Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dendritic cells (DCs), originally discovered in 1973 by Ralph M. Steinman and Zanvil A. Cohn and named after their distinct stellate morphology, are important regulators of immune responses. Specialized in antigen acquisition, processing, and presentation, the hallmark of DCs is their ability to induce naïve T cell activation and effector differentiation. DCs also interact with other immune cells, such as B cells, natural killer (NK) cells, innate lymphoid cells, and secrete immunomodulatory molecules, thereby mediating the induction and maintenance of immunity and tolerance. This chapter describes the current knowledge of DC ontogeny and function as well as the utility of DCs as immunotherapeutic agents in clinical applications.
DCs are highly heterogeneous and classified into subsets based on their developmental origin, anatomical location, function, expression profile of transcription factors, and phenotypic markers. DC development involves four stages, namely, hematopoietic precursors, DC precursors (pre-DC), immature DCs (imDCs), and mature DCs (mDCs). All DCs originate in the bone marrow and are continuously produced in a steady rate from CD34 + hematopoietic stem cells (HSCs) in a process primarily directed by Fms-like tyrosine kinase 3 ligand (Flt3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF). According to the classical model of hematopoiesis, HSCs give rise to common myeloid precursors (CMPs), excluding lymphoid lineage potential, and CMPs then develop into monocyte DC precursors (MDPs), excluding granulocyte potential. MDPs subsequently transition into common DC progenitors (CDPs). MDPs give rise to inflammatory DCs (iDCs), while CDPs give rise to pre-DCs that migrate out of bone marrow into lymphoid and non-lymphoid organs, where they differentiate into major DC subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). Notably, pDCs are also produced by the lymphoid progenitors in the steady state ( Fig. 20.1 ). Therefore, the progressive lineage specification that limits alternative fate potential, described by the classical hematopoiesis model, should not imply a solid state of cell fates.
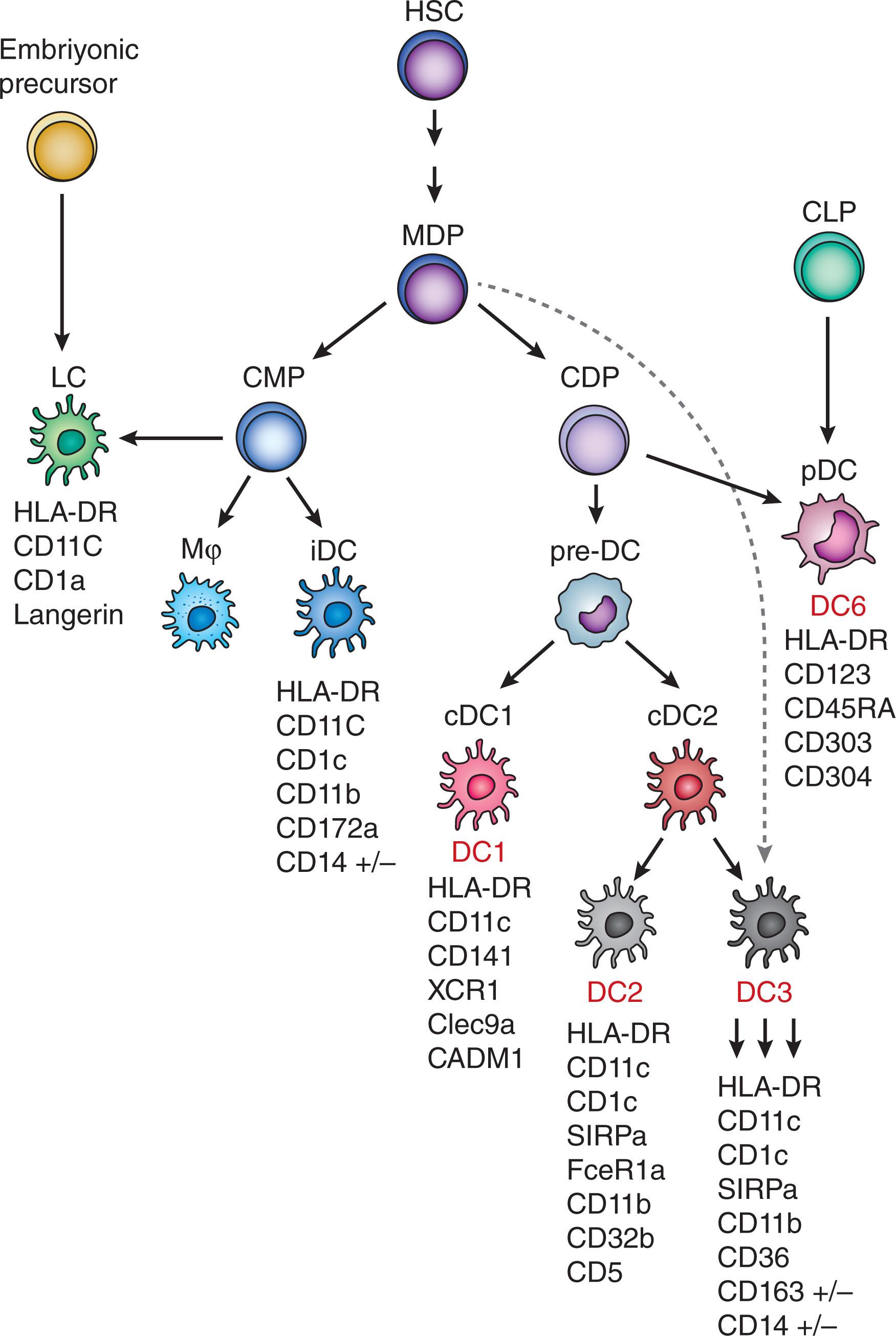
This section summarizes the transcriptional and phenotypical characteristics associated with distinct DC subsets while concentrating on human DCs with little reference to murine models. Readers are encouraged to seek additional information provided in other comprehensive reviews.
