Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
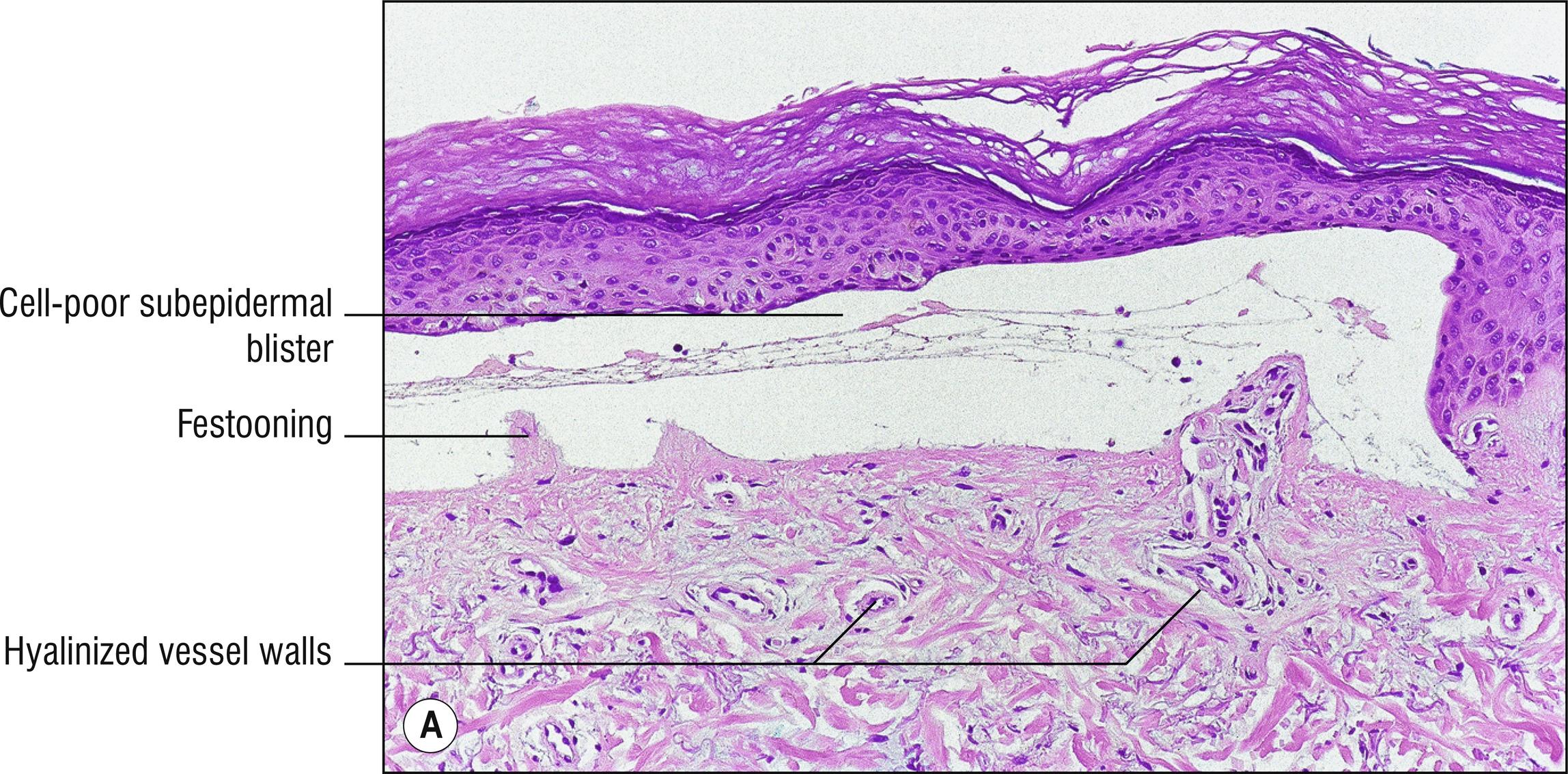
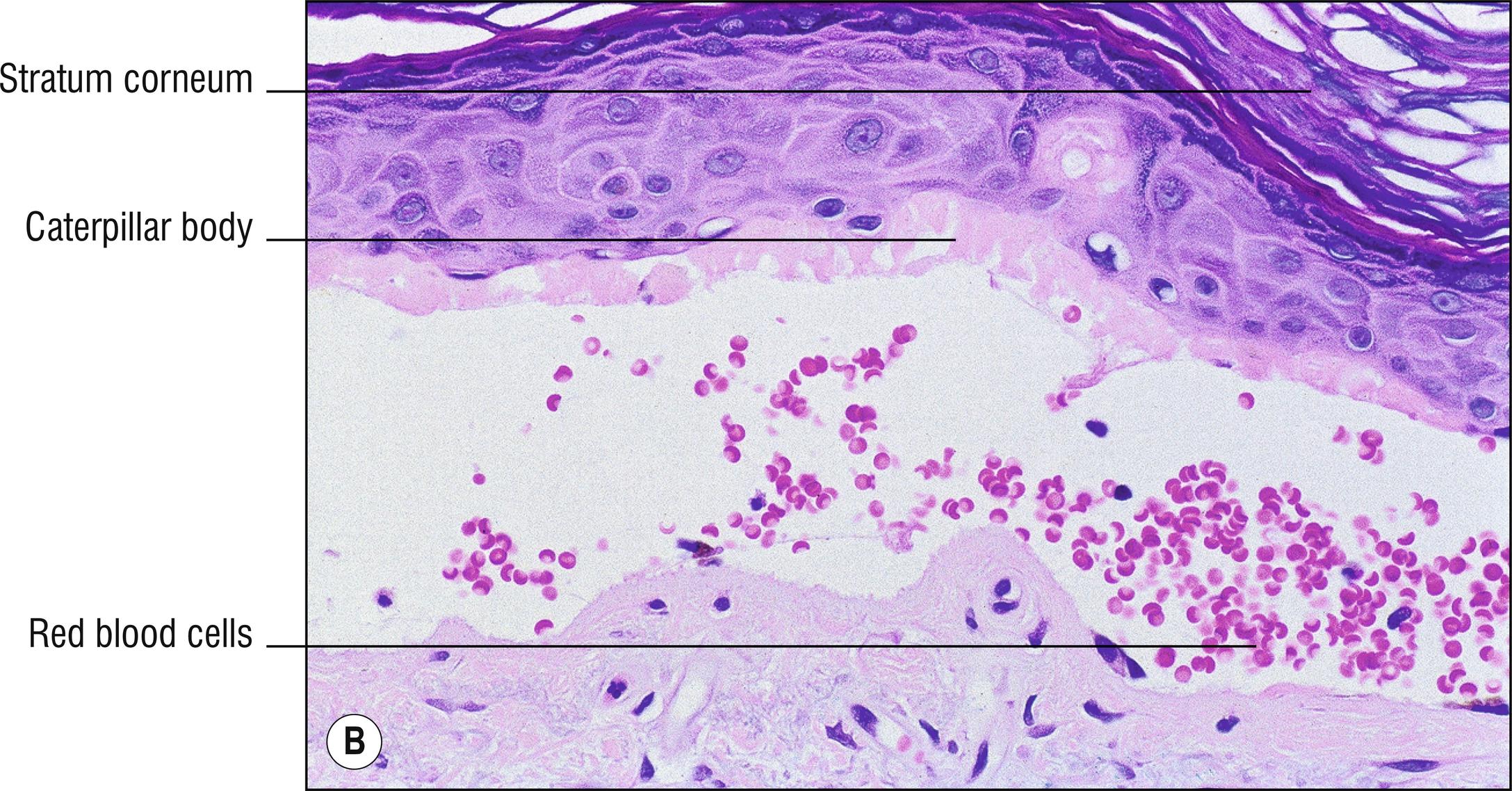
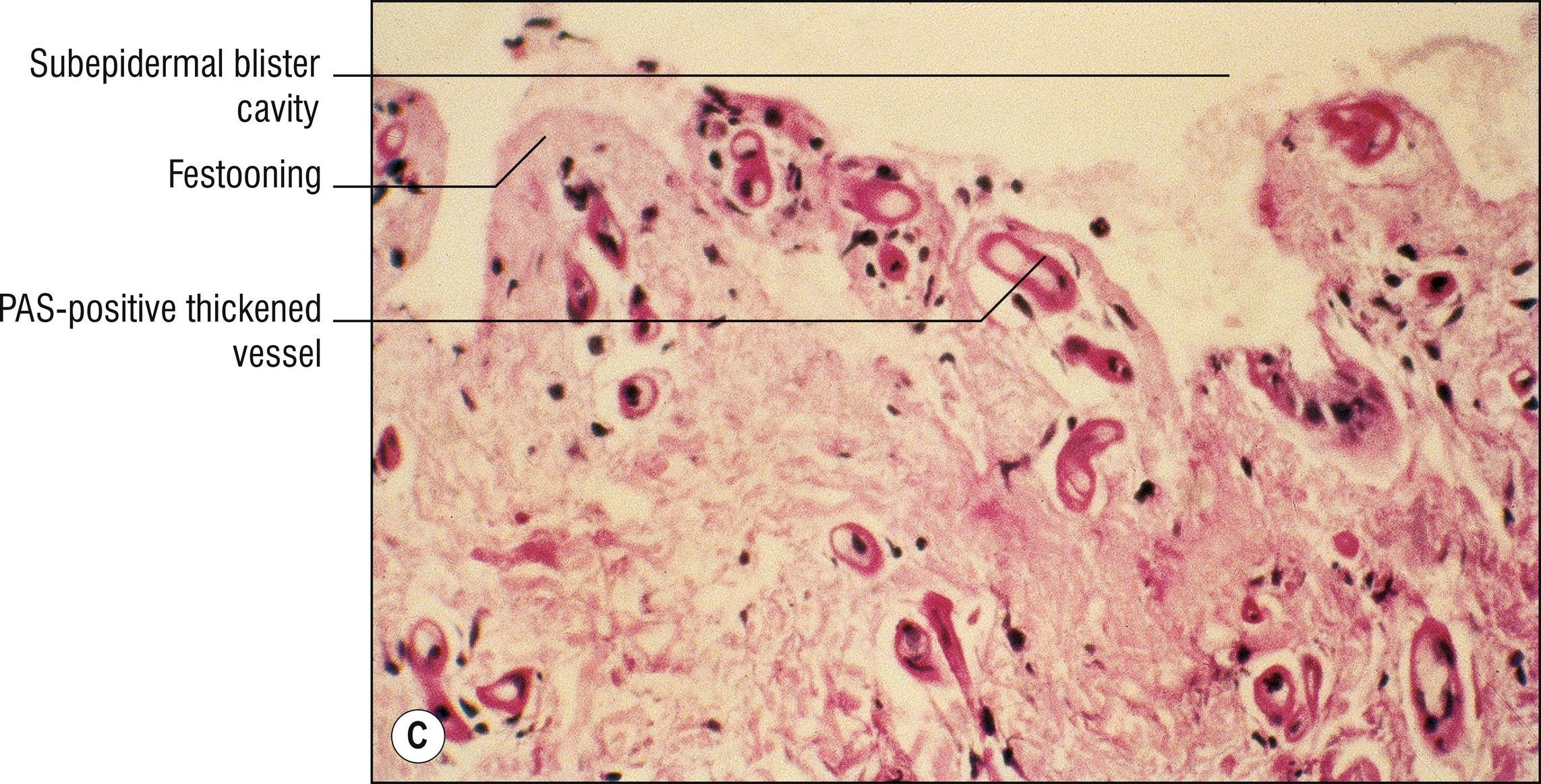
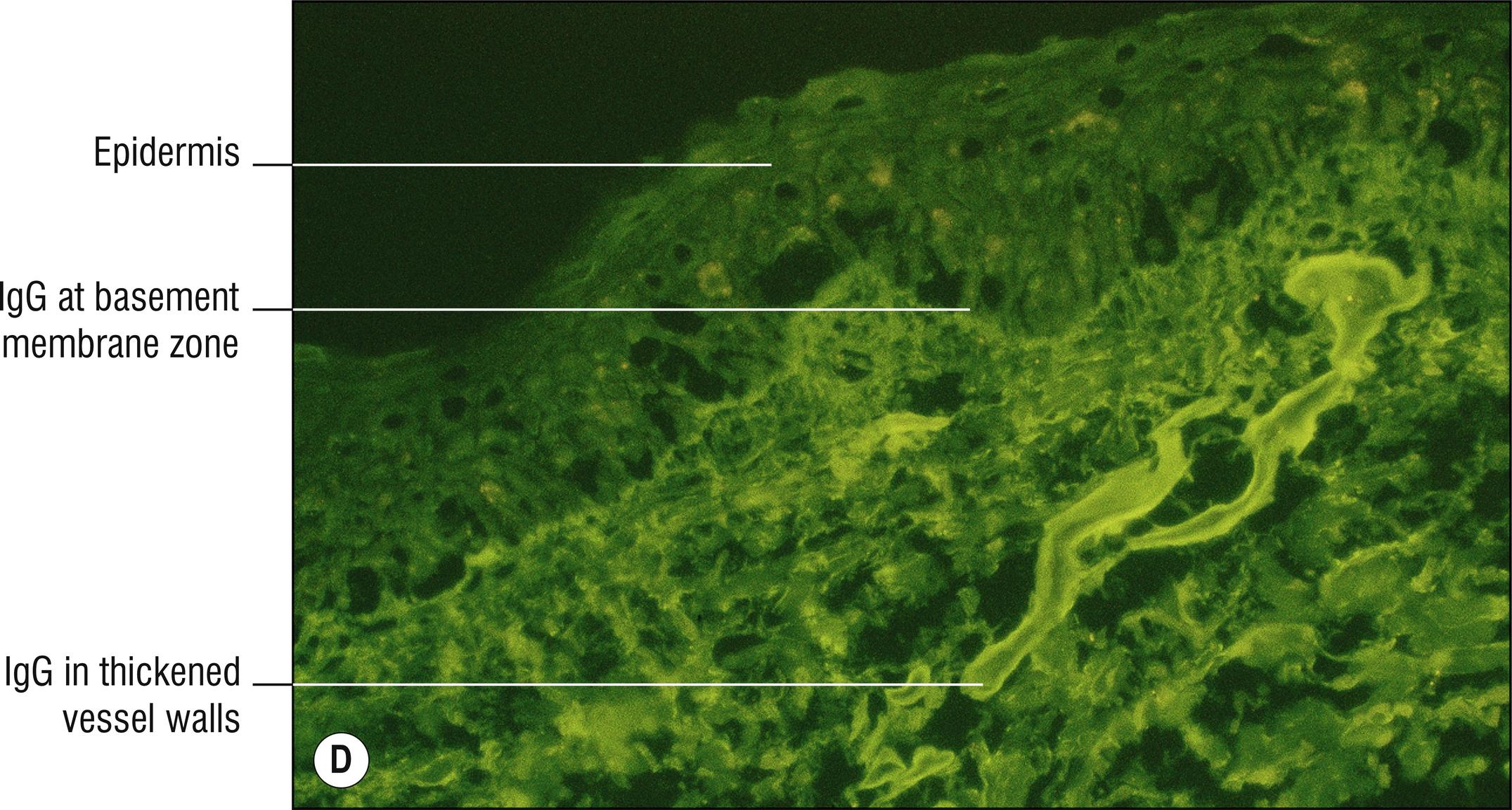
(see Fig. 8.1A–F )
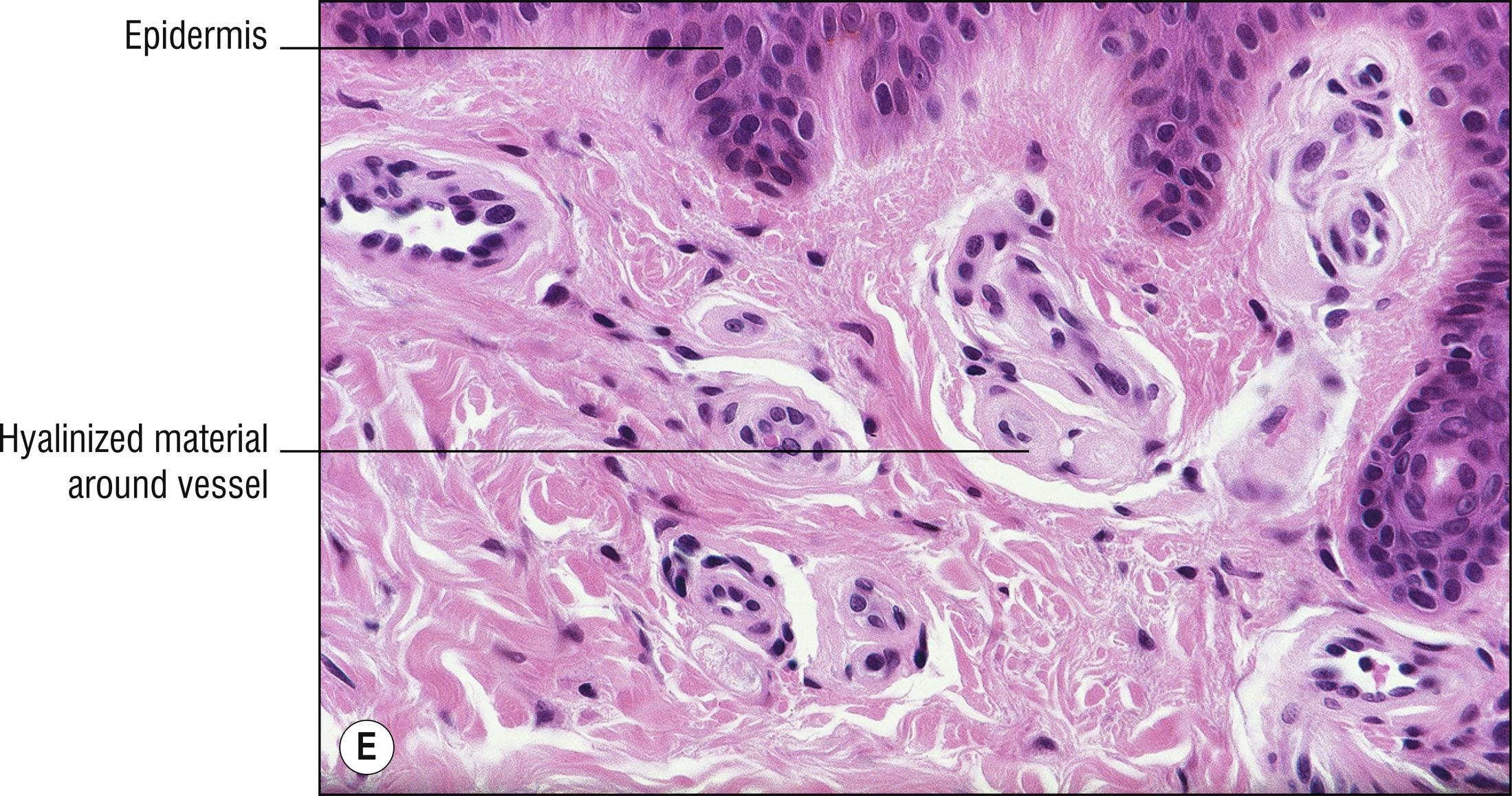
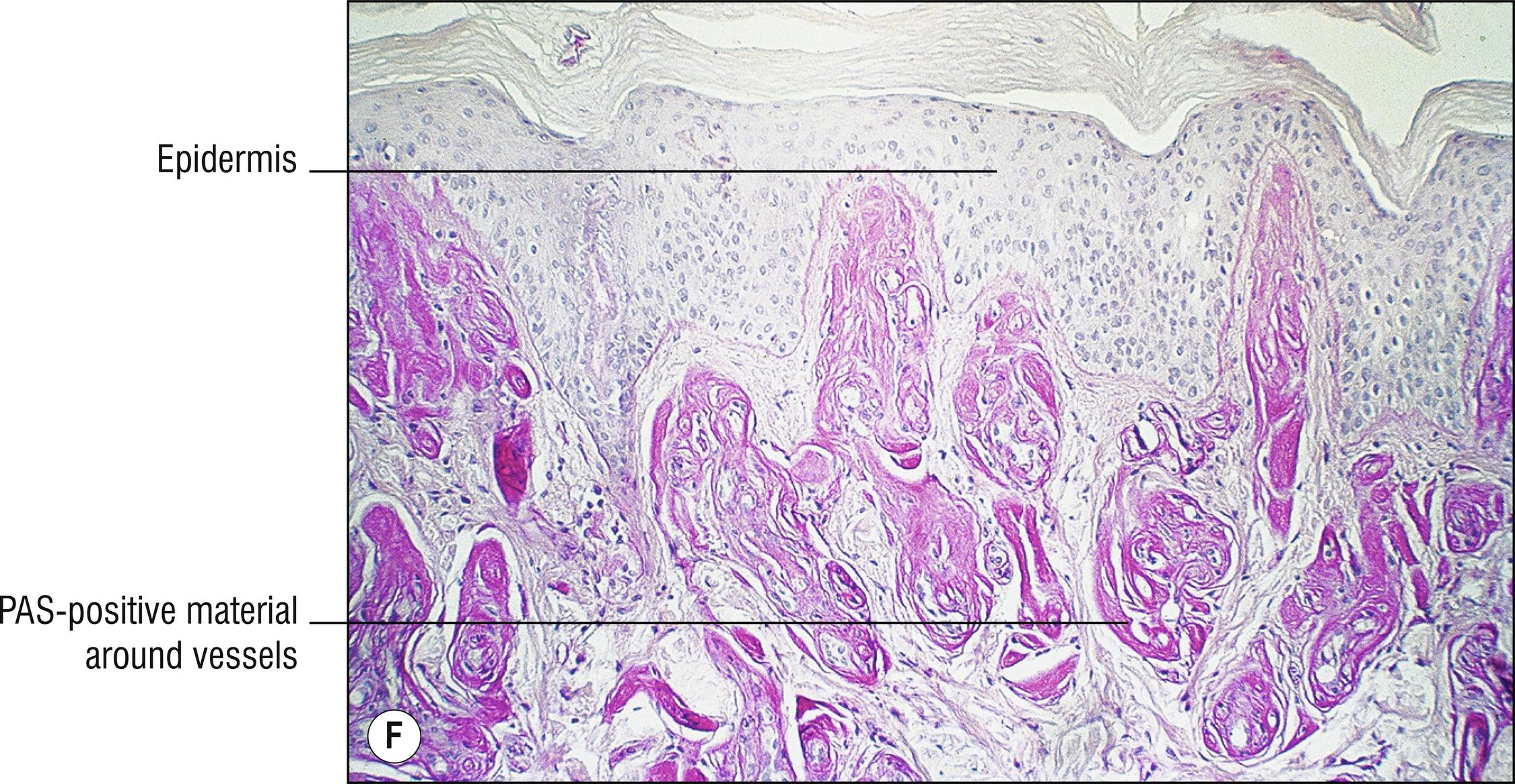
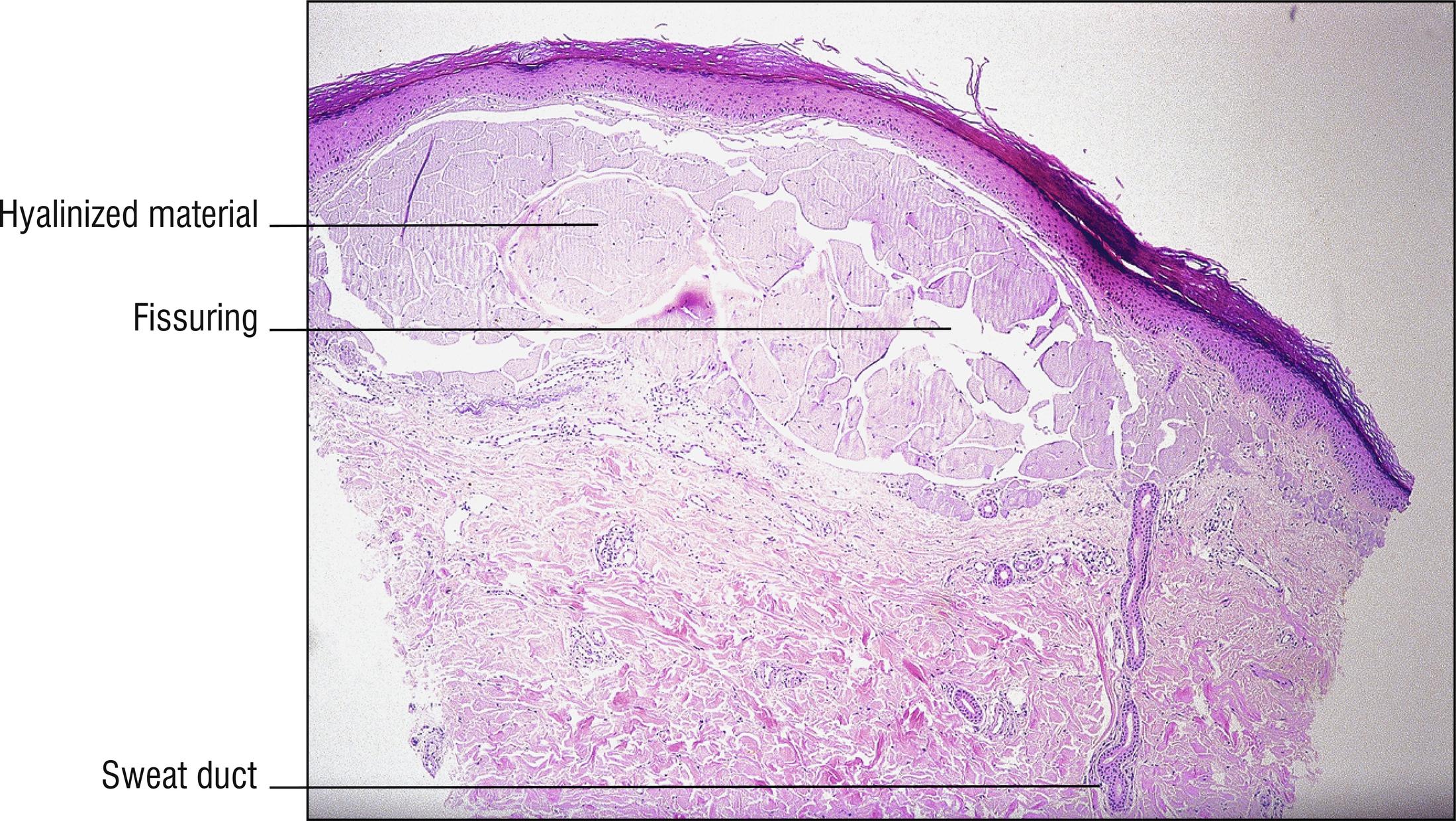
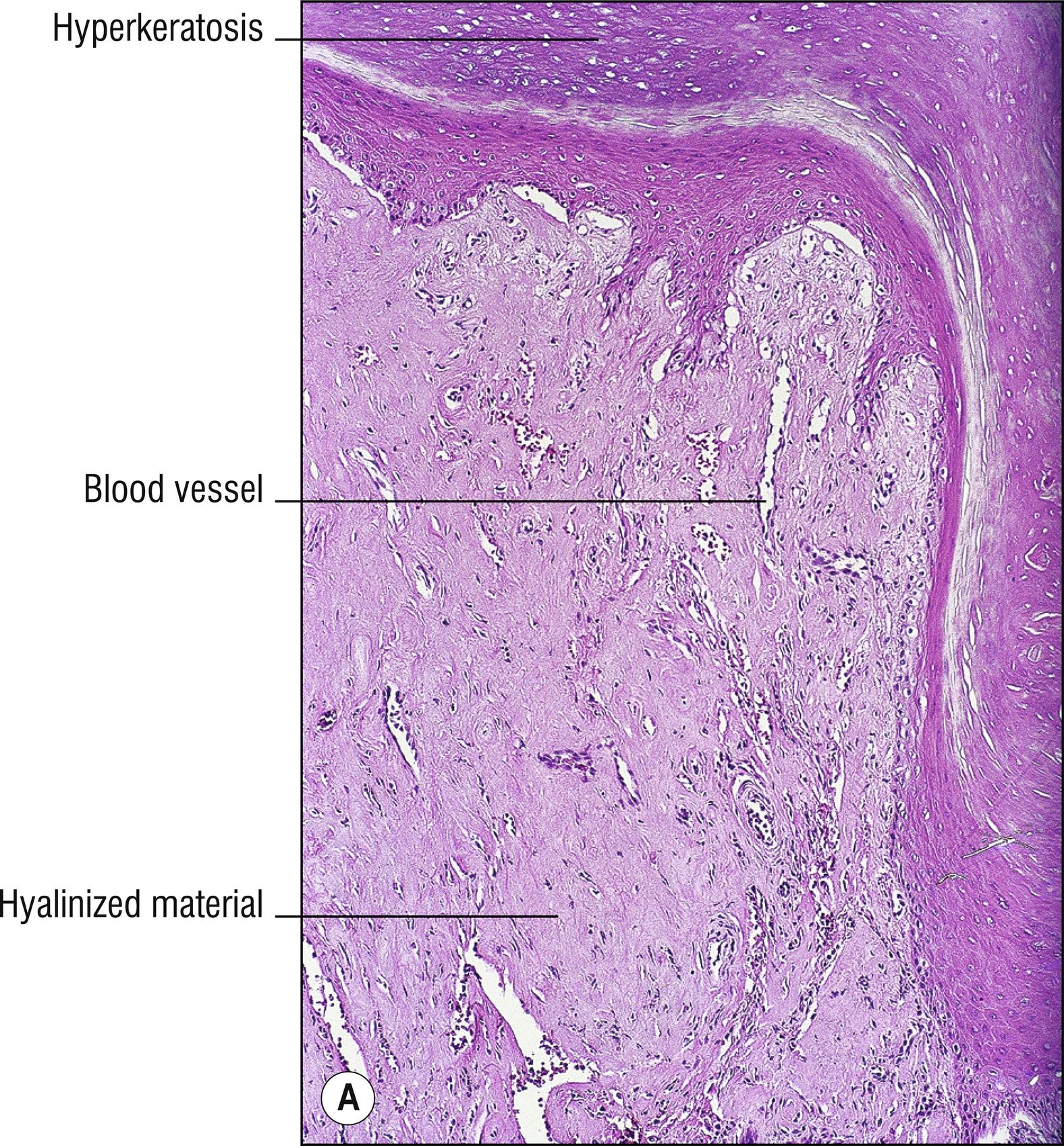
Uncommon to rare group of porphyrin metabolism disorders. Most of them present as photodermatitis (1.110) with papules or vesicles mostly on sun-exposed skin.
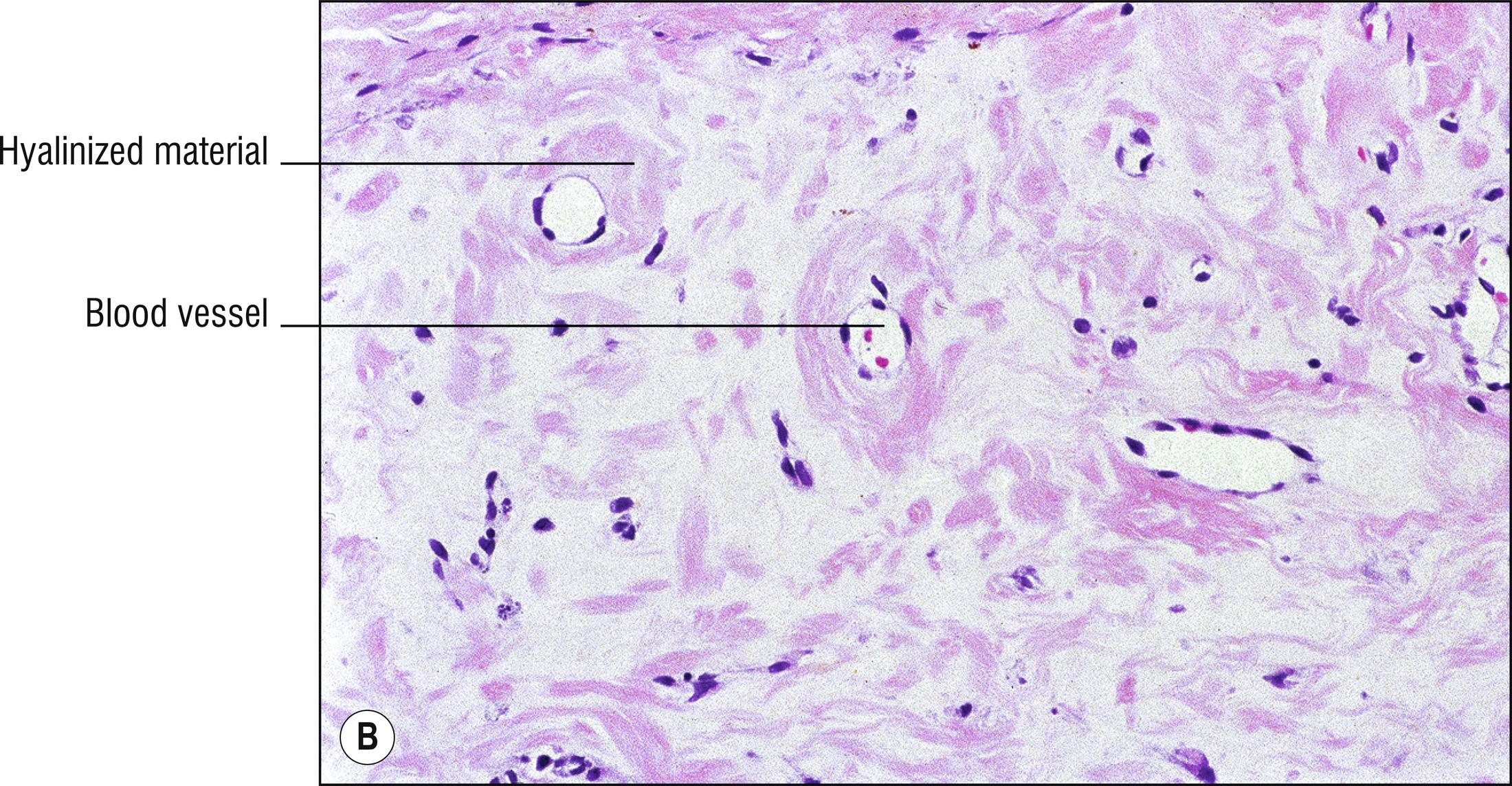
Porphyria cutanea tarda (PCT): most common type of porphyria, uroporphyrin decarboxylase deficiency, most cases acquired and only 20% due to UROD gene mutation, uroporphyrins highly elevated in the urine, “tarda” refers to “late” (adult) onset of vesicles , papules, crusts, and milia in areas of scarring, most common on the dorsum of the hands (1.56), malar hypertrichosis (1.62), sometimes associated with HIV, most cases associated with hepatitis C (14.7), alcohol abuse, and liver disease (1.75) with iron accumulation. Can be associated with hemochromatosis and HFE gene defect (8.17):
Subepidermal blister with viable roof, festooning of dermal papillae (very well-preserved papillae projecting into blister), and very little inflammation ( cell poor ).
