughout embryonic and fetal development, hepatocytes proliferate (mainly mediated by autocrine mechanisms). This proliferation gradually slows and is arrested with postnatal development. From then on, migration and proliferation of hepatocytes require extraneous growth factors, including epidermal growth factor (Egf) and hepatocyte growth factor (Hgf).
By day 26, a distinct endodermal thickening also forms on the ventral side of the duodenum just caudal to the base of the hepatic diverticulum and buds into the ventral mesentery (see Fig. 14.9 ). This cystic diverticulum will form the gallbladder and cystic duct . The gallbladder and cystic duct develop from histologically distinct populations of duodenal cells. How the extrahepatic ducts anastomosis with the forming intrahepatic ducts is still unclear.
As covered earlier in the chapter, cardiogenic mesoderm plays a significant role in specifying liver formation and hepatic gene expression within the foregut endoderm (see Fig. 14.5 ). However, additional signals promoting liver formation originate from the mesoderm of the septum transversum. As covered in earlier chapters, Bmp signaling has a major role in the development of lateral plate mesoderm, and this is true for the liver as well. Bmps are expressed in the septum transversum (see Fig. 14.5 ); in mice lacking Bmp4, the liver bud fails to grow or express albumin. As covered earlier in the chapter, Fgfs from the adjacent cardiogenic region stimulate albumin expression in the endodermal liver primordia. In explant cultures of the liver primordia, noggin (an antagonist of Bmps) inhibits this Fgf-induced albumin expression. Although Bmps alone are insufficient to induce hepatic development, blocking of Bmp signaling with noggin increases the expression of the pancreatic marker Pdx1 in this endoderm. Therefore, Bmps contribute to Fgf-mediated endodermal patterning by promoting the liver phenotype and repressing the pancreatic phenotype. What downstream targets invoked by Fgfs and Bmps that induce hepatoblast lineage and growth remain unclear but may include Gata4, hepatic nuclear factor-3 (Hnf3), and CAAT-enhancer binding proteins (C/EBP). Close contact with nascent endothelial cells promotes the migration of hepatocyte precursor cells into the surrounding mesoderm. In mice, ablation of VegfR2 inhibits this outgrowth with a loss in hepatic cell gene expression. Wnt signaling has also been implicated in liver development and is required for bile duct lineage specification in mice.
Much more needs to be learned about how hepatoblasts acquire a hepatocytic or bile-ductal cell fate. A number of factors have been implicated in mediating cell determination of these two lineages. Like the segregation of neuronal and glial precursor cells from the neuroepithelium (covered in Chapter 9 ), notch signaling seems to have a role in mediating the decision as to whether a hepatoblast becomes a hepatocyte or bile duct lining cell ( cholangiocytes ). As covered in Chapter 12, Chapter 13, Chapter 3, Chapter 5 , mutations in the NOTCH ligand JAGGED1 (or in the receptor NOTCH2) are associated with autosomal dominant Alagille syndrome . These patients exhibit a paucity of bile ducts. Double heterozygotic mice for a jagged1 and notch2 null mutation lack intrahepatic bile ducts at birth and mimic Alagille syndrome. Studies in mice also show that jagged1 is expressed in cells adjacent to notch2-positive epithelium at the site of biliary differentiation, and that notch2 represses the hepatocyte lineage. Hence, jagged1/notch2 interactions likely have critical roles in hepatocyte/cholangiocyte determination.
A major change in liver functions occurs near birth as the burden of hematopoiesis is shifted away from the liver and the liver begins taking on the metabolic and detoxifying burdens. One group of transcription factors important in mediating these functions is the hepatocyte nuclear factor (Hnf) family. Hnfs activate specific liver genes. Mice with the Hnf4α gene deleted from the liver primordium using a Cre-lox system (covered in Chapter 5 ) develop small livers that lack organized epithelia and fail to express almost all liver-specific genes. However, forced expression of Hnf4α in fibroblast cultures does not induce liver-specific gene expression, even though the fibroblasts take on an epithelial-like morphology. This suggests that other cell lineage–determining factors must be in place before Hnfs can initiate liver-specific gene expression.
