Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Unlike photons, protons deposit a localized peak of dose known as the Bragg peak. In the absence of exit radiation dose, nearby normal organ structures may be spared dose, which may translate to reduced toxicity (eg, neurocognition, endocrine dysfunction, secondary malignancies) or allow dose escalation to improve local control.
The benefits of proton therapy are best exemplified in medulloblastoma patients, who are treated to the craniospinal axis and posterior fossa. By sparing dose to the anterior midline structures of the chest, abdomen, and pelvis, in addition to the cochlea and pituitary, preliminary evidence suggests lower rates of acute toxicity and endocrinopathy. Patients are also predicted to have a lower risk of secondary malignancies.
Protons permit safe dose escalation of primary bone tumors (chordomas, chondrosarcomas) of the base of skull and spine, with 5-year local control (LC) rates of 70% and 80% to 90%, respectively. In addition to radiation dose, LC is modulated by treatment up front versus that based on recurrence, presence of hardware, and extent of residual disease.
Although no randomized studies exist on the impact of protons, early single-arm prospective studies are emerging: among patients with low-grade gliomas treated with protons, neurocognition after treatment remained stable without decrement in quality of life or ability to continue working.
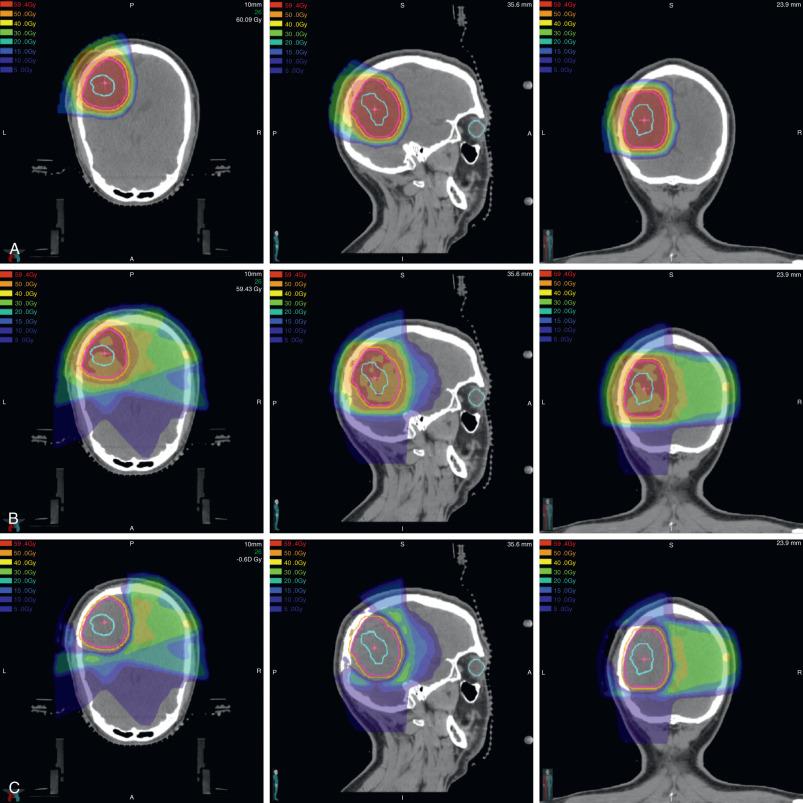
Unlike photons that are used for conventional external beam radiation therapy, protons are a heavy particle with mass, charge, and a superior dose distribution that was first recognized by Robert Wilson. Protons rapidly lose energy within the last few millimeters of tissue penetration, which yields a sharply localized peak of dose known as the Bragg peak ( Fig. 52.1 ). Distal to this, no additional dose is deposited. The Bragg peak can be precisely placed anywhere in the patient by modulating the proton energy, and several Bragg peaks can be shifted in depth and weighted to create a spread out Bragg peak (SOBP), which is useful for treatment. Precise delivery systems and patient positioning devices are used for proton therapy delivery ( Fig. 52.2 ).
Radiation side effects are associated with dose to and volume of normal tissue irradiated. In addition, risk of toxicity may be modulated by patient age, gender, and other treatments such as chemotherapy or surgery. The rationale for proton therapy is based on two premises: first, that sparing dose to normal organs improves treatment tolerability and late morbidity; and second, that by sparing dose to surrounding normal organs, radiation dose can be escalated to the tumor volume, if needed. The first premise has driven, in part, utilization of proton therapy for pediatric and some young adult tumors, including those of the cranium. Indeed, the hypothalamus/pituitary axis (HPA), cochlea, temporal lobes, and normal brain tissue lie in close proximity, and irradiation of these structures may cause endocrine dysfunction, hearing loss, neurocognitive dysfunction, and secondary malignancies. These are nontrivial late effects, especially as contemporary treatments have rendered overall survival (OS) rates upward of 85% to 90% for some histologies. Proton therapy decreases the low-dose radiation therapy outside of the target to decrease the risk of these late effects ( Fig. 52.3 ).