Caterpillar bodies (eosinophilic, linear, segmented basement membrane material resembling dyskeratotic cells; 1.27) sometimes found in the roof of the blister, not specific for porphyria.
Sparse hyalinized material around blood vessels (PAS positive, diastase resistant), especially in papillary dermis, sometimes throughout dermis.
Dermal sclerosis in late stages sometimes (1.125).
Direct immunofluorescence: IgG and C3 around papillary dermal vessels with lesser staining at dermal–epidermal junction in the lamina lucida.
Acute intermittent porphyria (AIP): rare, hydroxymethylbilane synthase (formerly porphobilinogen deaminase) deficiency, autosomal dominant, associated with HMBS gene mutation, increased porphobilinogen (PBG) in the blood, acute abdominal pain, psychosis, neurologic disease, no skin lesions .
Variegate porphyria (VP): protoporphyrinogen oxidase deficiency, autosomal dominant, usually PPOX gene mutation, combined features of AIP plus PCT .
Hereditary coproporphyria (HCP), autosomal dominant, and harderoporphyria, autosomal recessive: rare, mutation in CPOX gene, coproporphyrinogen oxidase deficiency, combined features of AIP plus PCT .
Erythropoietic protoporphyria (EPP): rare, ferrochelatase deficiency, autosomal dominant, often associated with FECH gene defect (X-linked dominant EPP may be associated with ALAS2 gene), photodermatitis with very few blisters begins in childhood , occasional fatal liver disease, free erythrocyte protoporphyrin elevated in the blood, impressive deposits of hyalinized material, PAS-positive, in the dermis, less blistering.