Another transcription factor that activates several liver genes and is involved in the functional change to mature liver function is C/EBPα. C/EBPα-deficient mice die at birth because of hypoglycemia. Although the liver tissue appears normal, hepatocytes are deficient in their ability to store glycogen and lipids. Other molecules implicated in late fetal liver maturation are oncostatin M and glucocorticoids. Oncostatin M stimulates the expression of hepatic differentiation markers, promotes liver-like morphology, and induces liver-specific gene expression in liver progenitor explant cultures. Glucocorticoids alone are capable of inducing most of the cellular responses typical of the liver differentiation promoted by oncostatin M, suggesting that they work together to promote liver maturation. Other factors involved in liver maturation likely include Hgf4α (upregulated on hepatic injury and hepatic regeneration) and Tgfβ (which may inhibit hepatocyte proliferation and promote differentiation).
On day 26, another duodenal bud begins to grow into the dorsal mesentery just opposite the hepatic diverticulum. This endodermal diverticulum is the dorsal pancreatic bud and will form the dorsal pancreas (see Fig. 14.9 ). As the dorsal pancreatic bud elongates into the dorsal mesentery, another endodermal diverticulum, the ventral pancreatic bud , sprouts into the ventral mesentery just caudal to the developing gallbladder. This bud will form the ventral pancreas as well as the bile duct (see Fig. 14.9 ).
Once specified, the pancreatic endodermal bud thickens and continues to expand into the closely apposed mesoderm. Branching of this bud occurs differently from the classic branching of other organs, such as the developing lung ( Fig. 14.10A ). Rather than expanding and folding the epithelium, solid epithelial clusters form, followed by the formation of intraepithelial microlumens. These microlumens soon coalesce to generate continuous lumens, forming an epithelial tree draining exocrine products into the duodenum (see Fig. 14.10B ). The pancreatic acinar cells that produce digestive enzymes, the pancreatic ductal cells that transport the digestive enzymes, and the pancreatic endocrine cells in the islets of Langerhans that produce insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin all differentiate from the endoderm of the pancreatic buds. The endocrine cell lineage proliferates within the endodermal epithelium, and soon these cells delaminate and subsequently aggregate into islets within the surrounding mesenchyme (see Fig. 14.10B ), where they continue to proliferate throughout the embryonic period.
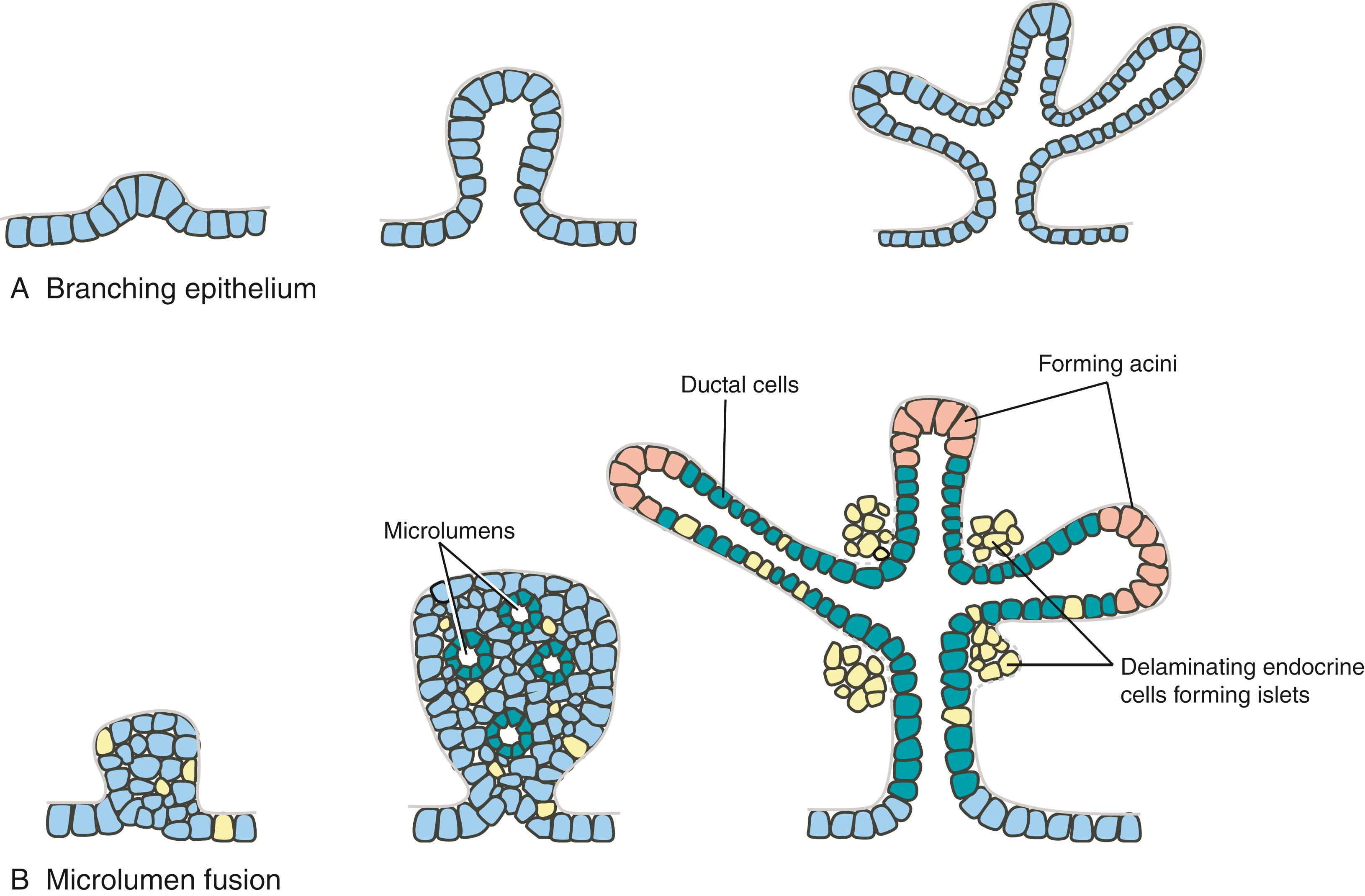
