Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Some of the work on which this chapter is based was supported by National Institutes of Health (NIH) grants R01-HD34618 and 1R01-HD-057100.
The blood-brain barrier (BBB) is a selective diffusion barrier that maintains central nervous system (CNS) homeostasis and limits the entry of substances that could potentially alter neuronal function. The main anatomic substrate of the BBB is the specialized cerebral microvascular endothelium. It and associated astrocytes, pericytes, microglia, neurons, and the extracellular matrix components constitute the classical components of the neurovascular unit (NVU) ( Figs. 126.1 and 126.2 ) . In addition, oligodendrocyte lineage cells that can express NG2 chondroitin sulfate proteoglycan have been demonstrated to also contribute to the NVU. , In contrast with other endothelium in the body, properties specific to the cerebral vascular endothelium include the presence of tight cell–cell junctions between the endothelial cells, a lack of fenestrations across the endothelial surface, specific transporters, and low exchange by transcytosis. The NVU is essential in coupling neuronal activity with other vascular/barrier functions in the CNS throughout development and in the adult . Although systematic measurements of BBB function in the fetus and newborn have been limited to date, understanding of the development of the barrier continues to increase.
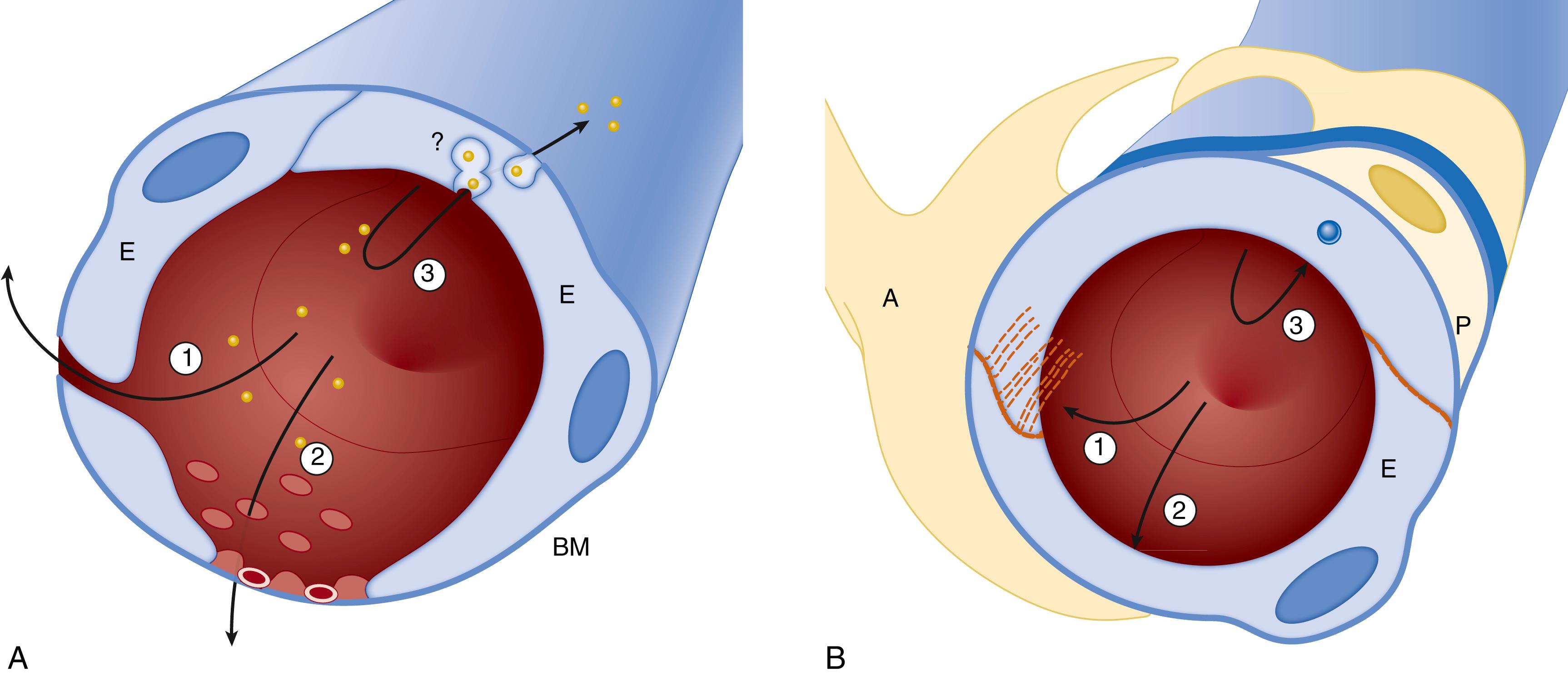
![Fig. 126.2, Neurovascular unit: The cell associations at the blood-brain barrier (BBB). The cerebral endothelial cells form tight junctions at their margins, which seal the aqueous paracellular diffusional pathway between the cells. Pericytes are distributed discontinuously along the length of the cerebral capillaries and partially surround the endothelium. Both the cerebral endothelial cells and the pericytes are enclosed by and contribute to the local basement membrane, which forms a distinct perivascular extracellular matrix (basal lamina 1 [BL1]), different in composition from the extracellular matrix of the glial end feet bounding the brain parenchyma (BL2). Foot processes from astrocytes form a complex network surrounding capillaries, and this close cell association is important for the induction and maintenance of the barrier’s properties. Axonal projections from neurons onto arteriolar smooth muscle contain vasoactive neurotransmitters and peptides and regulate local cerebral blood. BBB permeability may be regulated by the release of vasoactive peptides and other agents from cells associated with the endothelium. Microglia