Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Perception of odorous molecules is the oldest special sense in both phylogeny and ontogeny. Olfaction is the earliest special sense in evolution; even polyps and jellyfishes, possessing only a loose neural net without a brain or even a ganglion, can perceive noxious odors and chemicals in their surrounding water and retract in response, or respond positively to attractants (i.e., food), noted as early as 1849 by Thomas Huxley. However, it is difficult to know whether perception of soluble molecules in seawater should be designated olfactory or gustatory. The human fetus can perceive odors in the amniotic fluid that circulates through the nasal passages as soon as the nasal epithelial plug resolves in the early second trimester. Though olfaction and taste are mediated by different cranial nerves in remote parts of the brain, their functional distinction is blurred. Even the adult enjoyment of food and wine combines smell and taste. ,
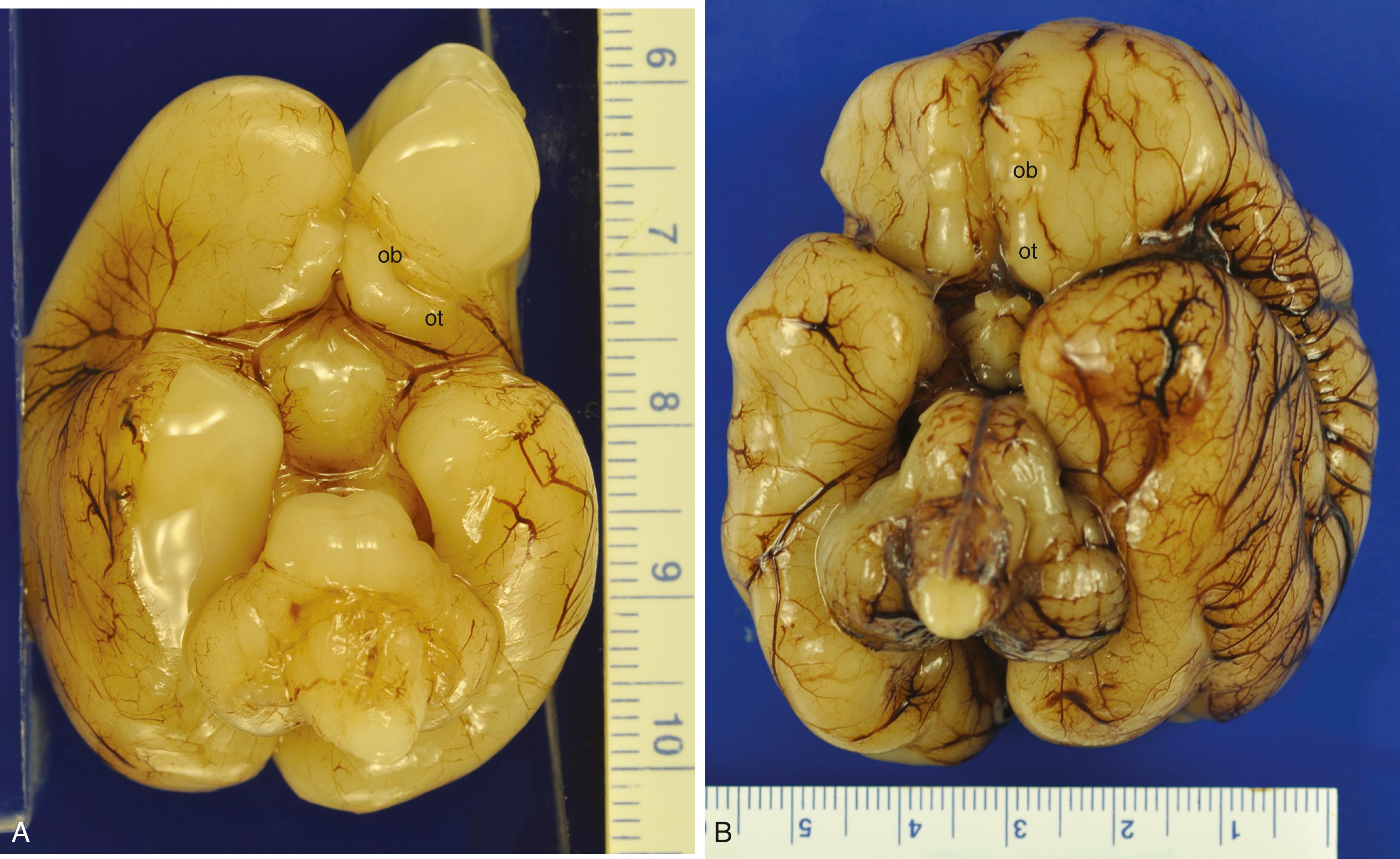
A primordial olfactory bulb appears at 41 days gestation as a ventrorostral outgrowth of the newly formed telencephalic hemispheres after prosencephalic cleavage. , It is a prominent structure of the ventral telencephalon with the volume ratio becoming relatively less as gestation progresses ( Fig. 136.1 ). The mature olfactory bulb exhibits laminar architecture unlike that of the cerebral, cerebellar, or other cortices. Its unique organization is, in part, because none of its neurons arise from the intrinsic neural tissue of the olfactory bulb itself during ontogenesis; neuroblasts stream into the olfactory bulb from the rostral telencephalon and then secondarily migrate radially. This rostral migratory stream is present in all vertebrate embryos. The olfactory tract also is unlike any other white matter fasciculus and is much more than a simple bundle of axons. It includes as much grey as white matter because granular neurons extend into it from the olfactory bulb at one end as do pyramidal neurons of the anterior olfactory nucleus from the other. In addition, resident progenitor “stem” cells capable of neuronal differentiation are present in the olfactory bulb and tract of both the fetus and the adult; the only other reservoir of such progenitor cells in the mature brain is in the polymorphic layer beneath the hippocampal dentate gyrus. Primary olfactory neurons residing in the nasal epithelium exhibit continuous turnover and extraordinary regenerative capacity in both the fetus and adult, unmatched in any other structure of the nervous system. If primary olfactory neurons could not regenerate, irreversible anosmia might ensue after a severe upper respiratory tract infection.