Congenital erythropoietic porphyria (CEP, EP, Günther’s disease): very rare, uroporphyrinogen III synthase deficiency, autosomal recessive, often UROS gene mutation, severe congenital mutilating “ werewolf ” form with onset in infancy, red fluorescent teeth, extensive hypertrichosis, hemolytic anemia, splenomegaly, more hyalinized material in dermis than other forms of porphyria (1.35).
Pseudoporphyria: related to hemodialysis or certain drugs (3.5), especially non-steroidal anti-inflammatory drugs, tetracycline, and furosemide, nearly identical to PCT histologically.
Other diseases with abundant dermal eosinophilic amorphous pink material (1.35) may resemble EPP, especially lipoid proteinosis (8.3) and amyloidosis (8.4).
Other subepidermal blistering diseases (1.147; Chapter 6 ), especially the more cell-poor ones such as epidermolysis bullosa (6.6), bullous amyloidosis (8.4), and some examples of bullous pemphigoid (6.1).
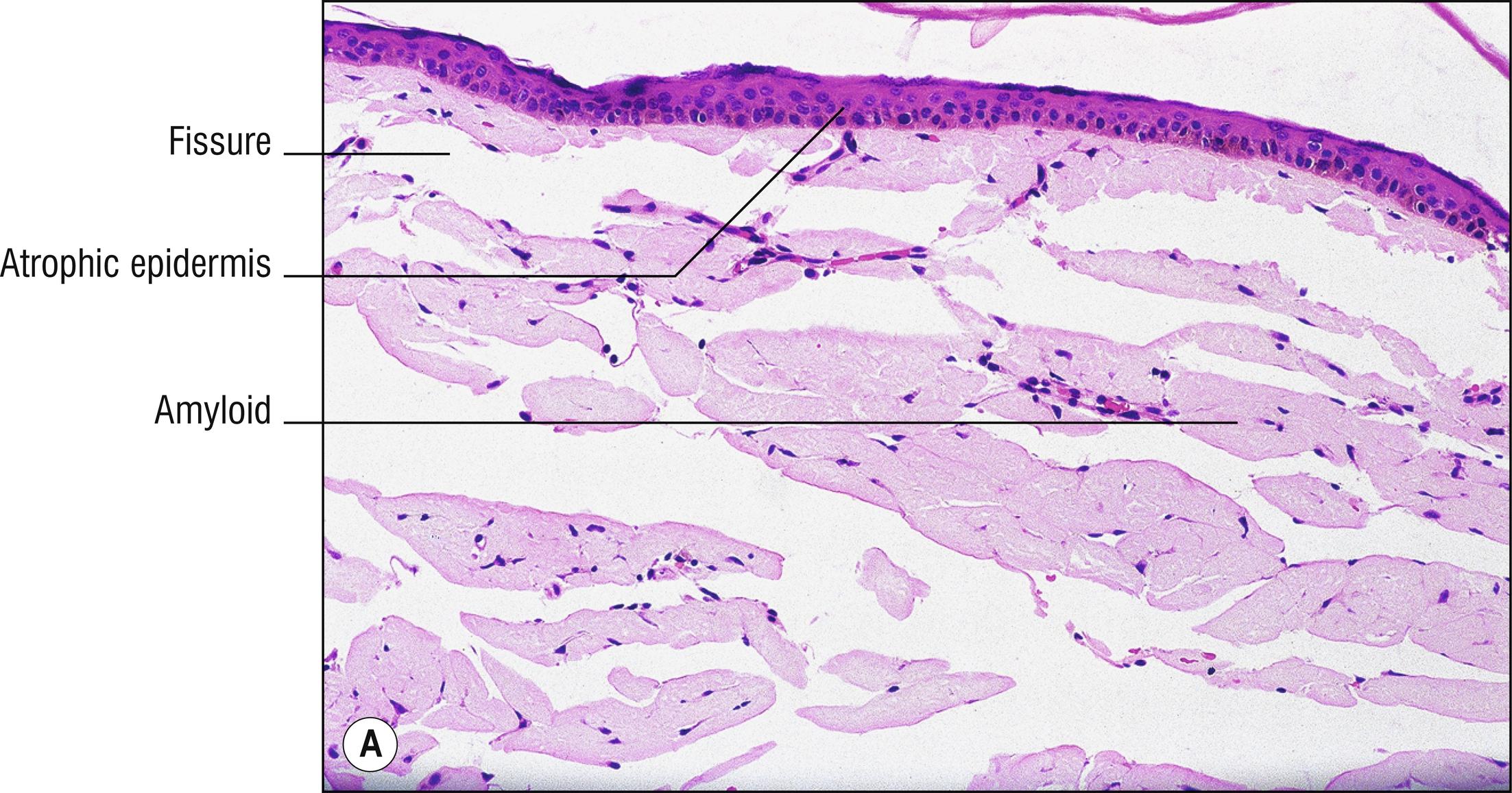
(see Fig. 8.2 )
Uncommon grouped whitish papules on sun-exposed skin (1.110) on the dorsum of the hands (1.56), face, neck, or ears in adults, or on the face in the very rare childhood form.