Interactions with the mesoderm play an essential role in growth and differentiation of the pancreas, and the expression of several growth-promoting transcription factors within the mesoderm is important in this. Fgf10 is expressed in pancreatic mesoderm, and Fgf10 knockout mice have hypoplastic dorsal and ventral pancreatic buds. Insulin gene enhancer protein-1 (Isl1) is expressed by mesoderm surrounding the pancreatic buds. If Isl1 is knocked out in mice, pancreatic mesenchyme is almost completely lost, and the expression of the pancreatic marker Pdx1 is greatly reduced.
In humans, while the common bile duct is forming and the ventral pancreatic bud is branching, proliferating, and differentiating, the mouth of the common bile duct and the ventral pancreatic bud migrate posteriorly around the duodenum toward the dorsal mesentery (see Fig. 14.9 ). By the early sixth week, the ventral and dorsal pancreatic buds lie adjacent to one another in the plane of the dorsal mesentery. Late in the sixth week, the two pancreatic buds fuse to form the definitive pancreas. The dorsal pancreatic bud gives rise to the head, body , and tail of the pancreas, whereas the ventral pancreatic bud gives rise to the hook-like uncinate process . Like the duodenum, the pancreas fuses to the dorsal body wall and becomes secondarily retroperitoneal.
When the ventral and dorsal pancreatic buds fuse, their ductal systems also become interconnected (see Fig. 14.9 ). The proximal portion of the duct connecting the dorsal bud to the duodenum usually degenerates, leaving the ventral pancreatic duct, now called the main pancreatic duct , as the only conduit for both the ventral and dorsal pancreas into the duodenum. The main pancreatic duct and the common bile duct meet and empty their secretions into the duodenum at the major duodenal papilla or ampulla of Vater . However, in some individuals, the proximal dorsal pancreatic duct persists as an accessory pancreatic duct that empties into the duodenum at a minor duodenal papilla .
Occasionally, the pancreas forms a complete ring encircling the duodenum, a condition known as annular pancreas . As shown in Fig. 14.11 , this abnormality probably arises when the two lobes of a bilobed ventral pancreatic bud (a normal variation) migrate in opposite directions around the duodenum to fuse with the dorsal pancreatic bud. An annular pancreas compresses the duodenum and may cause gastrointestinal obstruction ( duodenal stenosis ). Faulty hedgehog signaling may play a role in the development of annular pancreas. In mice, loss of Indian hedgehog (Ihh) signaling leads to ectopic branching of ventral pancreatic buds that then grow and wrap around the duodenum, forming an annular pancreas. Loss of Shh in some strains of mice also results in the development of an annular pancreas. Shh mutants exhibit duodenal stenosis and imperforated anus defects, both defects that are associated with annular pancreas in humans as well.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here