are the resident immunocompetent cells of the brain. The movement of solutes across the BBB is either passive, driven by a concentration gradient from plasma to brain, with more lipid-soluble substances entering most easily, or it may be facilitated by passive or active transporters in the endothelial cell membranes. Efflux transporters in the endothelium limit the central nervous system penetration of a wide variety of solutes. JAMS , junctional adhesion molecules; PECAM , platelet–endothelial cell adhesion molecule. Fig. 126.2, Neurovascular unit: The cell associations at the blood-brain barrier (BBB). The cerebral endothelial cells form tight junctions at their margins, which seal the aqueous paracellular diffusional pathway between the cells. Pericytes are distributed discontinuously along the length of the cerebral capillaries and partially surround the endothelium. Both the cerebral endothelial cells and the pericytes are enclosed by and contribute to the local basement membrane, which forms a distinct perivascular extracellular matrix (basal lamina 1 [BL1]), different in composition from the extracellular matrix of the glial end feet bounding the brain parenchyma (BL2). Foot processes from astrocytes form a complex network surrounding capillaries, and this close cell association is important for the induction and maintenance of the barrier’s properties. Axonal projections from neurons onto arteriolar smooth muscle contain vasoactive neurotransmitters and peptides and regulate local cerebral blood. BBB permeability may be regulated by the release of vasoactive peptides and other agents from cells associated with the endothelium. Microglia are the resident immunocompetent cells of the brain. The movement of solutes across the BBB is either passive, driven by a concentration gradient from plasma to brain, with more lipid-soluble substances entering most easily, or it may be facilitated by passive or active transporters in the endothelial cell membranes. Efflux transporters in the endothelium limit the central nervous system penetration of a wide variety of solutes. JAMS , junctional adhesion molecules; PECAM , platelet–endothelial cell adhesion molecule.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentoftheBloodBrainBarrier/1_3s20B9780323712842001269.jpg)
This chapter reviews the basic concepts of the BBB, summarizes the molecular biology of the BBB, introduces the concept of the NVU, and places these concepts in the context of the developing fetus and neonate. Finally, the BBB is discussed with special emphasis on the relevance of the barrier to select aspects of perinatal medicine.