Olfaction is the only special sensory system in vertebrates without synaptic relay via the thalamus, because the olfactory bulb incorporates its own thalamic equivalents: (1) the core of intrinsic granular neurons that lack axons and form dendrodendritic synapses, (2) the periglomerular inhibitory interneurons, and (3) the anterior olfactory nucleus, which serves as a relay to the amygdala (to govern behavior in response to odors) and to the entorhinal cortex (part of the parahippocampal gyrus). , Granular and periglomerular neurons are gamma-aminobutyric acid (GABA)-ergic, and the latter coexpress dopamine. Neurons of the anterior olfactory nucleus are mostly glutamatergic, but a minority are GABAergic.
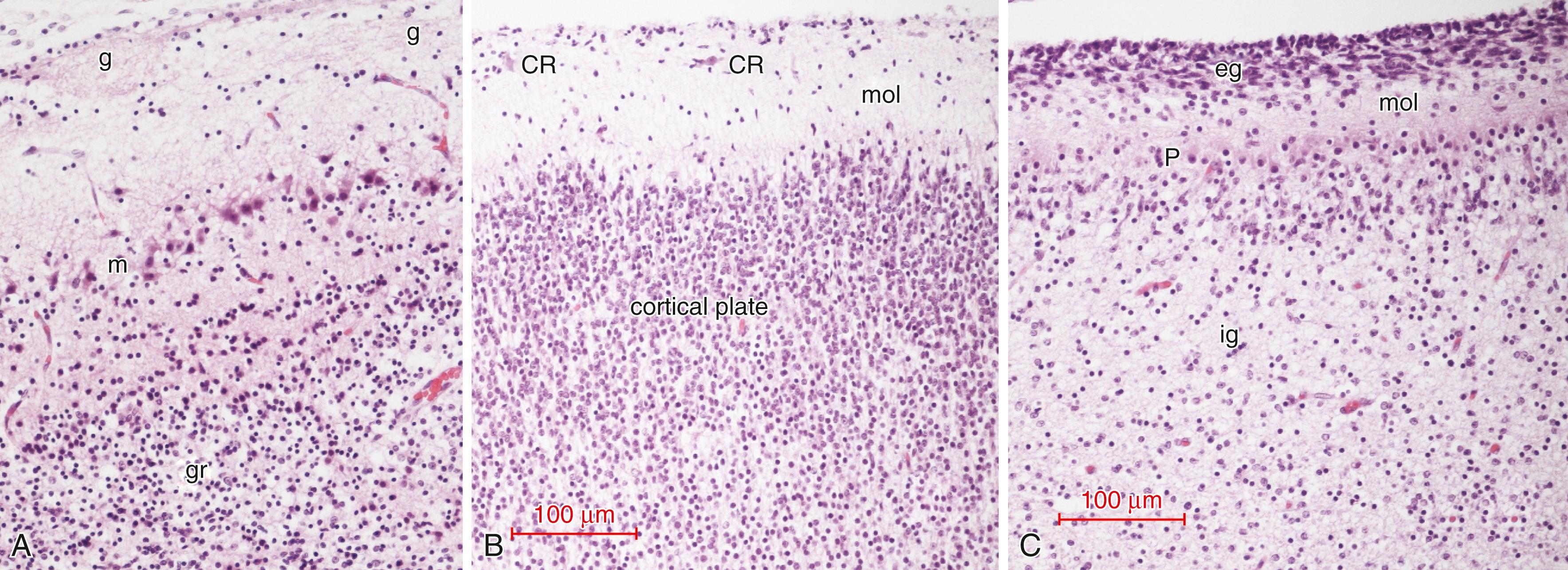
An understanding of olfactory function during development can only be fully understood in correlation with morphogenesis of the anatomic olfactory structures. Immunocytochemical reactivities and histochemical stains have greatly enhanced the microscopic observations of anatomic maturation, particularly in documenting synaptogenesis in the olfactory bulb, the maturation of individual neuronal types, and the distribution of progenitor “stem” cells within the olfactory bulb. Despite early conclusions based on histology that the human olfactory bulb is mature by 11 weeks gestation, and the functional evidence that neonates and fetuses respond to olfactory stimuli (see later), more recent immunocytochemical studies in our laboratory demonstrate that the olfactory bulb is far from mature at birth. Neuronal maturation, synapse formation, and myelination are incompletely developed at term. The transitory fetal olfactory recess of the lateral ventricle persists and does not involute until after birth. Even the nasal olfactory epithelium is not yet fully mature at birth. Secondary telencephalic sites of olfactory projections, such as the amygdala and entorhinal neocortex, also are not yet mature at birth, particularly in synaptic circuitry.
