Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malnutrition causes a wide spectrum of neurological disorders ( Table 85.1 ) . Despite socioeconomic advances, nutritional deficiency diseases such as kwashiorkor and marasmus are still endemic in many underdeveloped countries. The problem in Western countries is usually the result of dietary insufficiency from chronic alcoholism or malabsorption due to gastrointestinal (GI) diseases. Bariatric surgery has become an important risk factor of malabsorption due to its increased use in the treatment of obesity.
| Neurological Manifestations | Associated Nutritional Deficiencies |
|---|---|
| Dementia, encephalopathy | Vitamin B 12 , nicotinic acid, thiamine, folate |
| Seizures | Pyridoxine |
| Myelopathy | Vitamin B 12 , vitamin E, folate, copper |
| Myopathy | Vitamin D, vitamin E |
| Peripheral neuropathy | Thiamine, vitamin B 12 , vitamin E, and many others |
| Optic neuropathy | Thiamine, vitamin B 12 , and many others |
Individual vitamin requirements are influenced by many factors. The daily need for thiamine and nicotinic acid, important compounds in energy metabolism, increases proportionally with increasing caloric intake and energy need. For example, symptoms of thiamine deficiency may occur in at-risk patients during periods of vigorous exercise and high carbohydrate intake. Other factors such as growth, infection, and pregnancy may also worsen deficiency states.
The terms vitamin B 12 and cobalamin are used interchangeably in the literature. Cobalamins are abundant in meat, fish, dairy, and other animal byproducts. Vegetables generally contain only trace amounts of cobalamin ( ). Although only 1 μg/day of cobalamin is needed, strict vegetarians are at risk and may rarely develop clinically significant deficiency. Intestinal absorption of cobalamin requires the presence of intrinsic factor, a binding protein secreted by gastric parietal cells. Cobalamin binds to intrinsic factor, and the complex is transported to the ileum where it is absorbed into the circulation. A small amount of free cobalamin, about 1%–5%, is also absorbed through the entire intestine without intrinsic factor. Once absorbed, cobalamin binds to a transport protein, transcobalamin, for delivery to tissues. As much as 90% of total body cobalamin is stored in the liver. Even when vitamin absorption is severely impaired, many years are needed to deplete the body store. A clinical relapse in pernicious anemia after interrupting cobalamin therapy takes an average of 5 years to be recognized.
Two biochemical reactions depend on cobalamin. One involves methylmalonic acid as precursor in the conversion of methylmalonyl coenzyme A (methylmalonyl-CoA) to succinyl-CoA. The importance of this to the nervous system is unclear. The other is a folate-dependent reaction in which the methyl group of methyltetrahydrofolate is transferred to homocysteine to yield methionine and tetrahydrofolate. The reaction depends on the enzyme methionine synthase, which uses cobalamin as a cofactor. Methionine is converted to S -adenosylmethionine (SAM), which is used for methylation reactions in the nervous system.
The classic disease pernicious anemia is caused by defective intrinsic factor production by parietal cells, leading to malabsorption. These patients may have demonstrable circulating antibodies to parietal cells or lymphocytic infiltrations of the gastric mucosa, suggesting an underlying autoimmune disorder. A more common cause of malabsorption is food-cobalamin malabsorption ( ). Under some clinical settings, the normal digestive process fails to release cobalamin from food or intestinal transport protein. Cobalamin remains bound and cannot be absorbed even in the presence of available intrinsic factors. Predisposing factors include atrophic gastritis and hypochlorhydria, and malabsorption may be seen with Helicobacter pylori infection, gastrectomy or other gastric surgeries, intestinal bacterial overgrowth, and prolonged use of H 2 antagonists, proton pump inhibitors, or biguanides (e.g., metformin). Patients with human immunodeficiency virus (HIV) are often observed to have a low serum cobalamin level, usually with normal homocysteine and methylmalonic acid. The significance of this association is unknown.