In 1878, Paul Ehrlich, a German microbiologist, demonstrated in a seminal publication that there was a barrier between the vascular system supplying the peripheral circulatory system and the brain. Later experiments by Edwin Goldmann, an associate of Ehrlich, further established that this barrier was truly selective and not a result of the binding properties of the dye. Lewandowsky in 1900 introduced the descriptive term blood-brain barrier in this context. Although the concept of the BBB has been refined over the past few decades, the current understanding of its basic structure is built on the work of Reese, Karnovsky, and Brightman in the late 1960s. , Their work confirmed the presence of epithelial-like “tight junctions” that physically seal the interendothelial cleft, forming a continuous, impermeable membrane that constitutes the primary anatomic substrate of the BBB. , Reese and Karnovsky in 1967 proposed that the capillary lumen bridged by tight junctions formed a continuous, impermeable membrane that constituted the primary anatomic substrate of the BBB. , Studies by Brightman and Reese showed that horseradish peroxidase injected into the brain could diffuse through the approximately 20-nm gaps between the astrocyte end feet to the abluminal surface of the endothelium, indicating that astrocytes do not form the physical barrier. , Recent developments suggest that the BBB represents a dynamic interface devoted to the bidirectional control of the exchange of molecules and ions between the bloodstream and CNS, which also provides surveillance of immune-cell trafficking into the brain. , The BBB is now considered a component of a larger physiologic unit termed the neurovascular unit , which contributes to the dynamic function of this vital interface between the systemic circulation and the brain. , ,
In peripheral microvessels and in limited nonbarrier capillaries of the CNS, blood-borne polar molecules diffuse across the vessel wall into tissues through spaces between adjacent endothelial cells and through transcellular routes—specifically “endothelial fenestrations” and/or vesicular or tubulovesicular structures (see Fig. 126.1A ). In contrast, the BBB capillary is primarily formed by a “continuous” endothelium that is present in several other anatomically distinct structures. However, there are specialized junctional complexes found only in the brain that selectively prevent blood-borne molecules from freely passing through the interendothelial clefts. This endothelium, along with other perivascular cells, maintains an optimal microenvironment that couples neuronal activity with vascular-barrier function within the NVU (see Fig. 126.1B ). The BBB at the level of the endothelial cells is composed of three main features :
The “physical barrier,” formed by tight junctions between endothelial cells, prevents paracellular diffusion.
The “transport barrier” controls the movement of nutrients, ions, and toxins across the endothelium via specific metabolic transporters and efflux pumps located on the luminal and abluminal membranes of endothelial cells. , For example, the ATP-binding cassette (ABC) superfamily multidrug efflux pumps located on the luminal (apical) membrane serve as “gatekeepers” to extrude mainly xenobiotic lipophilic substances from the endothelial cytoplasm. ,