Olfactory epithelium lines the roof of the nasal cavities, part of the nasal septum, and the superior (and sometimes extending to the middle) turbinate bones. , Epithelial plugs obstruct the external nares in the first trimester but regress between 16 and 24 weeks gestation, a finding that was demonstrated as early as 1910. Volatile molecules must penetrate an aqueous mucus layer covering the olfactory epithelium to reach receptor sites on the cilia of primary olfactory dendrites. The number of bipolar primary olfactory neurons increases greatly with the formation of the turbinates, beginning at approximately 8 weeks gestation, which enables a greatly enlarged surface area of olfactory epithelium in the nasal cavity ( Fig. 136.2 ).
An active process of apoptosis occurs simultaneously with the generation of new neurons in the olfactory epithelium. The β-secretase enzyme BACE-1 is essential for axonal guidance of olfactory sensory neurons and for formation of synaptic glomeruli of the olfactory bulb. Neural precursors in the developing olfactory epithelium give rise to three major neuronal classes: olfactory receptors, vomeronasal receptors, and gonadotropin-releasing hormone secretory neurons, each associated with a variety of genes. These neuronal types are mixed rather than anatomically segregated, but different gene-expressing neurons reside in the medial and lateral portions. Specific olfactory marker proteins are identified in the human olfactory epithelium from 28 weeks gestation , and also in that of the fetal rat. Novel DNA microarrays recognize human olfactory receptor gene families and classes of neural precursors in the olfactory epithelium. , The olfactory epithelium with its primary olfactory receptor neurons is well characterized by transmission and scanning electron microscopy in humans , and is immature until near term.
Primary olfactory neurons have a high turnover rate throughout life. This regenerative capacity replaces cells lost during severe upper respiratory tract infections, for example, so the subject is not left anosmic afterward. The life span of olfactory neurons is modulated by activity-dependent histones. Olfactory ensheathing cells are specialized glial cells that secrete a large number of neurotrophic factors and cover the entire length of the olfactory nerve and bulb and are essential to preserve the structural integrity of the olfactory nerve, serving as do Schwann cells in other peripheral nerves, and also olfactory resident stem cells for neuroblast proliferation in physiologic turnover and regeneration. ,
The outgrowth of the incipient olfactory bulb includes a rostral extension of the primordial frontal horn of the lateral ventricle, the olfactory ventricular recess . , , This transitory structure begins to involute in the third trimester but is still present at term ( Fig. 136.3 ). It is not always located in the exact middle of the core of the olfactory bulb but sometimes is eccentric, though it remains within the granular layer and never lies superficial to the mitral cell layer. The olfactory recess initially is lined by primitive neuroepithelium but acquires an ependyma at midgestation, arresting neuroepithelial mitotic activity at the luminal surface as in other parts of the lateral ventricles. Its ependyma is a pseudostratified columnar epithelium that does not become a simple cuboidal even at term; it continues to exhibit reactivity to vimentin and glial fibrillary acidic protein even in the term neonate, unlike the mature permanent ventricular ependyma of the lateral, third, and fourth ventricles, in which these reactivities disappear with maturation in the third trimester. , Humphrey reported that the olfactory recess begins to involute by growth of its ependymal cells into the lumen starting at 14 weeks and completes this process by 18 weeks, but we found a well-formed recess with an ependymal-lined lumen even in the term neonate, though it no longer is evident within a few weeks postnatally. Magnetic resonance imaging (MRI) demonstrated remnants of this recess in 72 of 122 (59%) normal adult subjects. These adult remnants are confirmed histologically either as glial-lined microcysts or as small clusters of residual ependymal cells.