Nitrous oxide, a commonly used anesthetic gas, may cause a clinical syndrome of myeloneuropathy indistinguishable from that of cobalamin deficiency. It interferes with the cobalamin-dependent conversion of homocysteine to methionine. Prolonged exposure is necessary to produce neurological symptoms in normal individuals and is primarily seen in individuals who abuse the gas for its euphoric properties ( ). By contrast, patients who are already deficient in cobalamin may experience neurological deficits after only brief exposures during routine general anesthesia with nitrous oxide. Symptoms appear subacutely after surgery and resolve quickly with cobalamin treatment ( ).
The onset of symptoms is insidious, with paresthesias in the hands or feet experienced by most patients. Weakness and unsteadiness of gait are the next most frequent complaints. Lhermitte sign may be present. Mental slowing, depression, confusion, delusions, and hallucinations are common, and occasionally patients present with only cognitive or psychiatric symptoms.
On examination, signs of both peripheral nerve and spinal cord involvement may be present, although either can be affected first or disproportionately. Loss of vibration or joint position sense in the legs is common. If impaired position sense is severe, a Romberg sign may be present. Motor impairment, if present, results from pyramidal tract dysfunction and is most severe in the legs, ranging from mild clumsiness and hyperreflexia to spastic paraplegia and extensor plantar responses. Tendon reflexes are variably affected depending on the degree of pyramidal and peripheral nerve involvement. Visual impairment is occasionally present and may antedate other manifestations of vitamin deficiency. Ophthalmological examination may reveal bilateral visual loss, optic atrophy, and centrocecal scotomata. Brainstem or cerebellar signs, chorea, autonomic insufficiency, or even reversible coma may rarely occur.
Serum assays of vitamin B 12 and cobalamin-dependent metabolites provide direct measures of cobalamin homeostasis, although there are important limitations. Blood cobalamins are bound to two transport proteins, transcobalamin and haptocorrin. The cobalamin bound to transcobalamin, known as holotranscobalamin , is the fraction that is available to tissues, although it accounts for only 10%–30% of the serum level measured by standard laboratory methods. Serum levels are influenced by conditions that affect the concentrations of these transport proteins. Myeloproliferative and hepatic disorders may raise the concentration of haptocorrin and cause a falsely normal serum level. A misleadingly high serum level also may result from the presence of an abnormal cobalamin-binding protein. In contrast, pregnancy and contraceptives may give falsely low measurements in the absence of deficiency. Folate deficiency also causes a falsely low cobalamin serum level that corrects after folate replacement. These confounding factors diminish the sensitivity and specificity of the commonly used assay of total serum cobalamin in the diagnosis of deficiency state. Although measurement of holotranscobalamin is better in theory, available data suggest that its diagnostic sensitivity is approximately equivalent or only modestly better than that of total serum cobalamin, and its specificity is uncertain ( ).
Homocysteine and methylmalonic acid are precursors of cobalamin-dependent biochemical reactions. The concentrations of these metabolites increase during cobalamin deficiency. Measurement of these metabolites is especially useful when the serum cobalamin concentration is in the low range of normal, between 200 and 350 pg/mL, and in patients with suspected nitrous oxide abuse who may have normal serum cobalamin levels. Homocysteine level should be measured either at fasting or after an oral methionine load. The blood sample should be refrigerated immediately after collection because the level increases if whole blood is left at room temperature for several hours. Elevated levels of homocysteine and methylmalonic acid are not specific for cobalamin deficiency, as there are many other causes of increase in these metabolites ( Box 85.1 ).
In patients with autoimmune gastritis and intrinsic factor deficiency, antibodies against parietal cell and intrinsic factor may be elevated. Anti-parietal cell antibodies are nonspecific and are present in other autoimmune endocrinopathies as well as occasional normal individuals. Anti-intrinsic factor antibodies are less sensitive (50%–70%) but are specific for pernicious anemia. Elevated serum gastrin level is a marker of atrophic gastritis and hypochlorhydria and is a sensitive (up to 90%) but nonspecific indicator of pernicious anemia.