The “metabolic barrier” represents a combination of intracellular and extracellular enzymes, such as cytochromes P450 and monoamine oxidase that metabolize molecules capable of penetrating cerebral endothelial cells. This barrier-specific endothelial phenotype is determined by complex interactions of endothelial cells with other members of the NVU, including astrocytes, pericytes, smooth muscle cells, microglial cells, neurons, and the extracellular matrix molecules (see Fig. 126.2 ). , , Moreover, the components of the NVU contribute to the dynamic regulation of microvascular permeability and therefore regulate the function of the endothelial physical and transport barrier during normal and pathologic BBB activity . , ,
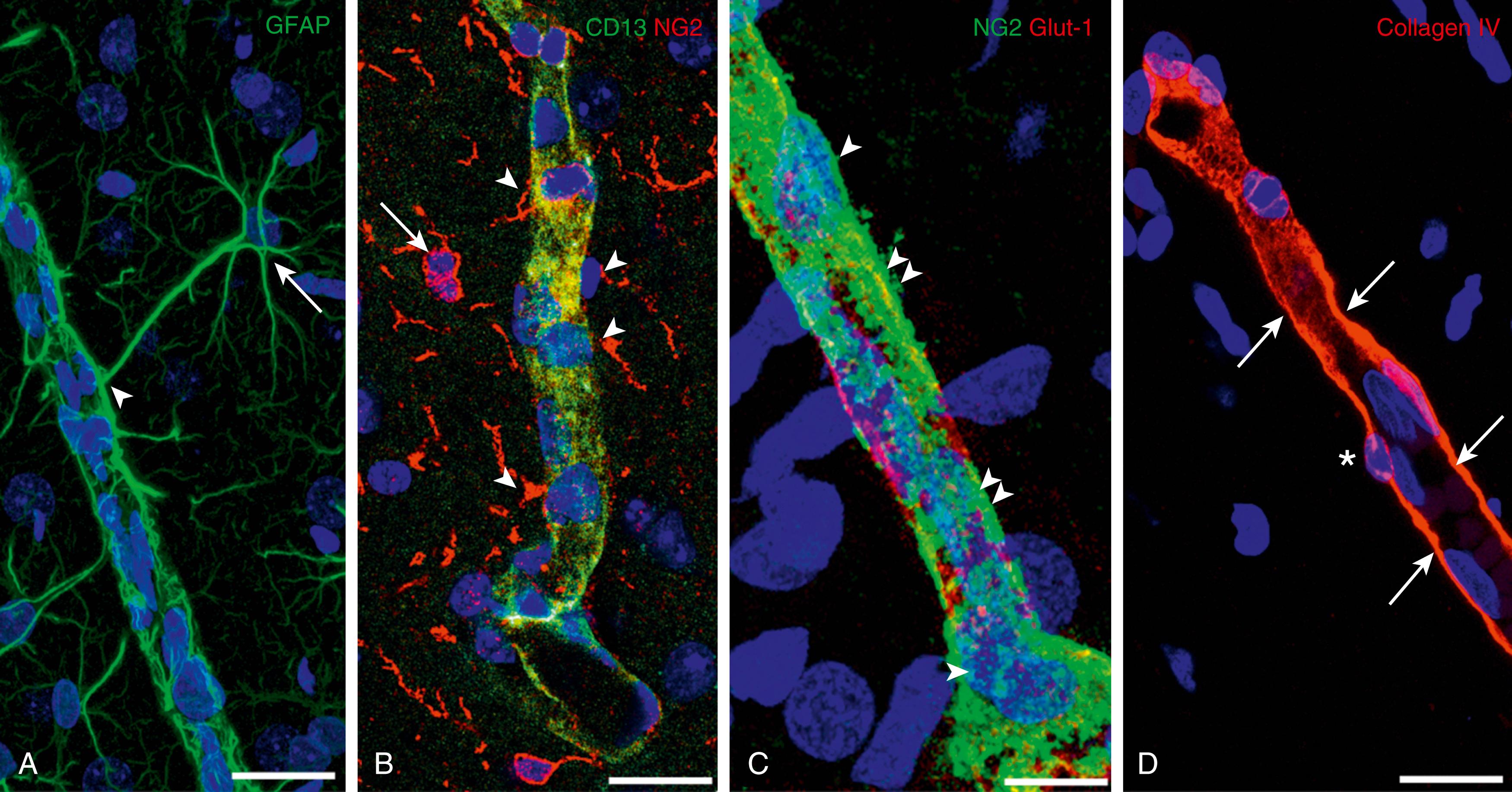
The endothelia of the brain’s blood vessels are interconnected by a continuous network of complex tight junctions that functionally fuse plasma membranes of adjacent endothelial cells and are responsible for the physical barrier of the BBB ( Fig. 126.3 ). , Tight junctions restrict the movement to small ions, such that the transendothelial electrical resistance can be as high as 1000 ohm/cm 2 in brain endothelium compared with 2 to 20 ohm/cm 2 in peripheral capillaries. ,
![Fig. 126.3, Structure of the blood-brain barrier’s tight junctions. The tight junctional complex comprises occludin, claudin 3 and 5, and possibly other claudins. Cadherins of the adherens junctions provide structural integrity and attachment between the cells and are necessary for the formation of tight junctions. The barrier to diffusion and the high electrical resistance of the blood-brain barrier appear to be largely due to the properties of claudin 3 and 5. The claudins associate and bind to each other across the intercellular cleft. A different ratio of the claudin mix may subtly alter tight junctional properties and the strength of the junction. Occludin has similar associations across the cleft but does not form the restrictive pore to small ions. The claudins and occludin are linked to the scaffolding proteins ZO-1, ZO-2, and ZO-3 and, in turn, via cingulin dimers to the actin/myosin cytoskeletal system within the cell. The role of the junction-associated molecules (junctional adhesion molecules [JAMs], members of the immunoglobulin superfamily) is unclear. JAM-A is localized on the lateral membrane of brain endothelial cells. ESAM, Endothelial cell-selective adhesion molecule; PECAM , platelet–endothelial cell adhesion molecule. Fig. 126.3, Structure of the blood-brain barrier’s tight junctions. The tight junctional complex comprises occludin, claudin 3 and 5, and possibly other claudins. Cadherins of the adherens junctions provide structural integrity and attachment between the cells and are necessary for the formation of tight junctions. The barrier to diffusion and the high electrical resistance of the blood-brain barrier appear to be largely due to the properties of claudin 3 and 5. The claudins associate and bind to each other across the intercellular cleft. A different ratio of the claudin mix may subtly alter tight junctional properties and the strength of the junction. Occludin has similar associations across the cleft but does not form the restrictive pore to small ions. The claudins and occludin are linked to the scaffolding proteins ZO-1, ZO-2, and ZO-3 and, in turn, via cingulin dimers to the actin/myosin cytoskeletal system within the cell. The role of the junction-associated molecules (junctional adhesion molecules [JAMs], members of the immunoglobulin superfamily) is unclear. JAM-A is localized on the lateral membrane of brain endothelial cells. ESAM, Endothelial cell-selective adhesion molecule; PECAM , platelet–endothelial cell adhesion molecule.