Often epidermal atrophy (1.9) with hyperkeratosis
Nodular fissured masses (1.23) of amorphous eosinophilic material in the superficial dermis
Separation between the masses by a thin rim of collagen, elastic tissue, or collarette of epidermal rete ridges
Special stains of the eosinophilic material (Congo red and crystal violet) often stain positive, as in amyloidosis
Solar elastosis common
Other diseases with amorphous pink material (1.35), especially amyloidosis (8.4) and nodular colloid degeneration (9.1).
(see Fig. 8.3A,B )
Very rare autosomal recessive deposition disorder, related to mutation in the extracellular matrix protein 1 gene (ECM1), beginning in childhood with laryngeal papules resulting in hoarseness , beaded papules of the eyelids (1.43) and nose, and verrucous plaques (1.146) of other areas of skin, especially over joints (1.92).
Hyperkeratosis, papillomatosis (1.102) sometimes
Amorphous eosinophilic deposits beginning around the vessels, later diffuse throughout the dermis, with a tendency to be perpendicular to the epidermis and to arrange around adnexal structures and blood vessels
Positive staining with colloidal iron, Alcian blue, Sudan black, PAS with or without diastase . Sudan black oil-red-O variably positive. Amyloid stains usually weakly positive or negative
Juvenile hyaline fibromatosis: very rare, autosomal recessive, due to CMG2 (capillary morphogenesis gene-2) or ANTXR2 (anthrax toxin receptor) mutation, deposition disorder affecting young children, mental retardation, no hoarseness, flexural contractures , gingival hypertrophy , papules or nodules on the lips (1.74), nose (1.95), ears (1.28), and perianal areas (1.108). PAS and Alcian blue positive hyalinized dermal deposits with a chondroid appearance.
Infantile systemic hyalinosis: somewhat like juvenile hyaline fibromatosis with same genetic defect reported, but there is more systemic involvement.
Other diseases with amorphous pink material (1.35), especially amyloidosis (8.4) and erythropoietic protoporphyria (8.1).
(see Fig. 8.4A–I )
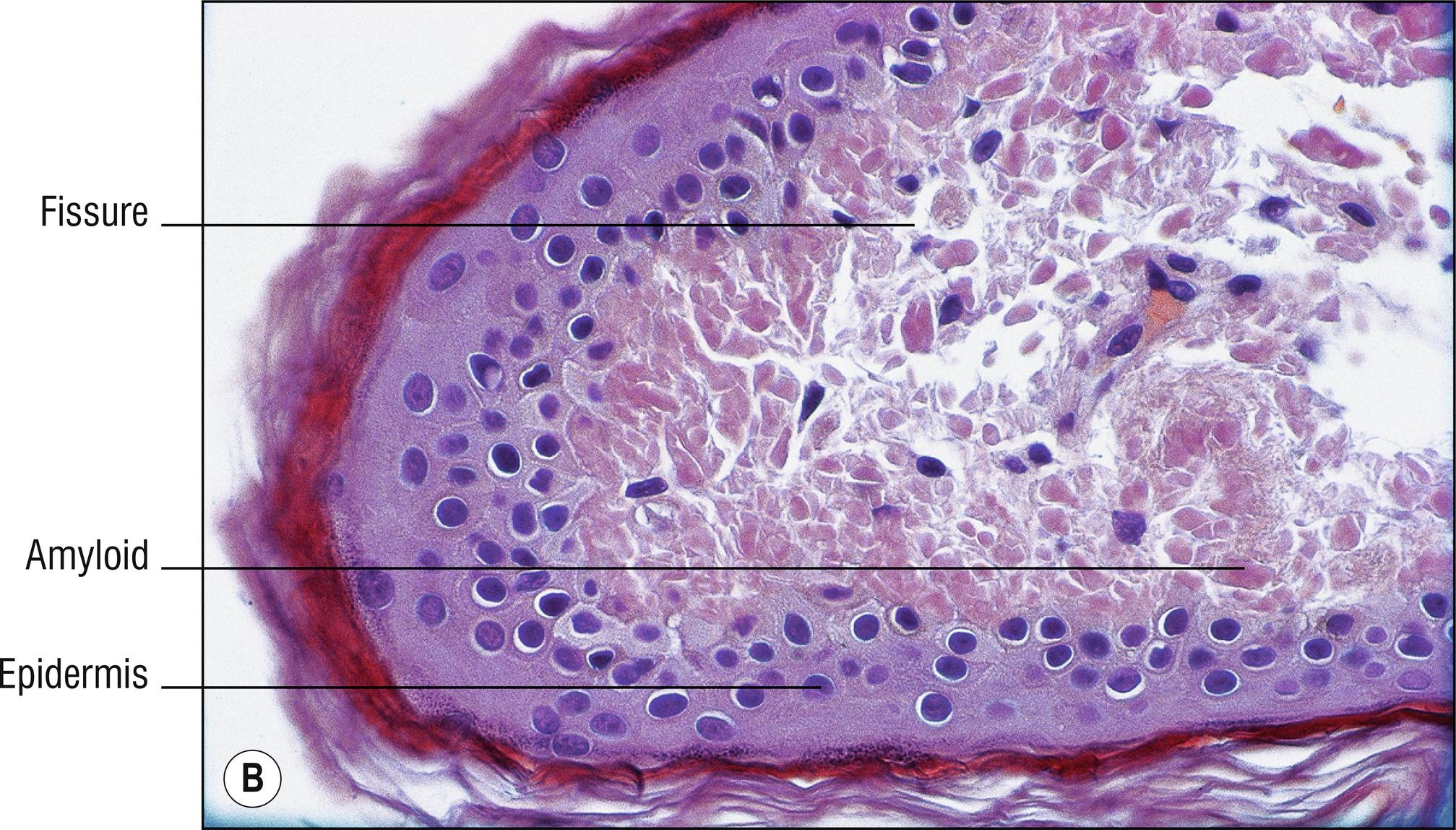
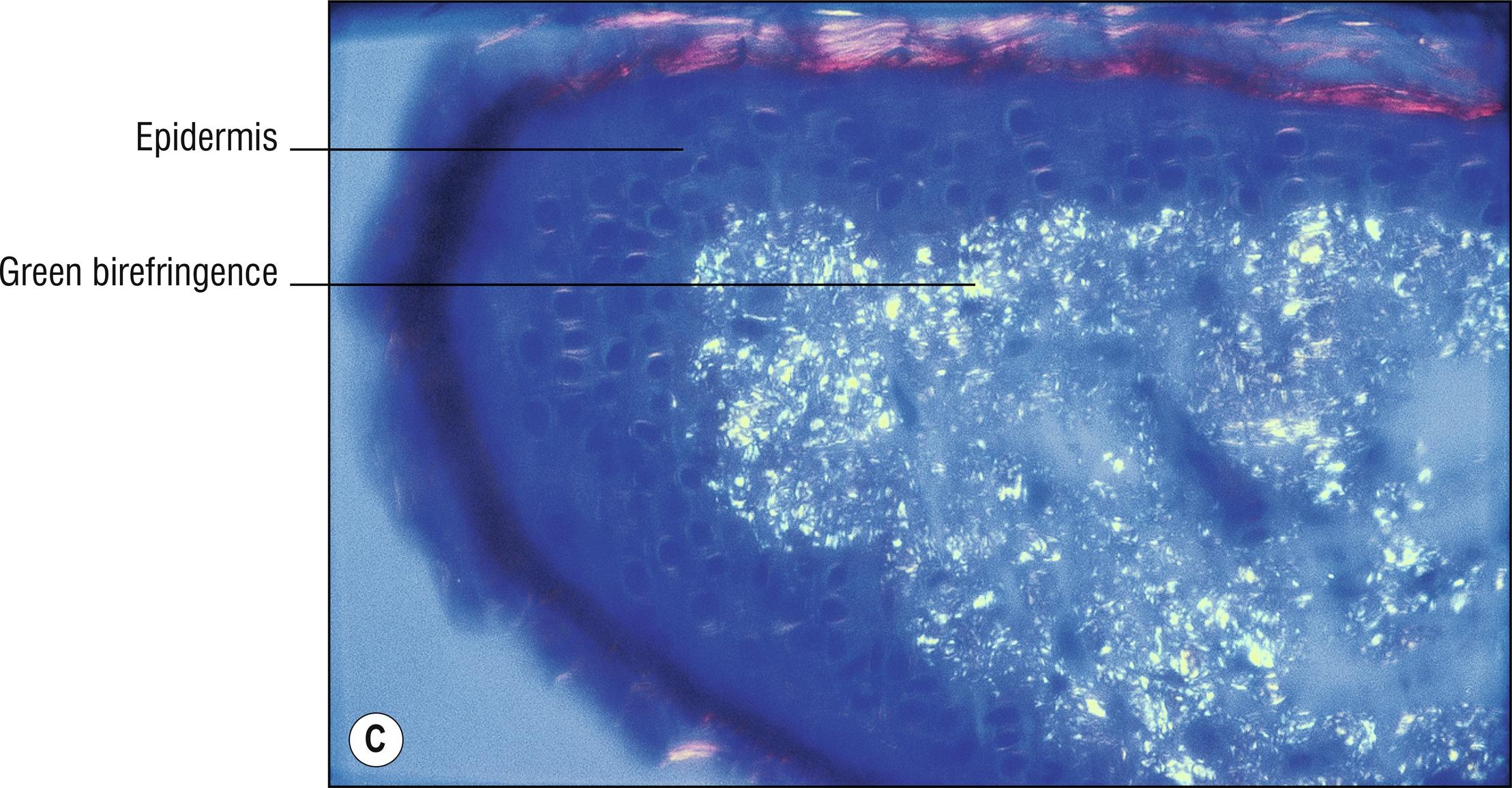
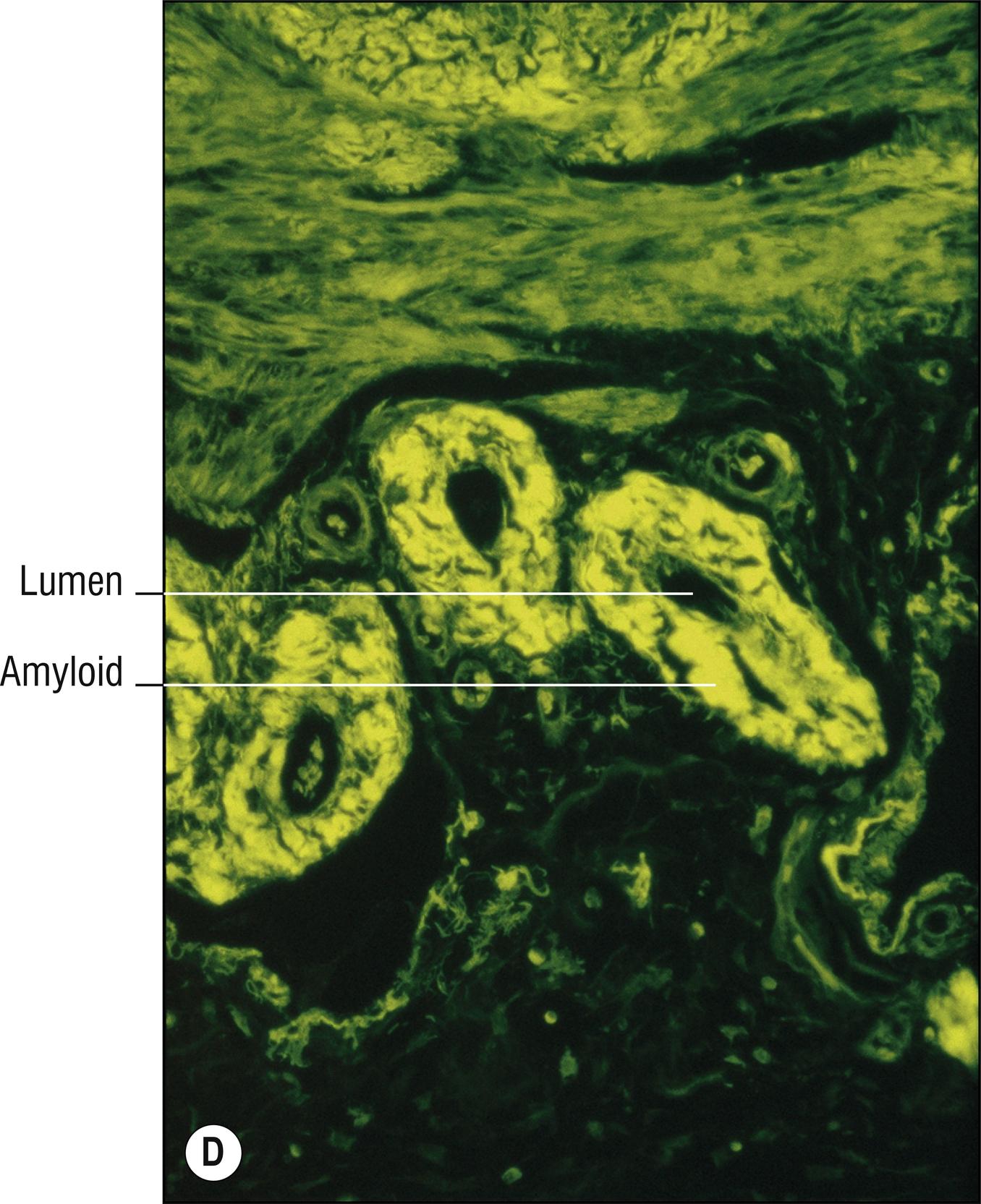