The classic hematological manifestation of pernicious anemia is a macrocytic anemia. Erythrocyte or bone marrow macrocytosis or hypersegmentation of polymorphonuclear cells may be present without anemia. Hematological abnormalities may be absent at the time of neurological presentation and are thus insufficiently sensitive for use in diagnosis.
Because most patients present with clinical features suggesting a myelopathy or encephalopathy, imaging studies are necessary to exclude structural causes. Results of magnetic resonance imaging (MRI) may be normal, or T2-signal abnormalities may be seen in the lateral or posterior columns in patients with subacute combined degeneration ( ) ( Fig. 85.1 ). Both gadolinium enhancement and spinal cord swelling have been described. Patients with encephalopathy or dementia often have multiple foci of T2 signal abnormalities in the deep white matter that may become confluent with disease progression. Diffusion tensor imaging (DTI) may be more sensitive in revealing brain changes that correlate with cognitive dysfunction ( ). Nonspecific abnormalities of electroencephalography, as well as visual and somatosensory evoked responses, are present in many patients with neurological abnormalities. Nerve conduction studies show small or absent rural nerve sensory potentials in approximately half of patients, providing evidence for an axonal polyneuropathy.
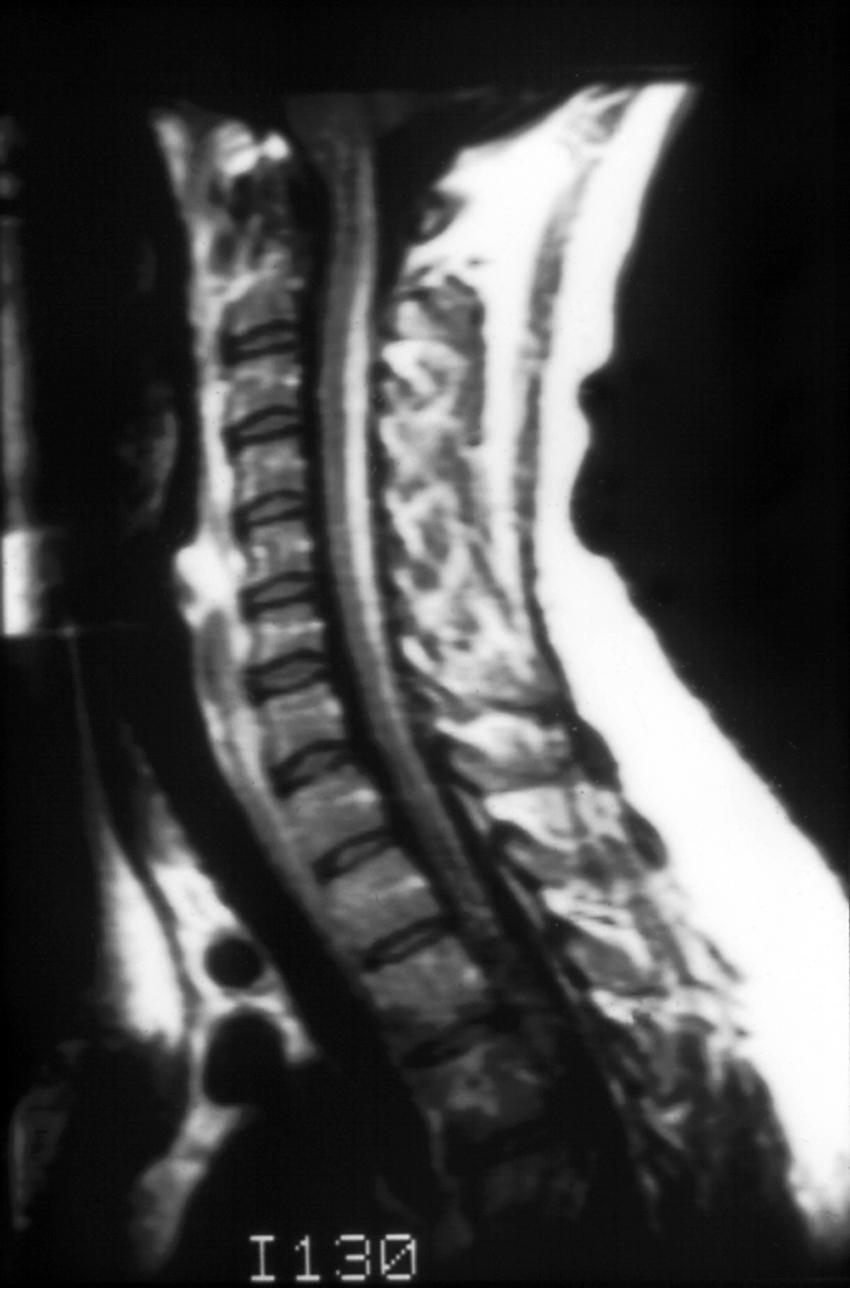