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DevelopmentoftheBloodBrainBarrier/2_3s20B9780323712842001269.jpg)
Tight junctions form a continuous circumferential belt separating apical and basolateral plasma membrane domains, thus working as a barrier within the intercellular space and as a fence within the plasma membrane to define cell polarity. , , The structural characteristics of these junctions were confirmed by transmission electron microscopy and freeze-fracture studies. They appear by transmission electron microscopy as focal contacts between membranes of adjacent cells. Freeze-fracture electron microscopy showed that these contacts correspond to branching fibrils of transmembrane particles. The main protein components of the tight junctions are transmembrane proteins that can be classified into three distinct groups: (1) the claudin family (encompassing 27 members in humans); (2) the tight junction–associated MARVEL proteins (TAMPs), including occludin; and (3) the junctional adhesion molecules (JAMs) (see Fig. 126.3 ). , , ,
Claudins and occludin have four membrane-spanning domains and two extracellular loops. Occludin was identified in 1993 as the first integral protein localized in the tight junction. It is a 60- to 65-kDa protein with a carboxyl-terminal domain. The main function of occludin appears to be in the regulation of tight junctions. , , , Likewise, the family of claudin proteins are 22-kDa phosphoproteins that have four transmembrane domains and contribute to the high electrical resistance of the tight junctions. , , , Twenty-seven members of the claudin family have been identified. , , Claudin-3, claudin-5, and claudin-12 are the main claudins that contribute to the high resistance of the BBB. , , Together, claudins and occludins form the extracellular components of tight junctions, and both are required for the formation and proper functioning of the BBB.
Transmembrane proteins are connected to the actin cytoskeleton by the submembranous junctional plaques. These are composed of adapter proteins that form multiple protein–protein interaction motifs. , These proteins are located at the cytoplasmic surface of endothelial cells and connect the transmembrane tight junction proteins to actin, which is the primary cytoskeleton protein for the maintenance of structural and functional integrity of the endothelium. A ring of actin microfilaments underlies the complex and has a role in regulating the permeability and structural integrity of the tight junction complexes situated on the apical cell membrane. Activation of the actin cytoskeleton may be initiated by a rise in intracellular calcium, for example, resulting from ligand binding to the B2 bradykinin receptor, which may change the configuration of claudins and occludin, thereby modifying the tight junctional properties.