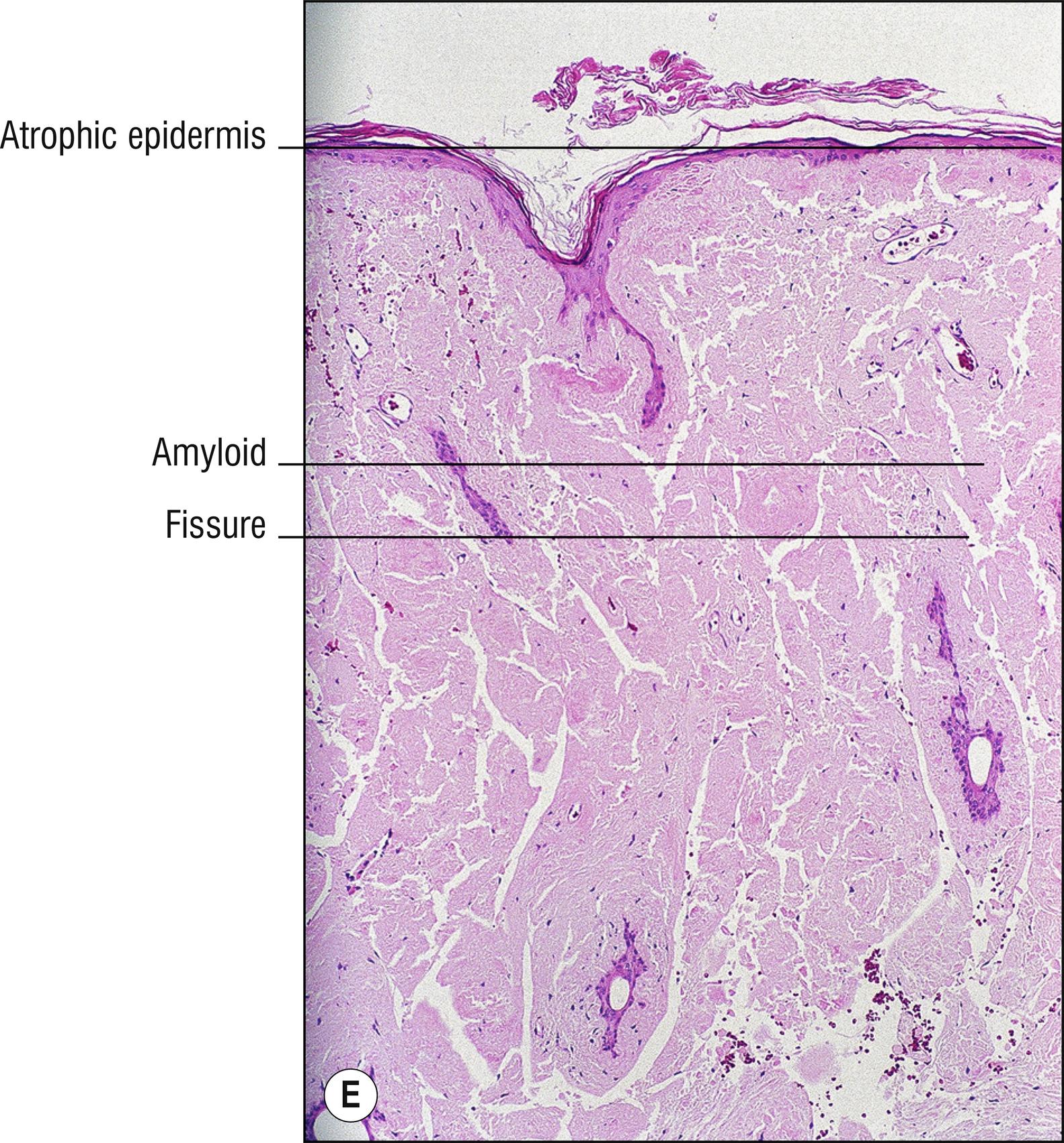
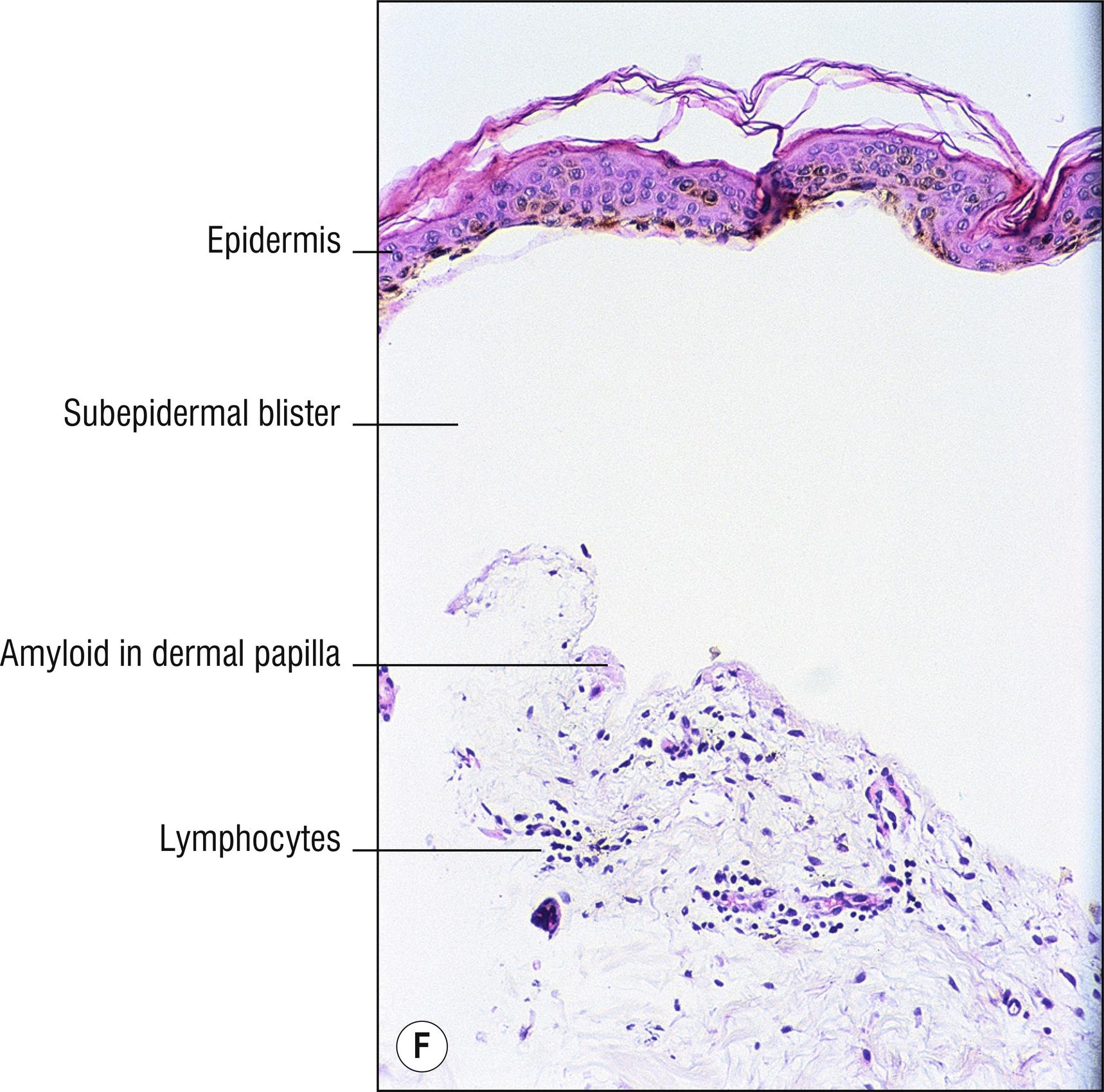
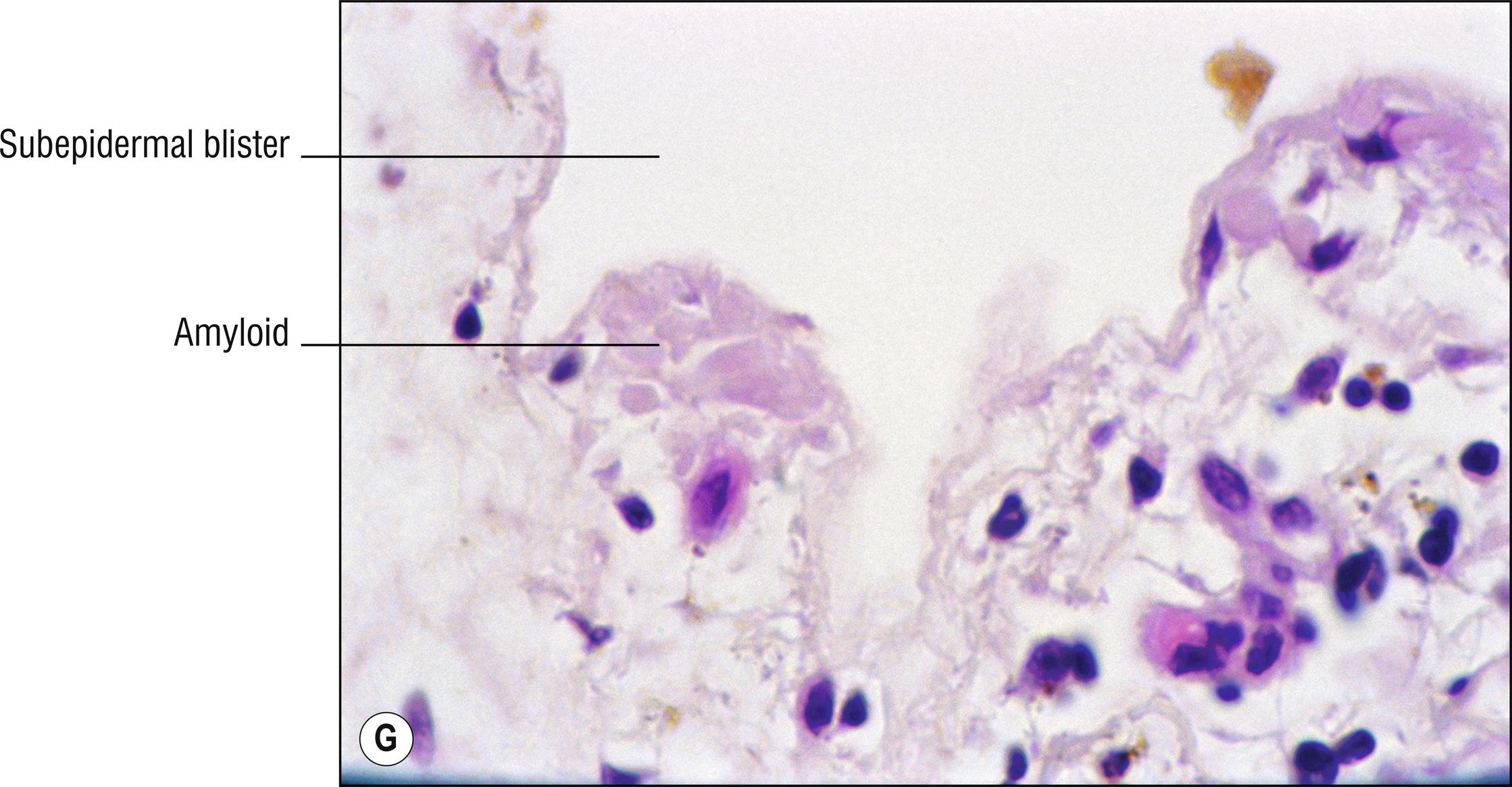
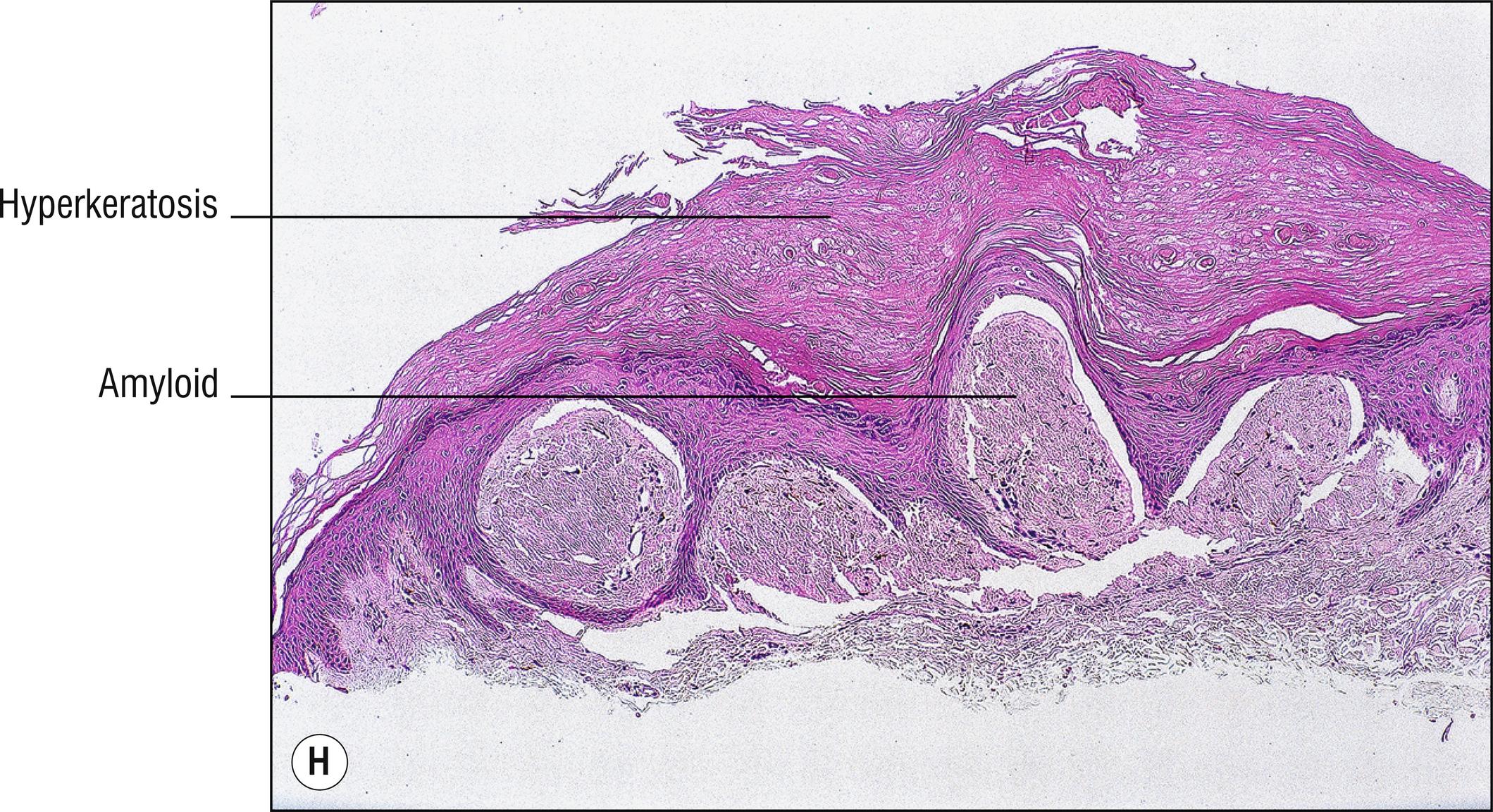
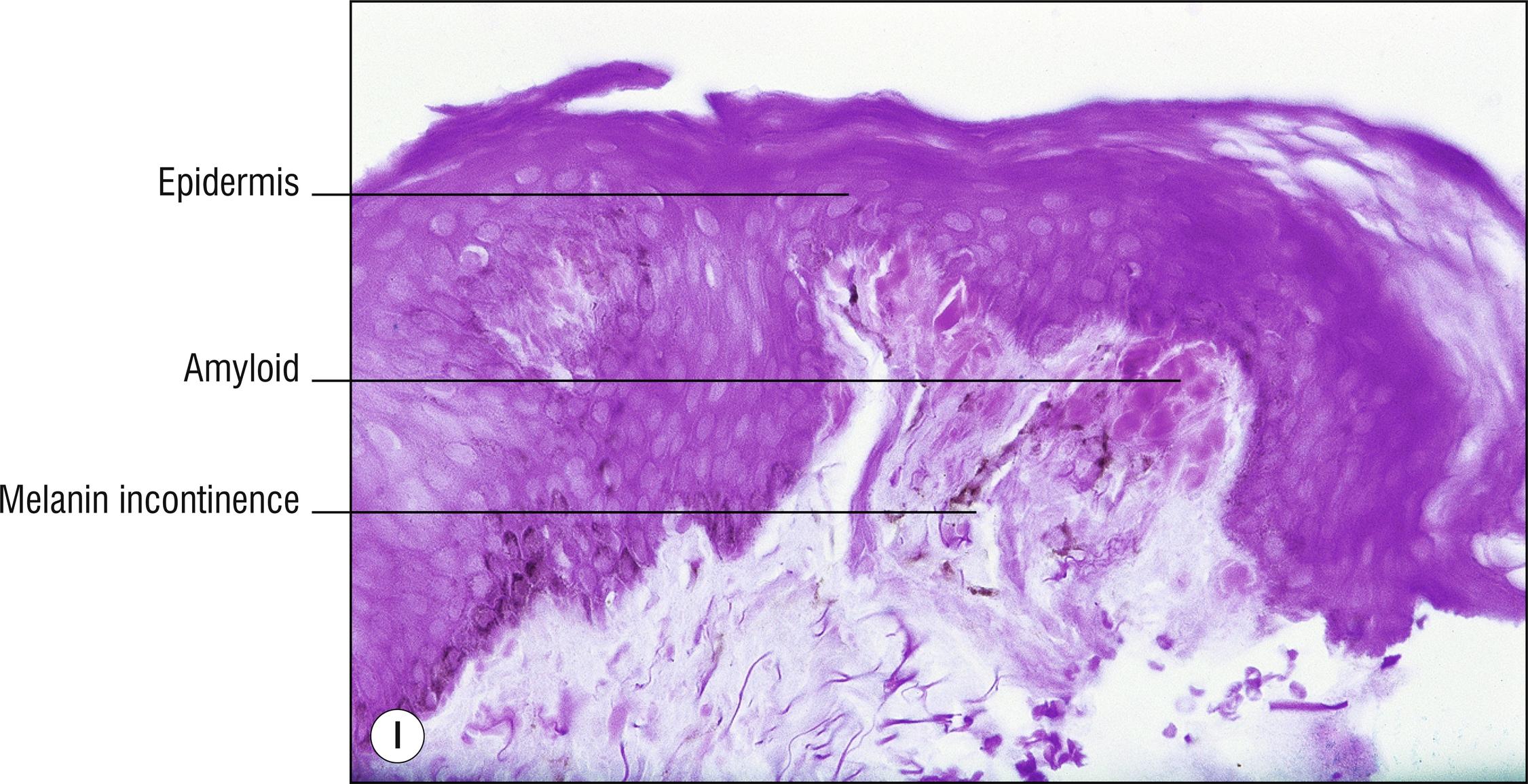
Uncommon deposits of eosinophilic amyloid protein, due to systemic disease involving kidney, heart, or liver, or in rubbed or lichenified skin from degeneration of keratinocytes without systemic disease (macular and lichen amyloid). Amyloid is a beta-pleated sheet by X-ray diffraction and infrared spectroscopy, and non-branching filaments with a diameter of 6–10 nm are seen by electron microscopy. The amyloid fibrils themselves can consist of a variety of proteins, and the deposits in the skin also contain small amounts of other substances such as glycosaminoglycans, apolipoprotein E (apoE), and serum amyloid P (SAP). In macular and lichen amyloidosis, there is evidence of keratin (immunostains EKH4 or EAB-903) or tonofilaments (by electron microscopy) in the papillary dermal amyloid deposits, which are thought to originate from the damaged keratinocytes in the epidermis.
Deposits of amorphous, eosinophilic, fissured material (1.23)
Special stains positive: crystal violet, Congo red, thioflavin T, pagoda red 9 (Dylon), scarlet red (RIT). PAS is moderately positive but is not used for this purpose. Immunostains for amyloid are available
Keratin stains such as EAB-903 may be positive in lichen and macular amyloidosis
Primary systemic amyloidosis: AL types of amyloid with immunoglobulin light chains (usually lambda) deposited, associated with myeloma (24.12), yellowish (1.151) or purpuric (1.120) macules or plaques, mostly in the elderly , especially on the eyelids (1.43), deposits diffuse in dermis and/or especially around blood vessels , sweat glands, and adipocytes, often with scattered lymphocytes, plasma cells (1.111), and extravasated erythrocytes (1.40). Special immunostains for light chains usually positive, but can also be positive by non-specific absorption in macular and lichen amyloidosis.