Broadly, DCs are defined as mononuclear immune cells that lack key lineage markers for T cells (CD3), B cells (CD19 and CD20), and NK cells (CD56). Additionally, DCs express high levels of major histocompatibility complex (MHC) class II molecules, such as HLA-DR. Expression of distinct surface markers and transcription factors are utilized to classically categorize DCs into four major terminally differentiated subsets: cDCs, pDCs, iDCs, and Langerhans cells (LCs).
cDCs comprise two major subsets, cDC1s and cDC2s. Both subsets express CD11c and MHC class II molecules. In humans, cDC1s are characterized by the expression of CD141 (BDCA-3), CLEC9A, XCR1 and CADM1, whereas cDC2s express CD1c (BDCA-1), SIRPα (CD172), and CD11b. In mice, cDC1s also express XCR1, CLEC9A, CADM1, as well as CD8a/CD103 and cDC2s express SIRPα, CD4, and CD11b. Although these markers define the cDC1 subset quite precisely, cDC2 markers are less specific and do not address the heterogeneity within the cDC2 subset. Recent studies by independent groups have identified that the classically defined cDC2 subset comprise multiple subpopulations, which are detailed in the “New Developments in DC Classification” section.
The development of cDC subsets is regulated by distinct transcriptional programs. cDC1s express interferon regulatory factor (IRF) 8, basic leucine zipper transcriptional factor ATF-like 3 (Batf3), and DNA-binding protein inhibitor 2 (ID2). cDC2s express IRF4 and Kruppel-like factor 4 (Klf4). Notch signaling also plays an essential role in the development and differentiation of cDCs. Evidence for the importance of Notch signaling in DC programming comes from in vitro cultures both in murine and human systems, in which DCs are generated in the presence of OP9 cells, a stromal cell line supporting the differentiation of HSCs. Specifically, in the context of human cells, expanding cord blood or peripheral blood CD34 + precursor cells with key factors for DC growth and differentiation, namely Flt3-L, SCF, IL-7, and thrombopoietin (TPO) and subsequently inducing their differentiation in the presence of OP9 feeder cells, generated primarily pDCs with small frequencies of cDCs. In the same system, when Notch ligand DLL1 expressing OP9-DLL1 cells were used as feeders, pDC generation was suppressed and the proportion of cDCs, particularly cDC1s, were greatly increased, which transcriptionally, phenotypically, and functionally resembled their primary blood cDC1 counterparts. Other studies in murine models have also demonstrated nonredundant roles for Notch signaling in cDC2 differentiation and function, particularly in T follicular helper (T FH ) cell differentiation and induction of B cell responses during infection.