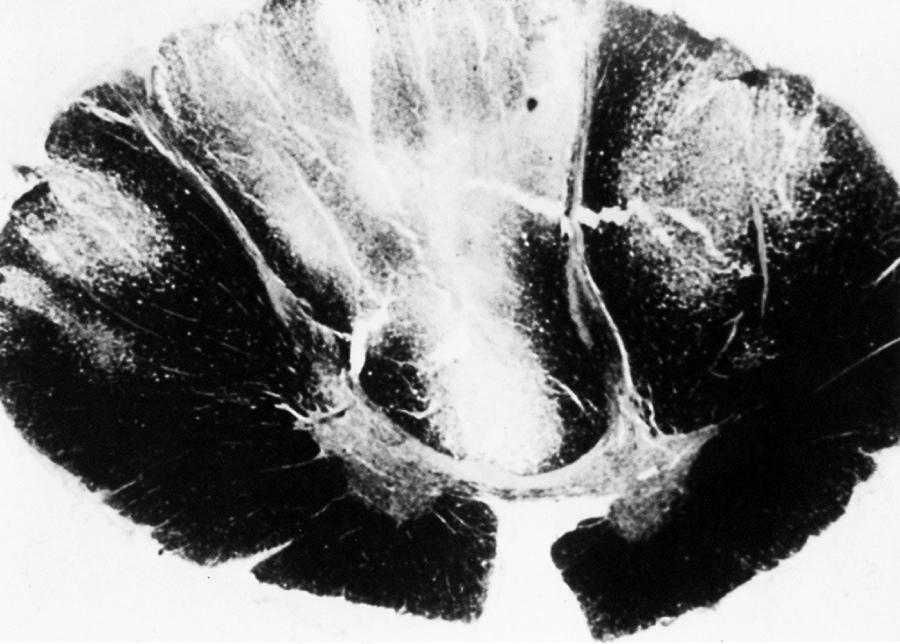
The term subacute combined degeneration of the spinal cord describes the pathological process seen in this disorder. Microscopically, spongiform changes and foci of myelin and axon destruction are seen in the white matter of the spinal cord. The most severely affected regions are the posterior columns at the cervical and upper thoracic levels ( Fig. 85.2 ). Pathological changes also are seen commonly in the lateral columns, whereas the anterior columns are involved in only a small number of the advanced cases. The pathological findings of the peripheral nervous system are those of axonal degeneration, but in some cases there is evidence of demyelination. Involvement of the optic nerve and cerebral white matter also occurs.
Recommendations for treatment of cobalamin deficiency vary widely. A typical regimen uses intramuscular daily injections of 1000 μg for the first week, followed by weekly 1000 μg injections for 1 month, and monthly injections thereafter. These parenteral doses provide quantities considerably higher than the body requirement. There is no evidence that overdosing can speed neurological recovery, but high doses of cobalamin appear to be safe. Oral supplementation at 1000 μg daily has also been used with some success, even in patients with suspected malabsorption, although close monitoring is necessary to ensure adequacy of treatment.