Secondary systemic amyloidosis: AA type of amyloid (serum derived) associated with chronic systemic diseases such as rheumatoid arthritis or leprosy, no skin lesions usually present clinically, but patients have deposits of amyloid in other organs. Dermatologists are sometimes asked to biopsy “normal” lower abdomen skin to find subtle amyloid around blood vessels, sweat glands, or fat cells, obviating the need for rectal biopsy or internal organ biopsy.
Nodular amyloidosis: large waxy nodule of diffuse light chain AL amyloid in the dermis, plasma cells usually present (1.111), with systemic amyloidosis or myeloma in a minority of cases.
Bullous amyloidosis: rare form of systemic amyloidosis with hemorrhagic (1.120) cell-poor blisters (1.147), amyloid deposits may be subtle or prominent. Amyloid can act as a sponge for IgM or C3, which may cause confusion with autoimmune blistering disorders such as pemphigoid when direct immunofluorescence is performed.
Lichen amyloidosus (technically the only form of amyloid spelled with the ending –us due to Latin derivation): pebbled lichenified plaques, mostly on the shins or scattered grouped papules in areas that are chronically rubbed, without systemic disease, small deposits of amyloid limited to dermal papillae , often melanin incontinence (1.79), may resemble or may be a variant of lichen simplex chronicus (2.3).
Macular amyloidosis: brown macules (1.17) with a rippled appearance (“hammered brass” or “corduroy pants”), most common on the upper back (1.141), without systemic disease, nearly normal epidermis, very subtle amyloid blobs in the papillary dermis in areas of melanin incontinence (1.79), biopsy may appear nearly normal (1.94).
Muckle–Wells syndrome: rare, autosomal dominant, mutation in CIAS gene encoding cryopyrin, urticaria, deafness, and AA amyloid in kidney.
Familial Mediterranean fever: rare, autosomal recessive, fever, peritonitis, pleurisy, arthritis, cellulitis-like plaques of the legs, AA amyloid in kidney.
Other diseases with amorphous pink material (1.35), especially porphyria (8.1), colloid milium (8.2), lipoid proteinosis (8.3), and Waldenström’s macroglobulinemia (24.12). Because special stains can be variable, electron microscopy may be desirable if proof of amyloid deposits is desired.