Both cDC subsets are known for their unique efficacy for priming naïve T cells and inducing their functional polarization. Additionally, cDC1s, expressing an array of immunoregulatory molecules, are key for tolerogenic regulation. cDC1s are superior to cDC2s at antigen cross-presentation, a process through which exogenous antigens are acquired and presented on MHC class I molecules, thereby inducing CD8 + T cell responses. While cDC1s can also activate CD4 + T cells and polarize them toward a Th1 phenotype, cDC2s are the main subset specialized in MHC class II-mediated antigen presentation. cDC2s efficiently activate CD4 + T cells and induce their polarization toward Th1, Th2, and Th17 phenotypes in specific contexts. cDC2 is the most frequent DC population and found in blood, lymphoid, and non-lymphoid tissues, while cDC1s are thought to primarily reside in the lymph nodes (LNs) and found in trace numbers in the blood. Another distinct feature between cDC1 and cDC2 subsets is the differential expression of pattern recognition receptors (PPRs), which recognize conserved molecules displayed by pathogens or damaged cells. cDC1s express Toll-like receptors (TLRs) 3 and 8. Due to TLR3 triggering, cDC1s have the unique ability to produce type III interferons, which contribute to antiviral and antitumor immunity. cDC2s express a wide range of PRRs, TLRs 1–8, and produce IL-23, a critical cytokine for innate defense. Hence, cDC2s mediate antimicrobial responses against a greater variety of pathogens.
pDCs are distinguished by high levels of CD123, CD303 (BDCA-2), and CD304 (BDCA-4) and the lack of CD11c, AXL, Siglec-6 expression. Transcription factor Tcf4 specifically regulates the development of pDCs. pDCs can also be recognized by their distinct morphology resembling secretory plasma cells with a round shape and well-defined endoplasmic reticulum (ER). Although small fractions of pDCs may be found in blood, they primarily reside in lymphoid tissues. The hallmark of pDCs is the ability to produce large quantities of type I interferons, IFN-α/β, although they have been shown to produce type III interferon, IFN-λ, as well. pDCs produce interferons in response to recognition of viral RNA or DNA through TLR7 and TLR9, respectively, and exert potent antiviral effects. The activation of TLR7/9 may also lead to the production of tumor-necrosis factor-α (TNF-α) and IL-6 by pDCs, which contributes to pDC maturation, antibody production by B cells, and generation of tolerogenic T cells.
pDCs lack the capacity for antigen presentation and T cell stimulation in an immature state, suggesting they are less important for the activation of naïve T cells. However, recent studies have shown that upon activation, a subset of pDCs differentiates into a more cDC-like state by adopting a dendritic morphology, upregulating HLA-DR, CD80, and CD11c and downregulating TCF4. This activated pDC subpopulation has antigen-presenting capacity and can prime T cells, highlighting the high degree of DC heterogeneity and plasticity.
iDCs appear under inflammatory conditions, accumulate at the inflammation site, and disappear upon resolution of inflammation. They are characterized by the expression of CD11c, CD11b, CD172a, CD14, and CD1c. iDCs also express the Zbtb46 transcription factor, which is specific to DC lineage. Functionally, iDCs can process and present antigens to T cells and induce T call activation and polarization. They can also produce inflammatory cytokines, including IL-6, IL-1β, and IL-23. Based on their gene signature similarities, iDCs are proposed to be in vivo equivalents of monocyte-derived DCs (moDCs), which are differentiated in in vitro culture systems most commonly with the addition of GM-CSF and IL-4 ( Fig. 20.2 ). However, the role of GM-CSF for the generation of iDCs in vivo is not fully understood. In mice, while GM-CSF or its receptor were dispensable, M-CSF receptor was shown to be essential for iDC differentiation and function.