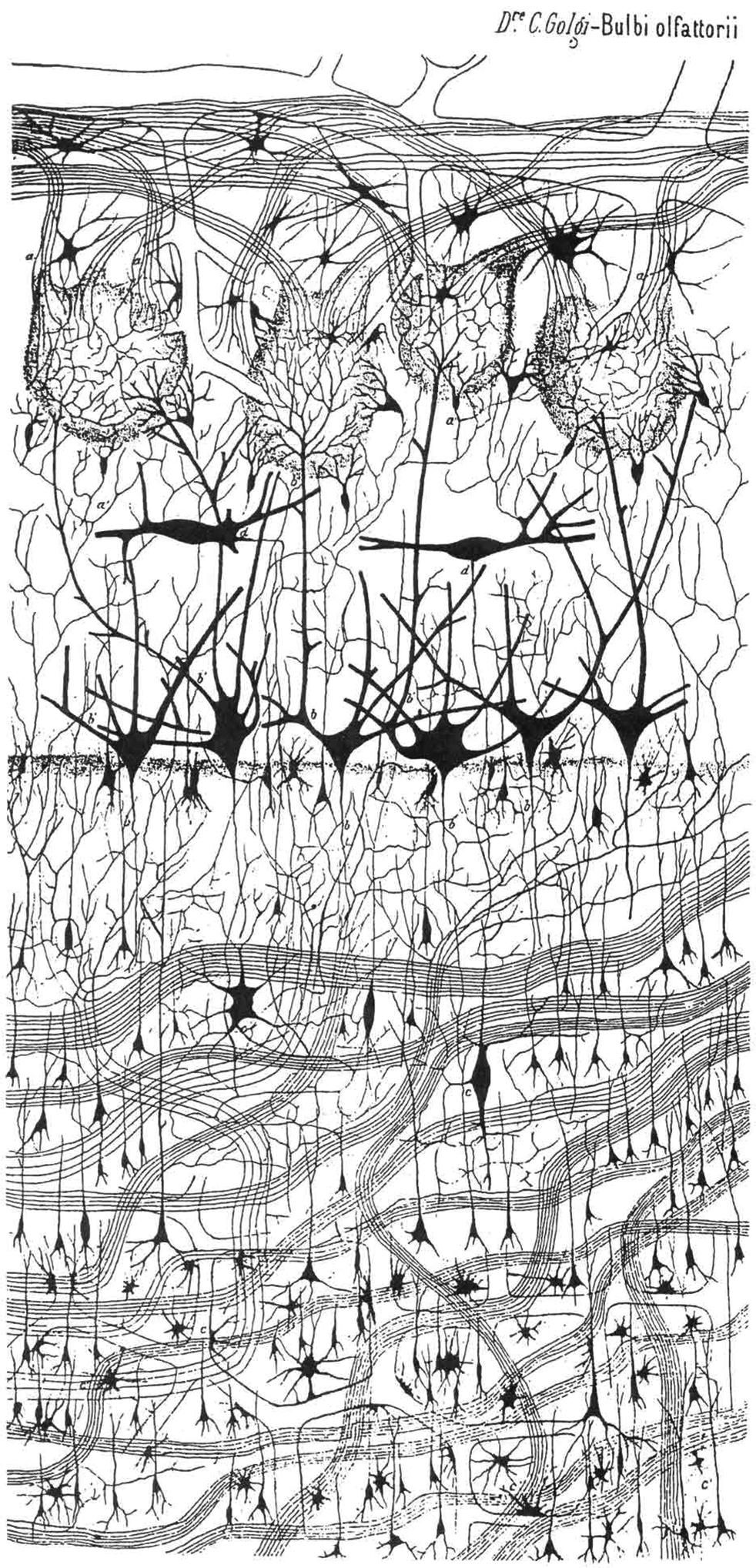
The olfactory bulb lacks the molecular zone, Cajal-Retzius neurons, a subplate zone, and a subpial granular glial layer of Brun that characterize the developing neocortex. Synaptic glomeruli lie superficial to the layer of large mitral cells, unlike the synaptic glomeruli of the internal granular layer of cerebellar cortex. The concentric laminar architecture of the mature olfactory bulb is well characterized, and the histology of the fetal olfactory bulb during development also is documented. The cortex of the olfactory bulb consists of six layers parallel to the surface of the bulb, none of which corresponds to those of the hexalaminar cerebral neocortex, the layers of the cerebellar cortex, the hippocampus, or even the loose lamination of the midbrain superior colliculus. Fig. 136.4 is the first Golgi depiction of the olfactory bulb by Golgi in 1875, and Fig. 136.5 illustrates a histologic comparison of olfactory, neocortical, and cerebellar cortices. The constituents of each layer are described as follows:
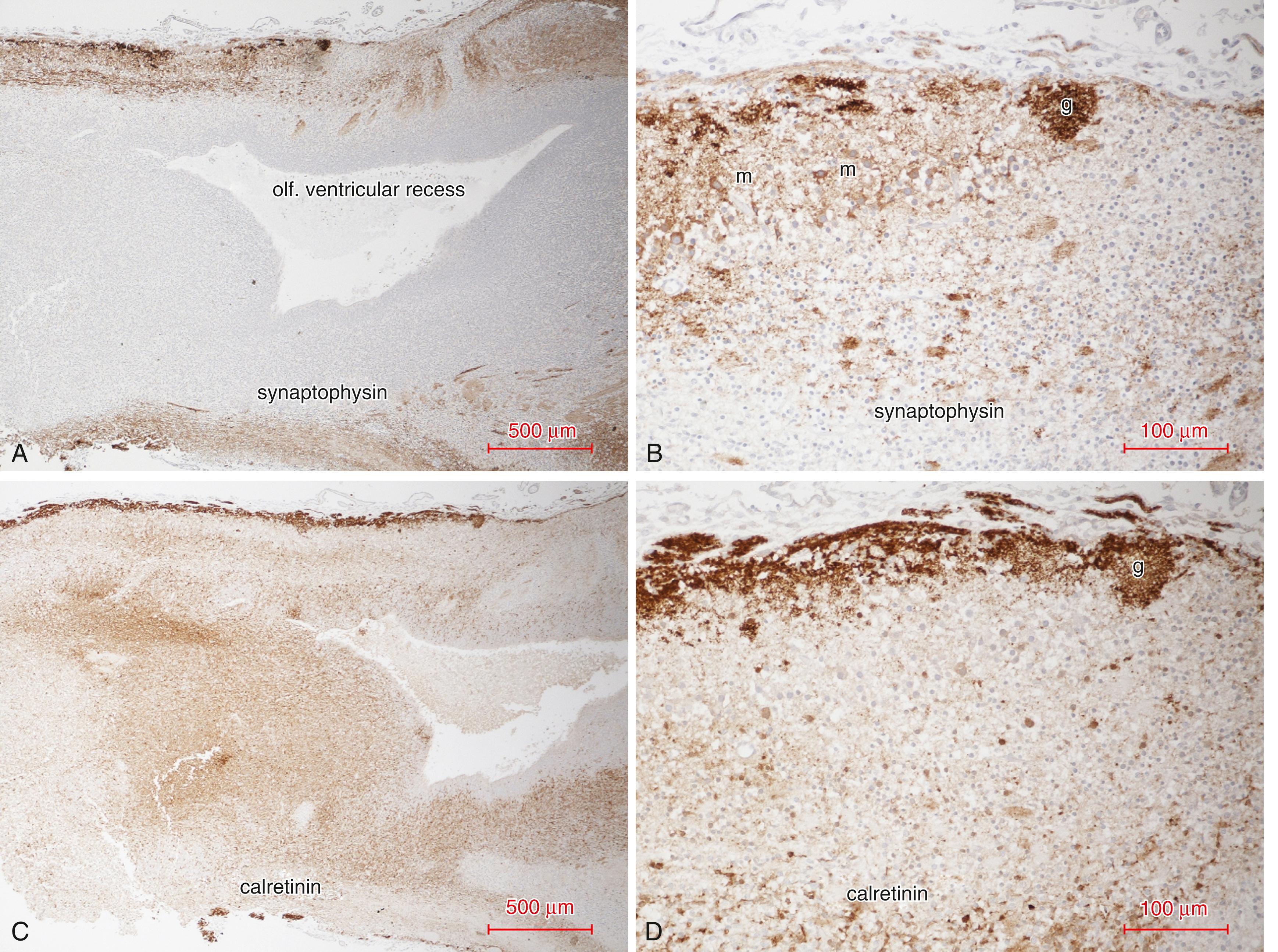
The most superficial layer of the olfactory bulb is acellular and composed of unmyelinated olfactory receptor cell axons that have passed through the bony cribriform plate. In rodents, a zonal organization of the olfactory epithelium is preserved in its somatotopic distribution in the olfactory bulb, as determined by zone-specific markers. Axonal transport of synaptophysin molecules within unmyelinated axons in this layer is well demonstrated by antisynaptophysin antibodies throughout the late first and second trimesters. These glycoproteins are thus immunocytochemically evident from their site of biosynthesis in the cytoplasm of the neuronal soma to their final site in the axonal terminal, where they contribute to the structure of the synaptic vesicular membrane. Late in gestation and the neonatal period they become reduced in the intensity of their reactivity, and only presynaptic terminals are strongly reactive.