(see Fig. 8.5A–E )
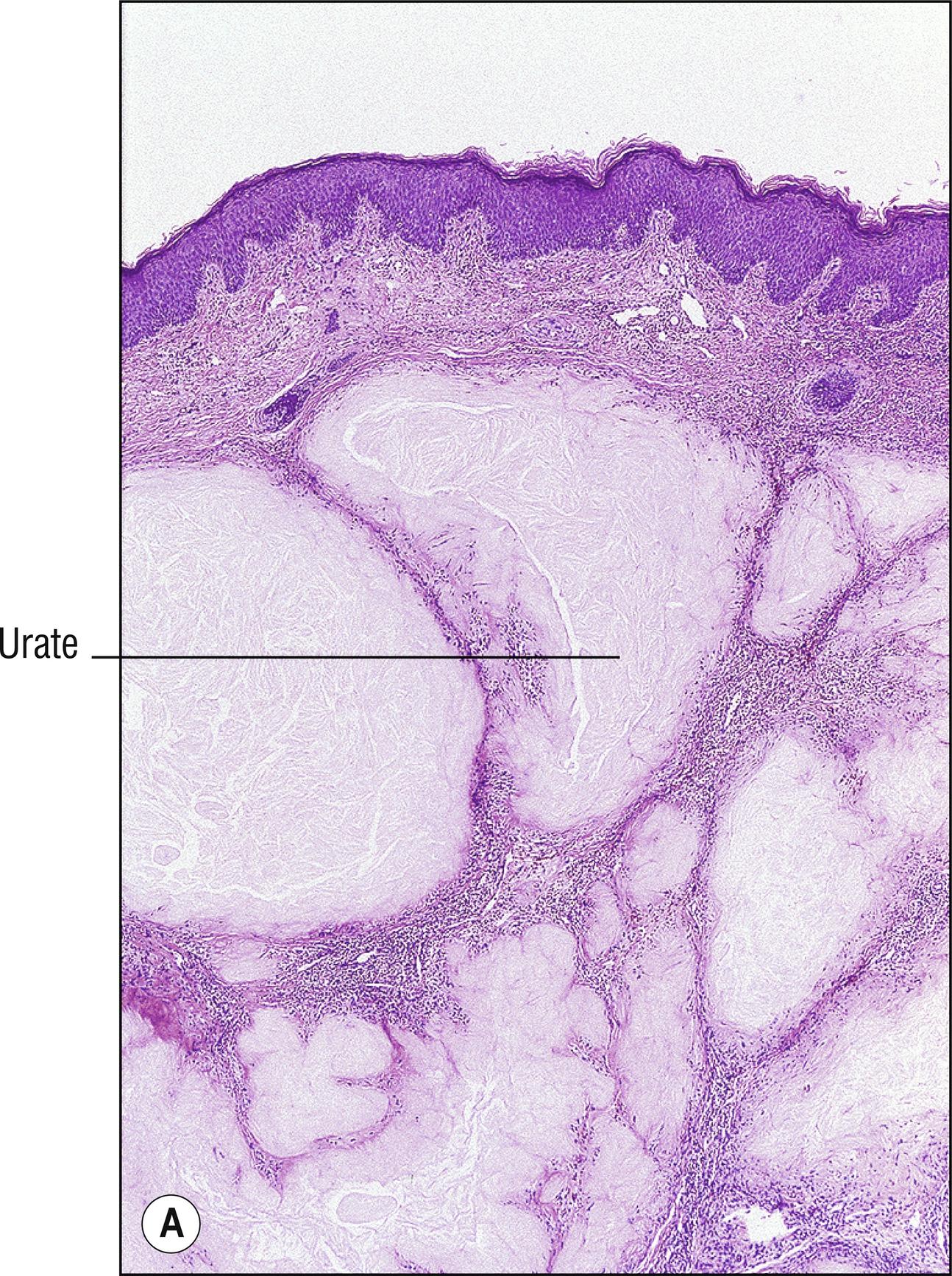
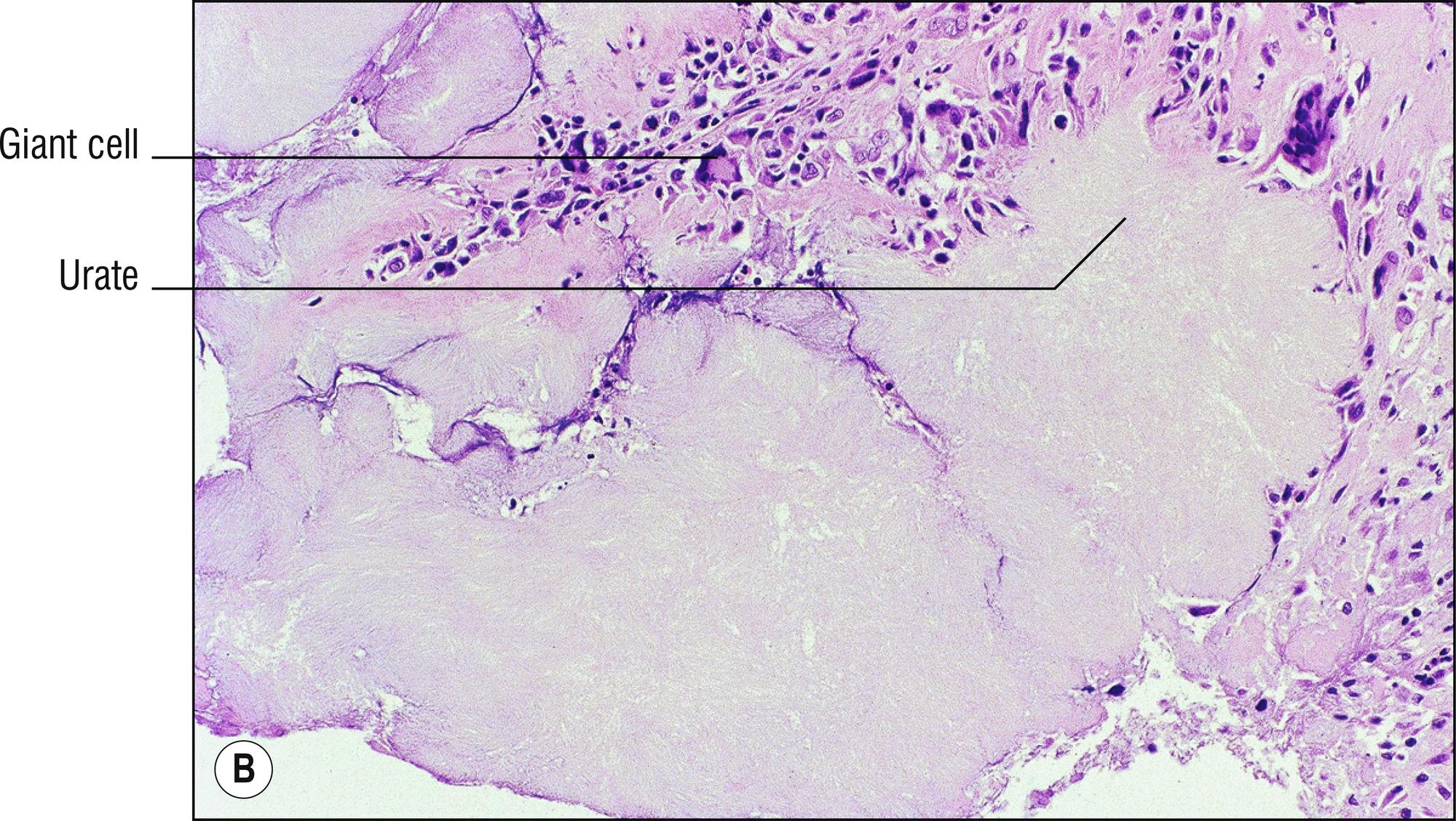
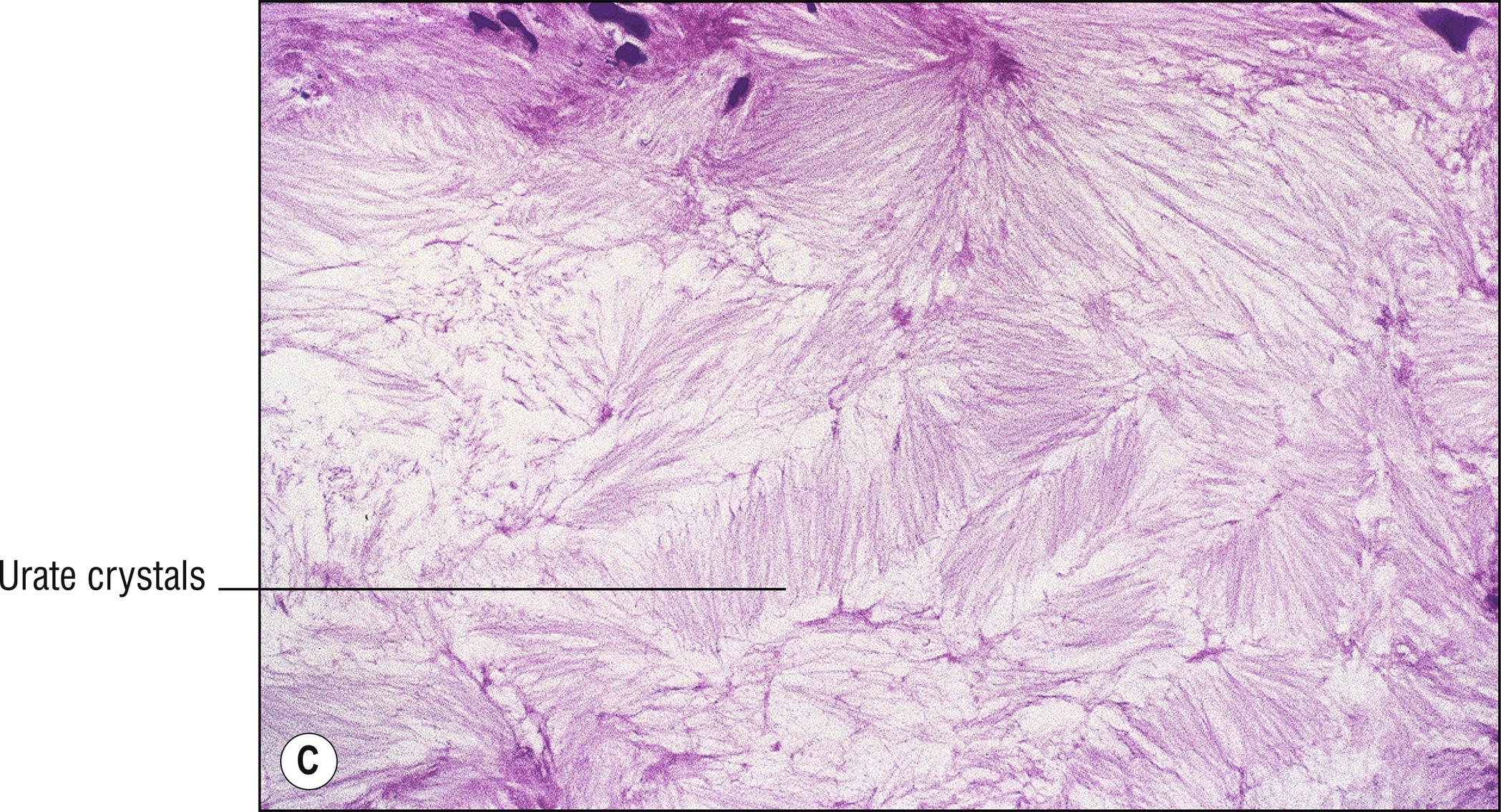
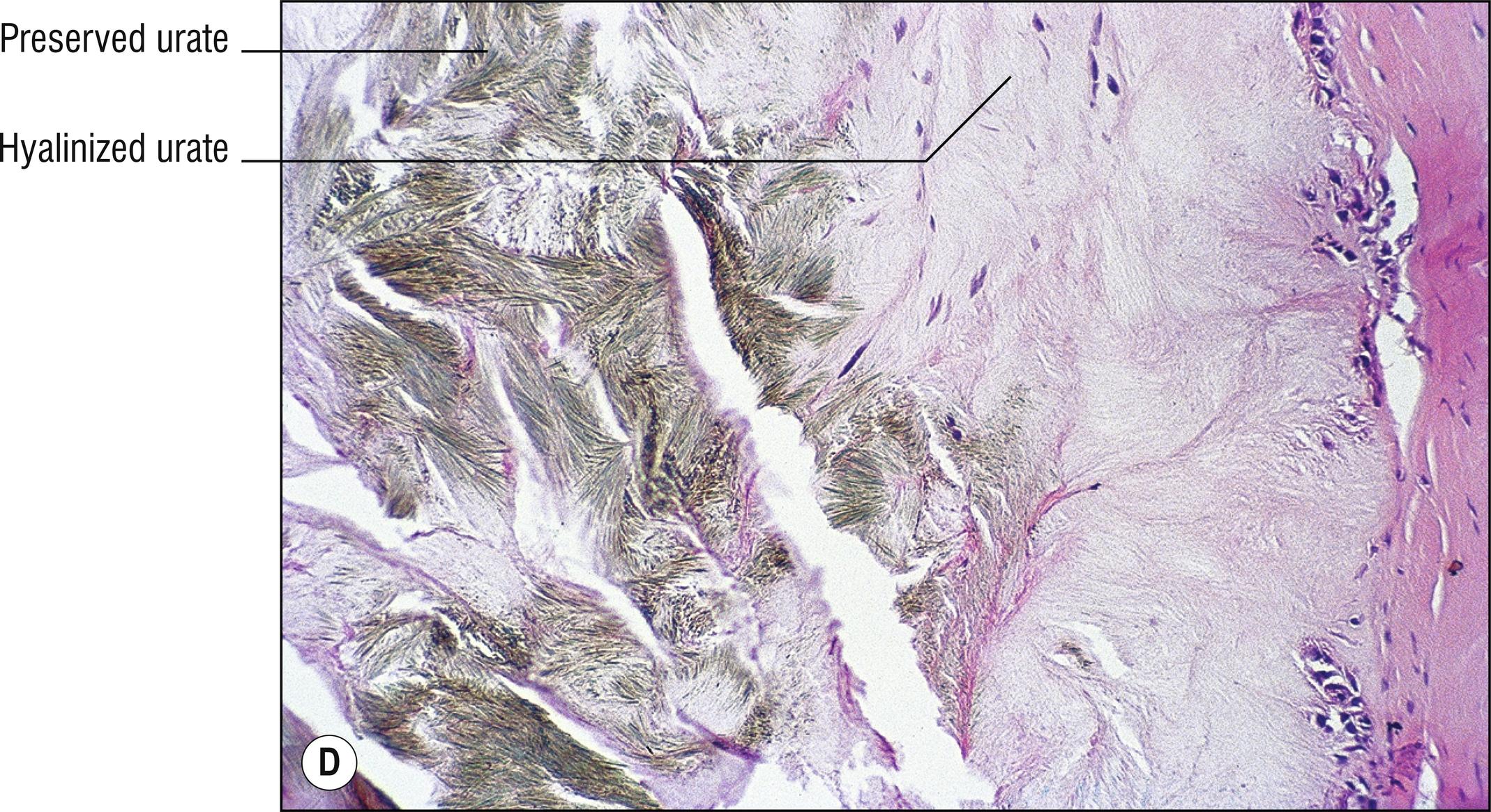
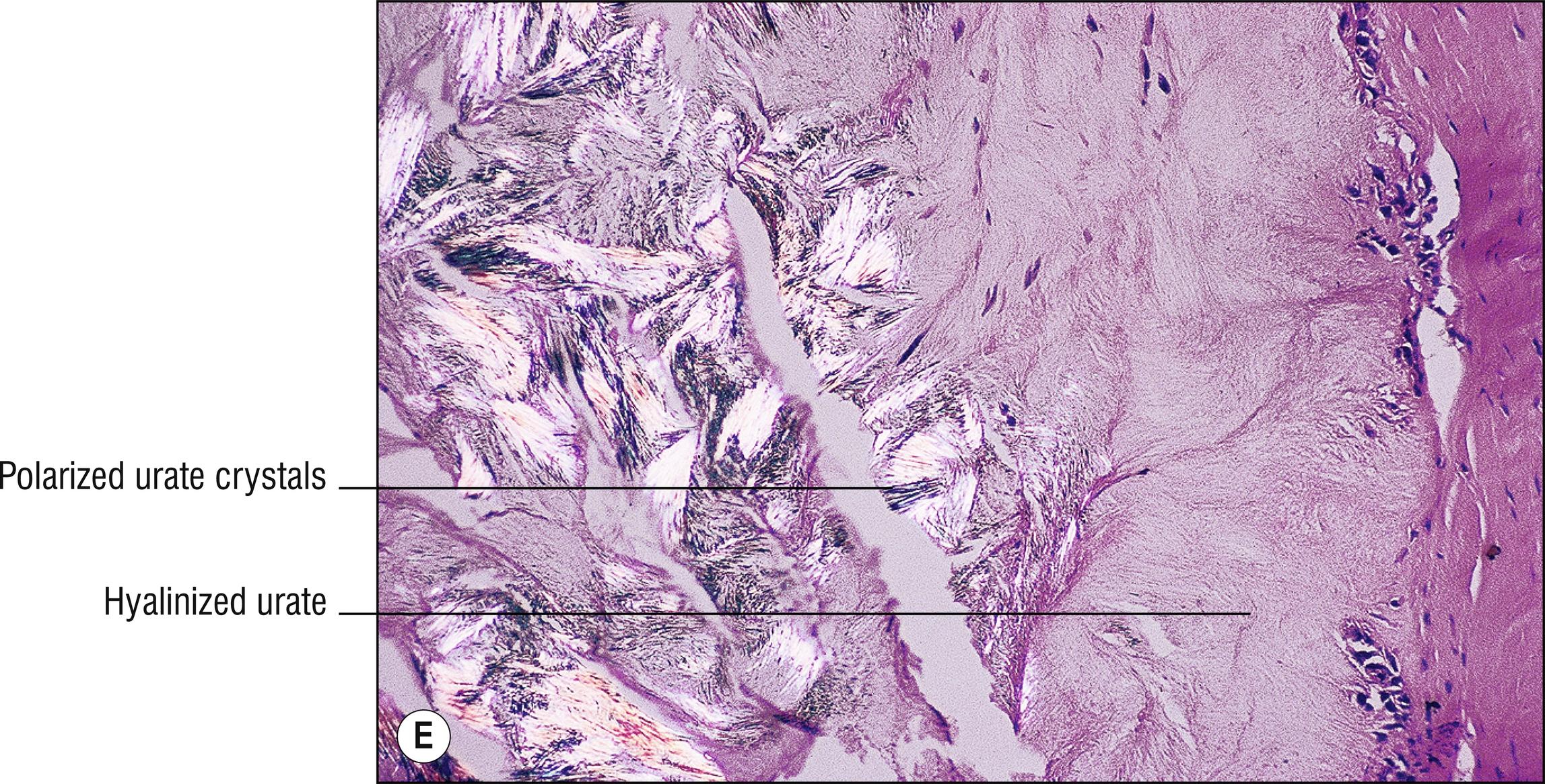
Common whitish – red nodules (1.150), especially on the digits and over joints , associated with uric acid accumulation and a metabolic defect. Patients often have arthritis (1.7).