The first components of the junctional plaque to be identified were the zonula occludens (ZO-1, -2, and -3). ZO proteins anchor the tight junction complex to the actin cytoskeleton, making them essential for the assembly of claudins, occludins, and JAM-A. , Other examples of scaffolding proteins implicated in this protein network are symplekin, cingulin, 7H6, ZONAB (ZO-1-associated n ucleic a cid– b inding protein), AF-6 ( A LL1- f used gene from chromosome 6 protein), and others (see Fig. 126.3 ). , , Additionally, regulatory molecules such as G proteins (Gαi, RGS5) have been localized to the tight junction structure and play a major role in the regulation of BBB permeability. For example, the heterotrimeric G proteins (Gαi) were identified in association with ZO-1. This regulatory protein contributes to the formation and maintenance of tight junctions in brain endothelial cells , and is involved in lymphocyte extravasation. ,
Adherens junctions are formed by junction-associated adhesion proteins (JAMs), members of the immunoglobulin (Ig) superfamily. JAMs are transmembrane proteins composed of two extracellular Ig domains and a short cytoplasmic domain. JAMs appear to regulate cell polarity and endothelial permeability, also acting as cell adhesion molecules for leukocytes. , In the resting state, JAM-A is typically localized on the lateral membrane of brain endothelial cells; under inflammatory conditions, tight junction complex remodeling results in JAM-A relocalization away from the lateral membrane. Platelet–endothelial cell adhesion molecules (PECAMs) and vascular endothelial cadherins are the two important proteins involved in the adherens junctions and are critical for the promotion of close physical contact between endothelial cells so as to facilitate the formation of tight junctions. , They also contribute to the high electrical resistance of tight junctions and play a role in inflammatory cell transmigration through junctional complexes. The disruption of adherens junctions leads to impaired BBB function, extravasation of blood components, and the influx of water, thereby predisposing to the formation of vasogenic edema.
Paracellular diffusion is prevented by tight junctions between endothelial cells; only small lipophilic molecules with molecular weights less than 400 Da can access the brain parenchyma by diffusion across the endothelial wall. Specific transporters for larger molecules are present in the endothelial cells that constitute the “transport barrier” of the BBB ( Fig. 126.4C ). They are located on the luminal and abluminal membranes of endothelia and control nutrients, ions, and toxins crossing between the bloodstream and brain. The solute carrier (SLC) family of transporters mainly regulates substrate uptake, whereas the ABC transporter family regulates efflux out of the brain parenchyma. However, some SLC transporters function both as uptake and efflux transporters.
A total of 395 members of the SLC superfamily have been identified and classified into 52 families. , The glucose transporter 1 (GLUT 1), monocarboxylate transporters 1 and 2 (MCT1 and MCT2), and the L-system neutral amino acid transporter 1 (LAT 1) belong to the SLC family and supply the brain parenchyma with glucose, ketone bodies, and neutral amino acids, respectively. Forty-eight known ABC transporters have been identified in humans. They are classified into seven subfamilies (A through G). They bind and hydrolyze ATP to translocate lipid-soluble molecules across the endothelial plasma cell membrane of the brain and therefore are classified as active transporters. This family includes the P-glycoprotein (P-gp, also termed the ABCB1 transporter ), the breast cancer resistance protein (BCRP, also termed the ABCG2 transporter ), multidrug resistance–associated proteins (MRPs), and transporters belonging to the C family. P-gp is commonly considered the primary transporter in the ABC family because it is responsible for transporting a large variety of substrates. P-gp is concentrated on the luminal membrane and functions as a barrier, preventing the entry of drugs and xenobiotics into the brain. , These efflux transporters maintain tight control, restricting the substances that are allowed to enter the CNS through the endothelial cell barrier.
High-molecular-weight solutes (e.g., large proteins and peptides) are able to cross the BBB to enter the intact CNS via endocytotic mechanisms called receptor-mediated transcytosis (RMT) ; they transport larger molecules such as insulin or act by adsorptive-mediated transcytosis (AMT), which transports albumin and similar compounds. Macromolecules bind to specific receptors during RMT. These receptors cluster with their ligands into vesicular caveolae that are internalized into the endothelial cell; they are subsequently transported across the cytoplasm and finally exocytosed at appropriate locations within the cell.
Brain endothelial cells express different systems responsible for the metabolism of drugs typically found in the liver, including the cytochromes P450 and phase II enzymes of metabolism. These drug and toxin-metabolizing enzymes contribute, along with the efflux transporters, to the detoxification activity of the BBB and participate in drug pharmacodynamics. In addition, enzymes regulate the concentration of signaling molecules and metabolize endogenous substrates such as fatty acids, hormones, steroids, and vitamins. Brain endothelial cells also express several enzymes that metabolize neurotransmitters such as monoamine esterases, cholinesterases, γ-aminobutyric acid (GABA), transaminases, aminopeptidases, and endopeptidases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here