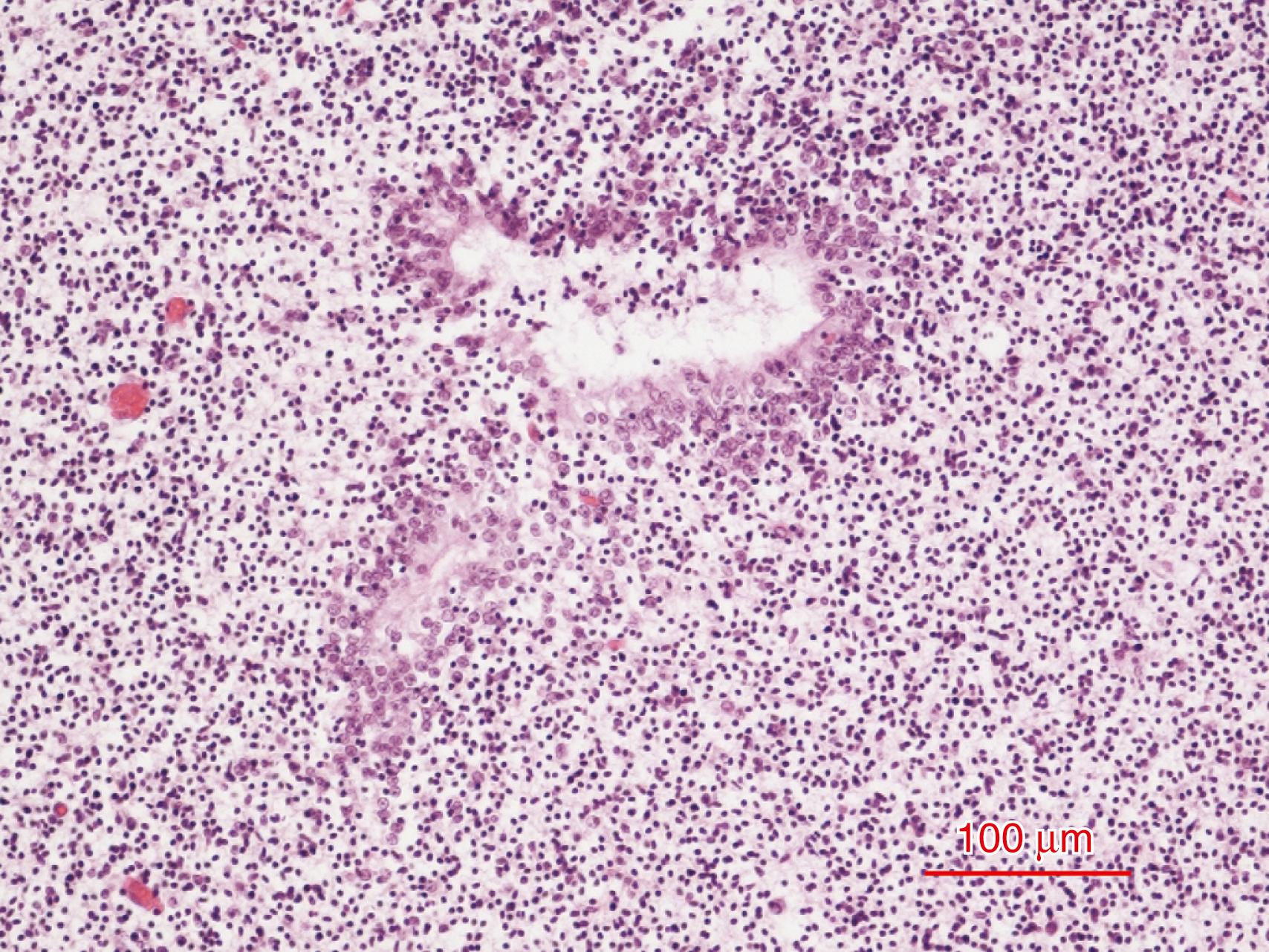
The glomerular layer contains the prominent synaptic glomeruli, generally arranged in a single layer but possibly two or three cells thick in some places. Periglomerular interneurons also occur in this layer. Small periglomerular neurons often form sheet-like processes that envelop other small periglomerular neurons. Spine-laden dendrites of periglomerular cells ramify within two, or occasionally more, glomeruli. Their axons extend across as many as six glomeruli as they contact local interneurons. These periglomerular neurons are heterogeneous in morphology, neurochemistry, and physiology; approximately 10% lack synapses with olfactory neurons. Periglomerular cells synthesize GABA and dopamine as transmitters, and these molecules are colocalized , ; these neurons also contain one of two calcium-binding proteins, calbindin or parvalbumin. Axons of primary olfactory neurons in the mucosa and dendrites of mitral cells form synapses within the glomeruli. Each glomerulus receives as many as 25,000 axons of olfactory nerve neurons (rabbit). A convergence of approximately 1000 receptor cell axons upon every second-order neuron (rabbit) results in major summation or amplification of olfactory stimuli. The olfactory bulb thus exhibits mathematical precision in its intrinsic synaptic relations. More than 20 known neurotransmitters or modulators have been identified in the olfactory bulb. It is not surprising, therefore, that a large array of genes is expressed during fetal life. The glomerular layer is one of the last layers of the olfactory bulb to develop, beginning at approximately 14 weeks. Other layers appear earlier, except for the concentric lamination of the granular zone of the core. Specific odor receptors project their axons to specific glomeruli, thus creating a topographic odor map within the olfactory bulb (zebrafish), programmed by the genes Robo2/Slit ; without these gene expressions from as early as the olfactory placode, growing olfactory axons are misrouted. Each primary olfactory neuron in the epithelium expresses only one specific receptor because all others are suppressed by a G-protein that couples to membrane receptors. These morphologic relations and constant ratios of cells and synapses suggest functional modules in the olfactory bulb, analogous to the functional columns (barrels; radial units) of visual (striate), somatosensory, motor, and insular neocortices. Indeed, a columnar arrangement with only limited lateral connectivity also is demonstrated in the olfactory bulb.