LCs are tissue-resident cells found in the epidermal layer of the skin. LCs share common precursors with macrophages and are classified in the same lineage as macrophages. Moreover, like other tissue-resident macrophages, LCs can proliferate in differentiated states. Importantly, LCs also have DC-like capabilities, such as acquiring antigens, migrating to LNs, and activating T cells. LCs express CD1a and Langerin (CD207). They also contain Birbeck granules in their cytoplasm, which distinguishes them from the other Langerin expressing dermal DCs. Ontologically, LCs arise from yolk sac and fetal liver progenitors. These progenitors seed the skin during embryogenesis and differentiate into LCs in a transforming growth factor-β (TGF-β) and M-CSF receptor-dependent process. Once established, LCs are maintained locally by self-renewal under steady state conditions. However, LCs also have a hematopoietic origin as circulating hematopoietic precursor cells have been shown to replenish the LC population during skin inflammation.
Serving as an immunological barrier to the external environment, LCs are efficient at recognizing foreign antigens since they express a variety of PRRs including Langerin and a large repertoire of TLRs. Upon recognition of foreign antigens, LCs upregulate MHC class II and other co-stimulatory molecules and migrate to LNs, where they prime CD4 + T cells and induce their polarization into Th1, Th2, Th17, and Th22 phenotypes. LCs can also prime CD8 + T cells under certain circumstances. LCs also play a role in establishing immune tolerance, for example by presenting self-antigens to T cells in the LNs in homeostatic conditions, as well as by inducing regulatory T cells in the skin that suppress allergic skin reactions.
The understanding of DC developmental pathways and subsets is continuously being improved due to advances in high-dimensional, single-cell technologies by more sensitively defining the identities of this highly heterogenous population. Utilizing blood cells isolated from healthy donors, which were enriched for HLA-DR expression and lack of lineage markers (CD3, CD56, CD19, CD14), Villani et al., performed single-cell RNA sequencing (scRNAseq) and unbiased clustering analysis to identify and compare DC populations. They identified six distinct DC subpopulations (DC1-6). Gene signatures of most of these clusters aligned well with traditionally defined subsets. DC1, DC6, and DC4 clusters mapped to cDC1, pDC, and CD16 + monocytes, respectively. Two distinct clusters, DC2 and DC3, mapped to cDC2 subset, suggesting a new subdivision within the classical cDC2 population. Additionally, a previously unknown subset was identified. Despite expressing markers in common, cells in this cluster (DC5) differed from pDCs by their morphological resemblance to cDCs and their potent capacity to activate T cells. This novel subset was defined by the expression of AXL and SIGLEC6, hence named “AS DCs,” and corresponded to 2% to 3% of blood DCs. Corroborating these results, Alcantara-Hernandez et al., unbiasedly evaluated the phenotypic heterogeneity of human blood and tissue DCs by utilizing mass spectrometry (CyTOF) and identified a cluster corresponding to AS DCs and subdivisions within the cDC2 population. Such subdivisions within the cDC2s were further delineated by Dutertre et al., demonstrating that cDC2s comprise a CD5 + and three CD5 − subpopulations (CD163 − , CD163 + CD14 − , and CD163 + CD14 + cells), with CD5 + and CD5 − cells resembling DC2 and DC3s, respectively, as described by Villani et al. Another recent scRNA-seq study, by Bourdely et al., also identified the DC3 subset in blood and defined DC3s as a separate subset bearing monocyte markers (e.g., CD163, CD14) and features of cDC2s. Further supporting the distinction of the DC3 subset, they identified a DC3-restricted progenitor population. Independently, Cytlak et al. found that the heterogeneity of DC2 and DC3 subsets was associated with distinct developmental pathways dependent on differential IRF8 requirements.
Under steady state conditions DCs commonly exist in an immature form. Upon detecting tissue disturbances and danger signals through their PRRs, DCs undergo phenotypical and functional changes, enhancing their capacity to stimulate T cells. This process is called DC “maturation” or “activation.”