Most of the other amorphous pink materials in Section 1.35 are not surrounded by a giant cell infiltrate, and do not show the subtle needle-shaped crystals.
Other juxta-articular nodules (1.92), especially calcinosis cutis (8.15) rheumatoid nodules (7.3).
Amorphous deposits of eosinophilic material in dermis and subcutaneous tissue with formalin-fixed tissue
Brownish (1.17), doubly refractive needle-shaped crystals in clefts (1.23) if alcohol fixed, or in the deeper aspects of incompletely fixed or processed tissue
Lymphocytes, histiocytes, and multinucleated giant cells around the deposits
Positive staining with von Kossa, but de Galantha is more specific for urates
Uncommon changes in the skin from hypothyroidism (1.138). Patients have skin that may look normal, but often is edematous (especially periorbital ), xerotic , or eczematous (1.29). Skin looks nearly normal with H rarely, the collagen bundles appear slightly separated by clear spaces or bluish deposits of mucin. Slightly increased acid mucopolysaccharide can sometimes be demonstrated between collagen bundles by special stains such as Alcian blue, colloidal iron, or toluidine blue.
Other diseases with increased mucin in the dermis (1.83), or a nearly normal appearance (1.94).
(see Fig. 8.7A,B )
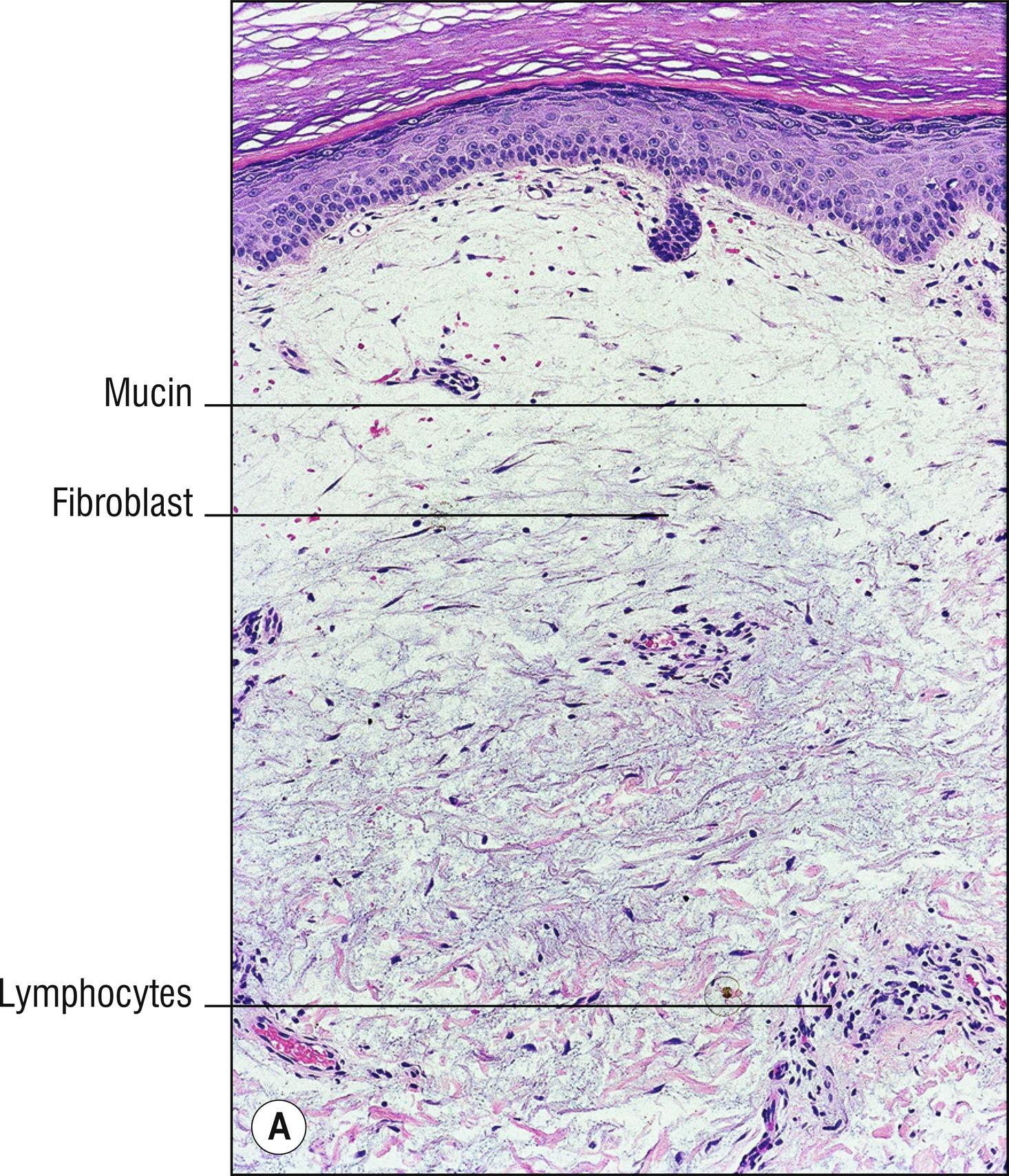
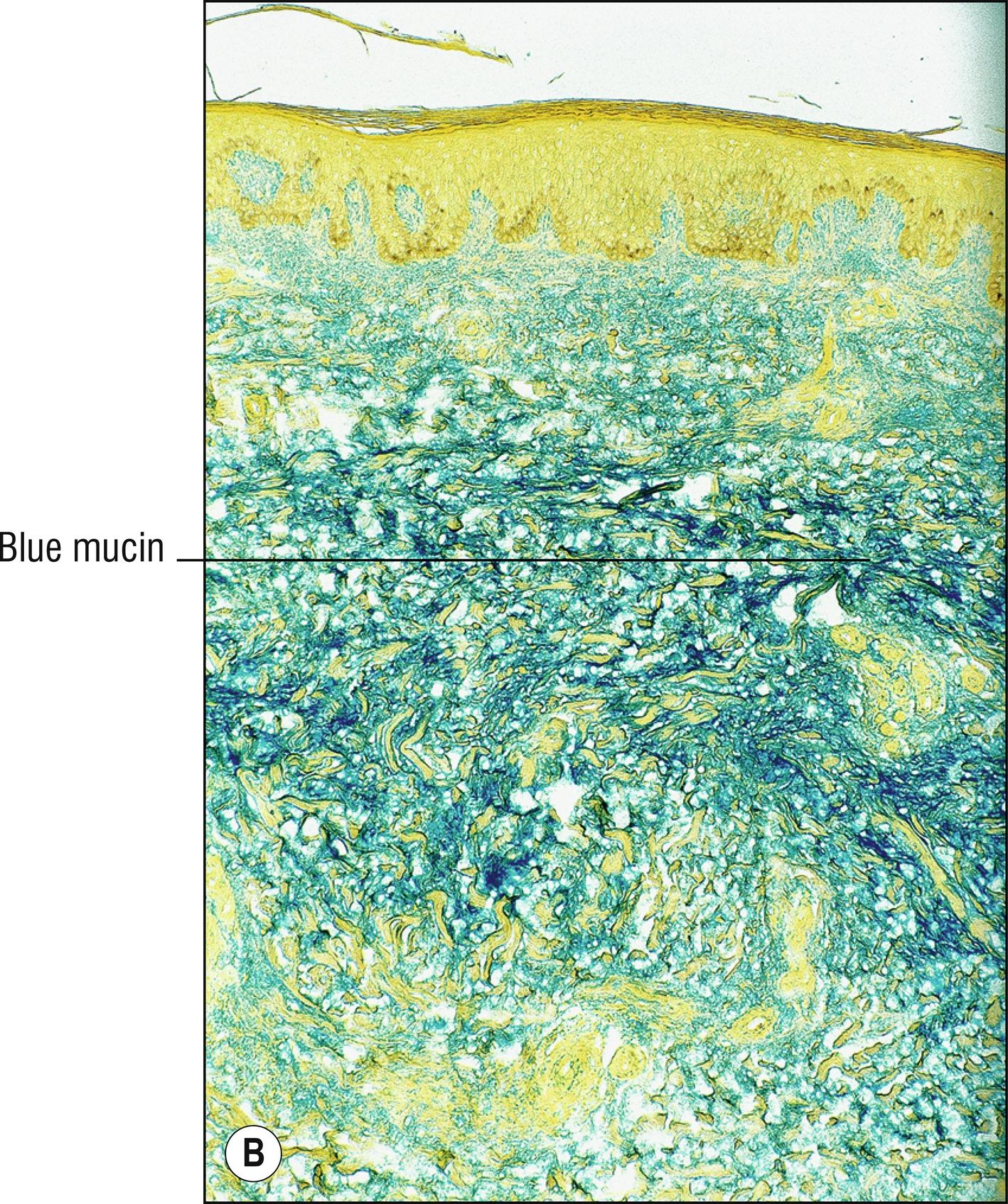
Uncommon edematous swelling (dermopathy), usually of lower legs (1.67), in patients with Grave’s disease (diffuse goiter, exophthalmos; 1.41). Patients often have LATS (long-acting thyroid stimulating) hormone and initially are often hyperthyroid (1.138), but at the time of development of pretibial myxedema they may have been treated and may be euthyroid or hypothyroid.
Large spaces between collagen bundles apparent with H&E
Abundant acid mucopolysaccharide between collagen bundles of the dermis can be stained with Alcian blue, colloidal iron, or toluidine blue
Normal or slightly increased numbers of fibroblasts
Sometimes increased mast cells (1.78)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here