An external plexiform layer is cell-sparse and formed largely by primary dendrites of granule cells and secondary dendrites of mitral and tufted neurons. Tufted neurons reside in this layer. These cells are similar to mitral neurons in morphology (though smaller), synaptic connections, and transmitter synthesis (glutamate). Approximately 80% of synaptic contacts between neurons are organized as reciprocal pairs: mitral-to-granule cell synapses are excitatory and granule cell-to-mitral neuronal synapses are inhibitory.
This is a single layer of large mitral neurons. There is heterogeneity in the neurochemistry and electrophysiologic functions amongst several classes of mitral neurons. , During development, mitral neurons can be identified as early as 8 weeks gestation, at which time neuronal differentiation and cytoplasmic growth of these large neurons occurs. Tufted neurons are similar to mitral neurons, though smaller and found only in the most rostral regions of the olfactory bulb and more superficially than the mitral cells. They also project their axons caudally into the olfactory tract. The mitral cell layer is initiated at 9.5 weeks, is well developed by 11 weeks, and is more prominent at 18.5 weeks than in the adult.
An internal plexiform layer of neurites but with few synapses.
The innermost cellular layer of the olfactory bulb consists of granule cells in a ratio of approximately 50 to 100 granule cells for each mitral cell. These small interneurons are GABAergic and possess no extrinsic connections; they are unique in lacking a definite axon, but rather form dendrodendritic synapses with mitral and tufted neurons. Lamination in the granular layer, and also in the mitral cell layer, is at least partly regulated in fetal mice by the zinc-finger gene Fex in olfactory neurons. The neuroepithelium surrounding the olfactory ventricular recess shows proliferative activity. Massive apoptotic cellular death occurs in the murine olfactory bulb, especially amongst granular neurons, if thiamine is deficient.
The intrinsic thalamic equivalent of the olfactory bulb is in its granule cells and periglomerular interneurons, which “modulate” intrinsic olfactory bulb synaptic circuits but have no extrinsic afferent or efferent connections. They are GABAergic interneurons intensely immunoreactive in tissue sections with calcium-binding soluble proteins such as calretinin and parvalbumin. The anterior olfactory nucleus also contributes to thalamic-like function in serving as a relay between the olfactory bulb and telencephalic targets, particularly the amygdala and entorhinal cortex. , , , Portions of the anterior olfactory nucleus extend into the olfactory tract (see later).
Olfaction also is rare amongst sensory systems because the primary receptors are naked dendritic endings within an epithelium, though pain receptors in the skin share this characteristic. The ganglion cells of these primary olfactory neurons lie outside the central nervous system (CNS), as with sensory ganglia of other cranial nerves and spinal dorsal roots. Axonal terminals of these primary olfactory neurons enter the olfactory bulb and form synaptic glomeruli ( glomerulus = Greek; ball of threads ) with dendrites of the layered mitral and tufted cells that give origin to long axons that extend into the olfactory tract and project to diencephalic structures in the hypothalamus and telencephalic structures.