Measuring the impact of radiation on cognitive function can be challenging. Many patients with intracranial tumors already have baseline deficits in various measures of cognitive performance prior to any treatment. In addition, detection of radiation-associated neurocognitive deficits may be subtle and not readily captured with abbreviated measures such as IQ. For example, among 60 pediatric patients treated with protons (47% cranial spinal irradiation [CSI], 53% partial brain) for medulloblastoma, gliomas, craniopharyngioma, and ependymoma, there was no significant change in mean Wechsler Full Scale IQ, verbal comprehension, perceptual reasoning/organization, or working memory. However, processing speed scores significantly declined by a mean 5.2 points, which was more significant in younger children (<12 years) and those with the highest baseline score. This likely reflected a slowing in skill acquisition relative to peers rather than a loss of skills.
No significant decline in cognitive functioning was noted after proton therapy for low-grade glioma (LGG) among pediatric (median age at treatment, 11 years) or adult patients (median age, 37.5 years). Again, young age (<7 years) and higher dose to the left temporal lobe/hippocampus were associated with a significant decline in verbal comprehension and IQ. All together, these results suggest that neurocognition appears stable after proton treatment for older patients, but young patients are vulnerable even when brain dose can be reduced.
A single, retrospective study has attempted to compare neurocognitive effects in children after protons versus photons. Patients treated with protons had no significant temporal decline in IQ, in contrast to photon patients, who experienced an average 1.1-point decline in IQ per year. However, no statistically significant change in IQ over time was detected between protons and photons: −0.7 versus −1.1 points per year; p = .509. Although these findings remain to be confirmed in other cohorts, several considerations should be highlighted, including potentially small numbers limiting power, photon and proton patients being treated across different time periods with different follow-up times, and the use of an abbreviated measure (ie, IQ) with limited sensitivity. In addition, photon patients were younger at treatment (median age, 8.1 vs. 9.2 years), which is notable given that radiation effects are more pronounced for younger children.
Risk of endocrinopathy depends on dose to the HPA and hormone of concern, with growth hormone being the most radiosensitive. Among 29 pediatric intracranial LGG patients treated with protons, 9 (31%) were suspected to have baseline endocrine abnormalities due to tumor involvement of the HPA. All endocrinopathies, except one, occurred in patients who received >40 Gy (relative biologic effectiveness [RBE]) to the HPA. Thus even with a conformal technique such as protons, dose to an organ at risk (OAR) is unavoidable when the target is adjacent to or involves an OAR.
Secondary cancers from radiation tend to arise within tissue exposed to low or intermediate doses. Of note, although proton therapy can minimize these doses, it is associated with neutron scatter dose, which has also been associated with second cancers. Comparison of proton therapy and other conformal photon techniques, including intensity modulated radiotherapy (IMRT) and volumetric arc therapy (VMAT), suggests that protons yield a lower risk of radiation-associated malignancy for patients with LGG, craniopharyngioma, and medulloblastoma.
The most dramatic differences in second malignancy risks are likely among medulloblastoma patients, who have a large volume exposed to radiation (ie, CSI). In a study of 17 pediatric medulloblastoma patients, lifetime attributable risk (LAR) after proton or photon CSI was calculated. The predominant cancer risks were from lung, with photon CSI conferring higher risks to all organs of all patients. The ratio of LAR from protons to LAR from photons (RLAR) ranged from 0.10 to 0.22 for second cancer incidence (ie, lower risk associated with proton therapy). RLAR of cancer incidence and mortality both decreased with increasing age at radiation exposure. In addition to tissue susceptibility, another factor influencing lower cancer risk in older, fully grown children is the observation that the entire vertebral body is not included within the CSI target volume. As a result, anterior organ tissues are farther away from the radiation target volume.
As secondary cancers from radiation have a 10- to 15-year latency before presentation, clinical confirmation of the preceding modeling data may not be available for several more years, although preliminary data are promising. More than 500 patients treated with protons at the Harvard Cyclotron Laboratory were matched to patients treated with photons in the Surveillance, Epidemiology, and End Results registry. The 10-year cumulative incidence rate for second malignancies after protons and photons was 5.4% and 8.6%, respectively. However, in the absence of radiation dose and fields data, the authors could not determine which second malignancies were radiation associated. Among retinoblastoma patients treated with photons or protons, the 10-year cumulative incidence of radiation-induced second malignancies was lower with proton therapy: 0% versus 14% ( p = .015), although there was no significant difference in the 10-year cumulative incidence of all second malignancies (5% vs. 14%; p = .12).
The second premise for protons is the ability to escalate dose given that nearby organ structures can be spared dose. For chordomas and chondrosarcomas, primary tumors that arise from the base of skull or vertebral bodies, a dose-response exists in which higher radiation doses are associated with improved local control. Indeed, given the location of these tumors, gross total resection may be difficult to achieve. Therefore proton therapy has been frequently incorporated as part of local adjuvant or primary treatment (see the Chordomas and Chondrosarcomas section).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here