With proper treatment, serum levels of homocysteine and methylmalonic acid return to normal in about 2 weeks. Neurological improvement is more delayed and may be incomplete. Most of the symptomatic improvement occurs during the first 6–12 months of therapy. The need for early diagnosis and treatment is underscored by the observation that remission correlates inversely with the time lapse between onset of symptoms and initiation of therapy.
Folate deficiency may produce the same neurological deficits as those seen in cobalamin deficiency because of its central role in the biosynthesis of methionine, SAM, and tetrahydrofolate (see the previous section Cobalamin [Vitamin B 12 ]). Overt neurological manifestations are rare in folate deficiency, probably owing to alternative cellular mechanisms that are available to preserve SAM levels in times of folate scarcity.
Absorption of folate occurs in the jejunum and to a lesser extent the ileum. Chronic alcoholism is an important cause of folate deficiency. Folate deficiency also may complicate small-bowel disease (e.g., sprue, Crohn disease, ulcerative colitis). Other populations at risk are pregnant women and patients receiving anticonvulsant drugs that interfere with folate metabolism. Sulfasalazine, methotrexate, triamterene, and oral contraceptives also can cause folate deficiency. Intrathecal methotrexate, in particular, causes a leukoencephalopathy associated with marked elevation of homocysteine levels in the cerebrospinal fluid (CSF).
The majority of patients with laboratory evidence of folate deficiency do not have overt neurological findings. The classic syndrome of folate deficiency is similar to subacute combined degeneration seen in cobalamin deficiency. Presenting symptoms are limb paresthesias, weakness, and gait unsteadiness. These patients have megaloblastic anemia, impaired position and vibration sense, pyramidal signs, and possibly dementia. Chronic folate deficiency may result in mild cognitive impairment or increased stroke risk in adults. Although low folate level is present in many elderly asymptomatic people, the prevalence seems to be higher in the psychiatric and Alzheimer disease populations. Moreover, a low folate level appears to correlate with depression and cognitive impairment. Even in healthy older adults, a low folate level is associated with subtle deficits in neuropsychological test performance. Chronic folate deficiency during pregnancy leads to an increased frequency of neural tube defects in babies.
Serum homocysteine is an important surrogate marker for folate metabolism, although there are other causes of elevated homocysteine levels (see Box 85.1 ). Hyperhomocysteinemia is a risk factor for vascular diseases and venous thrombosis. For cerebrovascular disease, the association is strongest for multi-infarct dementia and white-matter microangiopathy. Even a modestly increased serum level in the range of 15–20 mmol/L engenders a recognizable increase in vascular risk. A meta-analysis of randomized control trials suggests a modest 10% reduction in stroke and 4% reduction in cardiovascular risk with long-term folate supplementation (0.5–15 mg/day, mean duration 3.2 years) ( ).
Clinical observations in two inborn errors of metabolism reinforce our understanding of the role of homocysteine in neurological diseases. Hereditary deficiency of cystathionine β-synthase leads to hyperhomocysteinemia and hyperhomocysteinuria. The homozygous form presents with markedly elevated homocysteine levels, mental retardation, premature atherosclerosis, and seizures. Heterozygous individuals have milder elevations of homocysteine and also have increased risk of vascular disease. A much more common condition is a C-to-T substitution at codon 677 in the gene coding for N5, N10-methylenetetrahydrofolate reductase (MTHFR). Some 5%–10% of the White population are homozygotes for this C677T mutation. These individuals have mildly elevated homocysteine levels and increased risk of vascular disease.
Plasma and erythrocyte folate levels may be measured directly. Erythrocyte level is generally more reliable than plasma level because it is less affected by short-term fluctuation in intake. Serum homocysteine is increased in folate deficiency. Its measurement is discussed in Laboratory Studies under the previous section, Cobalamin (Vitamin B 12 ).