DC maturation can be induced by a variety of stimuli such as microbial products, danger signals released by damaged cells, cytokines, and cellular crosstalk (e.g., ligation of CD40). To sense such diverse stimuli, DCs express a wide array of PRRs, localized both at the cell membrane and the cytoplasm. PRRs recognize pathogen-associated molecular patterns (PAMPs), derived from microorganisms, or danger-associated molecular patterns (DAMPs), secreted by damaged cells. Membrane-bound PRRs include TLRs and C-type lectin receptors (CLRs). Cytoplasmic PRRs include NOD-like receptors (NLRs) and retinoic acid (RA)-inducible gene (RIG)-I-like receptors (RLRs). Other receptors such as scavenger receptors (SRs) and integrins can also serve as PRRs. Another major group of PRRs is secreted proteins, including complement receptors and collectins. PRRs are further classified into subgroups based on their ligand specificity and function. Table 20.1 summarizes the properties of PRRs.
| PRRs | Localization | Natural Ligand | Source | |
|---|---|---|---|---|
| TLRs | TLR2/TLR1 | Plasma membrane | Triacyl lipopeptides | Bacteria |
| TLR2/TLR6 | Plasma membrane | Diacyl lipopeptides | Bacteria, viruses, fungi, parasites | |
| TLR3 | Endolysosome | dsRNA | Viruses | |
| TLR4 | Plasma membrane | LPS, HSPs | Bacteria, viruses, self | |
| TLR5 | Plasma membrane | Flagellin | Bacteria | |
| TLR7 | Endolysosome | ssRNA | Viruses | |
| TLR8 | Endolysosome | ssRNA | Viruses | |
| TLR9 | Endolysosome | CpG ODNs | Bacteria, viruses | |
| TLR10 | Endolysosome | Unknown | Unknown | |
| CLRs | MMR | Plasma membrane | Mannose | Bacteria, fungi, parasites |
| DEC-205 | Plasma membrane | CpG ODNs, Keratins | Bacteria, viruses, self | |
| Dectin-1 | Plasma membrane | β-Glucan | Fungi | |
| DC-SIGN | Plasma membrane | Mannose, fucose | Bacteria, viruses | |
| CLEC9A | Plasma membrane | F-actin | Self | |
| NLRs | NOD1 | Cytoplasm | iE-DAP | Bacteria |
| NOD2 | Cytoplasm | MDP | Bacteria | |
| NLRPs | Cytoplasm | LT, particulate matters | Bacteria, viruses, self | |
| ALRs | AIM2 | Cytoplasm | dsDNA | Bacteria, viruses, self |
| IFI16 | Cytoplasm | dsDNA | Bacteria, viruses, self | |
| RLRs | RIG-I | Cytoplasm | Short dsRNA | Viruses |
| MDA5 | Cytoplasm | Long dsRNA | Viruses | |
| LGP2 | Cytoplasm | dsRNA | Viruses | |
| STING | ER | cyclic dinucleotides | Bacteria, viruses | |
a A list of some of the PRRs expressed by dendritic cells (DCs), their cellular localization at steady state, and their natural ligands.
imDCs continuously sample their surroundings and capture antigens via endocytosis, a process by which cells internalize extracellular substances. DCs utilize multiple endocytic mechanisms to acquire external antigens: phagocytosis (ingestion of large particles, e.g., pathogens, apoptotic cells), micropinocytosis (engulfing extracellular liquid and dissolved molecules), and receptor-mediated endocytosis. Upon engagement of PRRs by inflammatory stimuli and induction of maturation, DCs downregulate endocytosis, restricting antigen capture after the initial stimulation. Concomitantly, DCs upregulate cell surface expression of MHC class II molecules and co-stimulatory molecules (e.g., CD80, CD86), which are facilitated by DC-LAMP and CD83, two key markers that distinguish mDCs. Further augmenting the efficacy of antigen presentation to T cells, DC maturation increases the half-life of MHC molecules and enhances lysosomal acidification and antigen loading onto MHC-II. Mature DCs also upregulate CCR7, a homing receptor, and migrate to LNs in response to chemokines such as CCL-19 and CCL-21. In the LNs, mDCs present antigens to T cells, both non-self and self, and induce T cell polarization into cytotoxic or tolerogenic states, respectively. The immunogenic or tolerogenic function of DCs also depends on the type of stimuli that induce their maturation. DCs may produce pro-inflammatory, for example, IL-12, IL-6, IL-1β, or anti-inflammatory, for example, IL-10, TGF-β, cytokines in response to different stimuli, which impacts T cell polarization. Tolerogenic DCs may induce the formation of regulatory T cells (Tregs), suppressing immune responses. Therefore, DCs are key mediators of both immunity and tolerance.