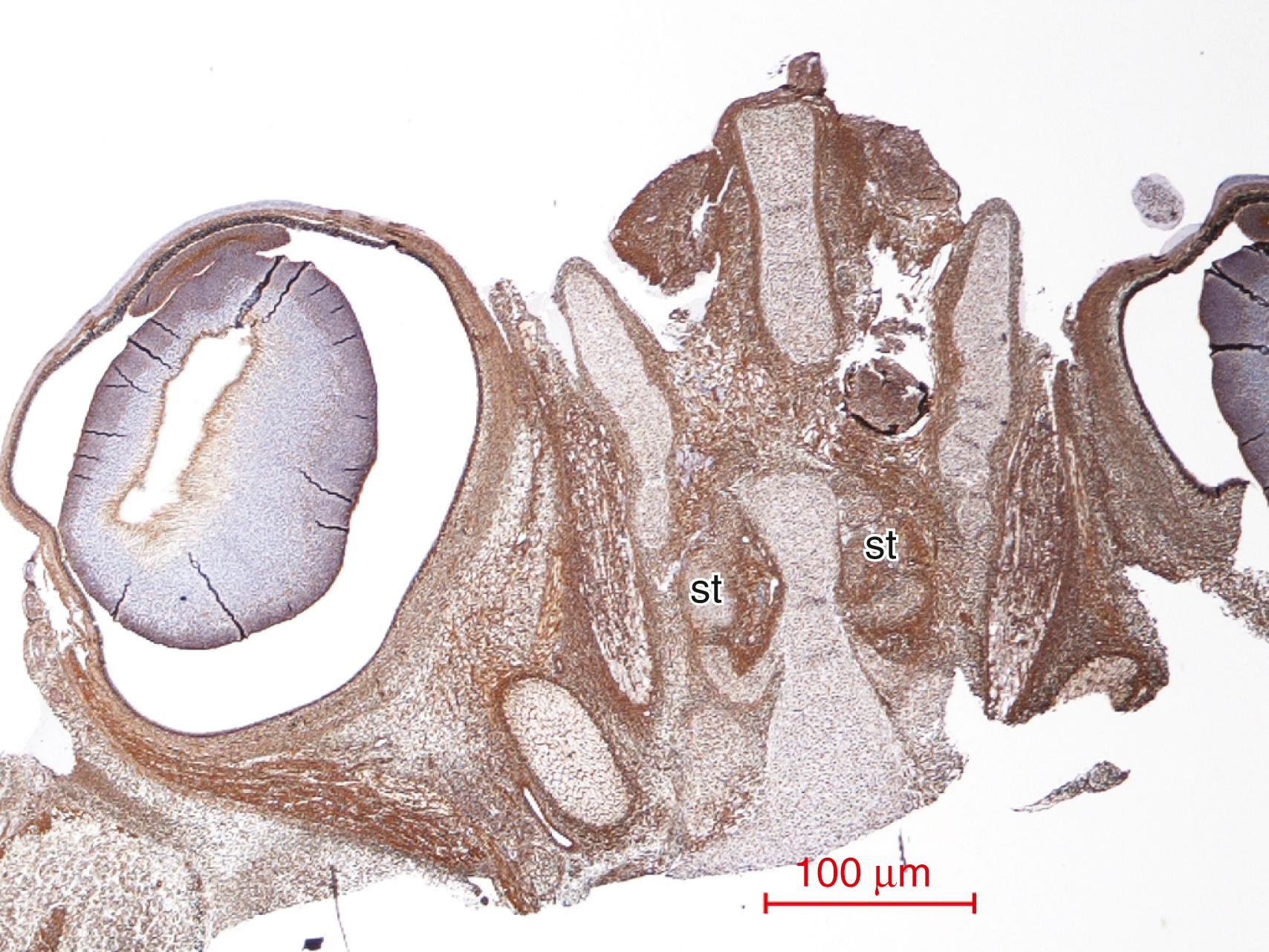
The deepest core of the mature olfactory bulb corresponds to subcortical white matter and contains the axons of mitral and tufted neurons that pass caudally to form the olfactory tract and project to other structures of the brain. However, it also contains many granular cells and progenitor cells that extend into the olfactory tract (see below). The immunocytochemical profile of synaptophysin and calretinin in the maturing olfactory bulb in the human fetus is illustrated in Fig. 136.6 .
The olfactory tract is much more complex than a simple fasciculus of longitudinal ascending and descending axons. The axonal bundles are surrounded by granule cells that extend caudally from the olfactory bulb. At the other end of the olfactory tract, there is an extension rostrally of pyramidal neurons of the anterior olfactory nucleus and one to several clusters of such neurons within the olfactory tract, which are separated parts of the anterior olfactory nucleus. , In addition, long, slender processes of bipolar progenitor cells residing mainly in the granular layer of the olfactory bulb extend caudally within the olfactory tract in parallel with the descending axonal pathway.
Axons of the tufted and mitral neurons form the olfactory tract and pass caudally to terminate mainly in the anterior olfactory nucleus but also directly to other telencephalic and diencephalic regions. One of the principal telencephalic centers for processing of odors is the amygdala. Postsynaptic axons of anterior olfactory neurons enter the anterior commissure, as shown by DiI axonal transport in the rat fetus. Some presynaptic olfactory tract axons also cross the midline in the anterior commissure. Olfactory contributions form the peripheral rim of fibers of the anterior commissure, with axons of the anterior temporal neocortex forming most of the deep core; these olfactory fibers of the anterior commissure myelinate postnatally, the sharpest contrast with unmyelinated anterior commissural axons seen at approximately 4 months of age using Luxol fast blue myelin stain.
Cortical areas of olfactory perception are poorly defined and are not assigned a Brodmann area number (unlike the gustatory system) but mainly are localized to the insula and nearby ventral part of the postcentral gyrus, where they may be integrated with gustatory input.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here