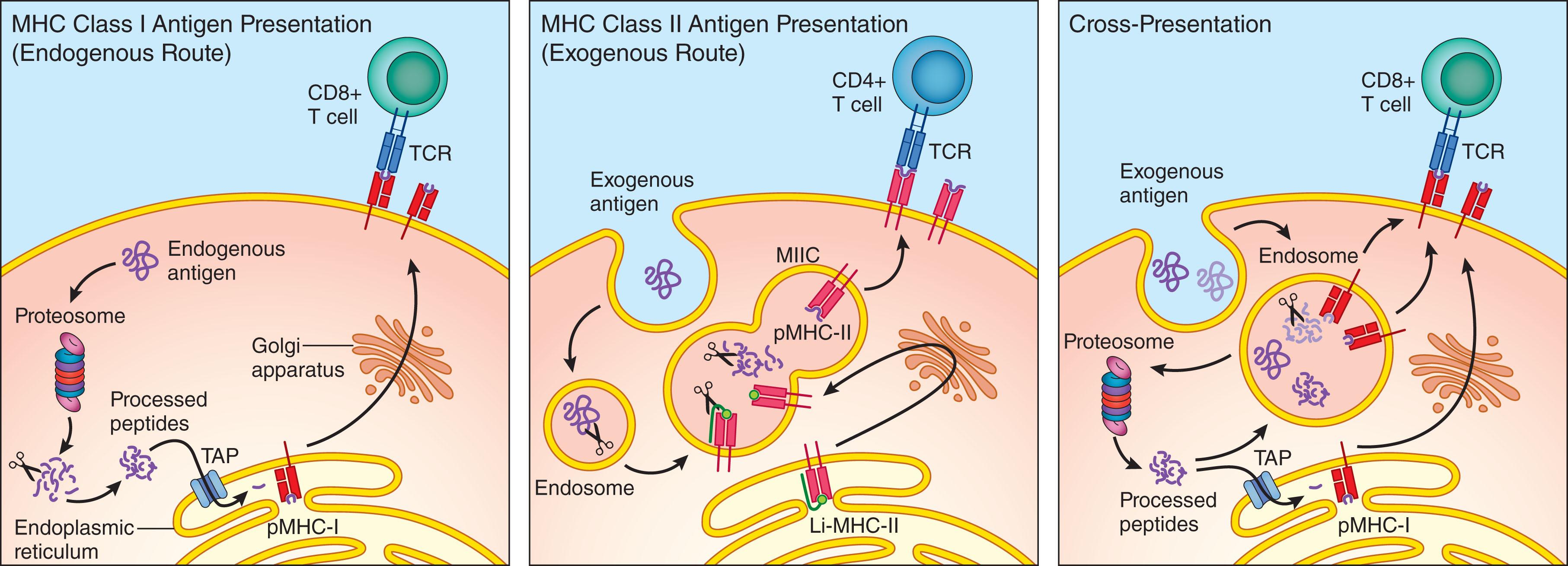
DCs are the most efficient antigen-presenting cell (APC) population in vivo. Through specialized mechanisms, DCs capture both exogenous and endogenous antigens, process these antigens, and present them in the context of class I and class II MHC molecules to CD8 + and CD4 + T cells, respectively ( Fig. 20.3 ). DCs also present lipid antigens to T cells by CD1 molecules.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here