In patients with documented folate deficiency, the initial dose is usually 1 mg of folate several times per day, followed by a maintenance dose of 1 mg/day. For acutely ill patients, parenteral doses of 1–5 mg may be given. Even with oral doses as high as 15 mg/day, there is no report of toxicity. In women of childbearing potential with epilepsy, daily folate supplementation of 0.4 mg or more is recommended as prophylaxis against neural tube defects. Since 1998, in an attempt to lower the incidence of neural tube defects, the US Food and Drug Administration (FDA) has mandated fortification of grain products with folate. The fortification translates to an increased daily intake of 0.1–0.2 mg in a typical adult.
Vitamin E refers to a group of tocopherols and tocotrienols, of which α-tocopherol is the most important. It is a free-radical scavenger and an antioxidant and has attracted attention for its potential in the prevention and treatment of a wide range of diseases. Unfortunately, the value of vitamin E for these indications has yet to be proven. We limit discussion here to the neurological manifestations of vitamin E deficiency.
Like other fat-soluble compounds, vitamin E depends on the presence of pancreatic esterases and bile salts for its solubilization and absorption in the intestinal lumen. Neurological symptoms of deficiency occur most commonly in patients with fat malabsorption ( Box 85.2 ). A reduced bile salt pool may be caused either by reduced hepatic excretion, as in congenital cholestasis, or by interruption of the enterohepatic reabsorption of bile, as in patients with extensive small-bowel resection. Pancreatic insufficiency contributes to malabsorption. Another setting is cystic fibrosis.
Gastrointestinal diseases
Biliary atresia, chronic cholestasis
Intestinal resection
Crohn disease
Pancreatic insufficiency (e.g., cystic fibrosis)
Blind loop syndrome and bacterial overgrowth
Bowel irradiation
Celiac disease
Other causes of steatorrhea
Hereditary diseases: abetalipoproteinemia, hypobetalipoproteinemia, Anderson disease, α-tocopherol transfer protein mutation
A number of rare familial disorders lead to chronic diarrhea, abnormal blood lipid profile, and malabsorption of fat and fat-soluble vitamins. In addition to vitamin E deficiency, these patients also have deficiency of vitamins A and D. Abetalipoproteinemia or Bassen-Kornzweig syndrome is an autosomal recessive disorder due to mutation in the microsomal triglyceride transfer protein (MTP) gene. This results in impaired absorption of fat and fat-soluble vitamins ( ). In addition to a neurological syndrome similar to that seen in other vitamin E-deficient states, spiky red blood cells (acanthocytes) and retinal pigment changes are characteristic. Two other disorders are also characterized by chronic fat malabsorption and vitamin E deficiency. SAR1B gene mutation leads to chylomicron retention disease or Anderson disease. Familial hypobetalipoproteinemia presents with variable degrees of malabsorption and symptoms, and about 50% are due to mutation in the APOB gene ( ).
Another rare syndrome of ataxia with isolated vitamin E deficiency (AVED) occurs in patients without GI disease or generalized fat malabsorption. Mutations in the α-tocopherol transfer protein gene (TTPA) on chromosome 8q are responsible ( ; ). This condition is inherited in an autosomal recessive manner. The defect appears to be impaired incorporation of the vitamin into hepatic lipoproteins that are necessary for delivery to tissues.
Clinical symptoms typically do not begin until many years of malabsorption deplete the vitamin reserves. This takes 15–20 years in adults, but clinical onset as early as age 1–2 years may occur in children because of their small vitamin reserves. The usual presenting symptoms are weakness or gait unsteadiness. Neurological examination reveals a syndrome of spinocerebellar degeneration accompanied by peripheral nerve involvement. Some patients are diagnosed erroneously with Friedreich ataxia. The most consistent abnormalities are limb ataxia, areflexia, and loss of vibration and position sense. Cutaneous sensation usually is spared or affected to a lesser degree. About half of patients have nystagmus, ptosis, or partial external ophthalmoplegia. Mild to moderate proximal weakness is common, and some patients may have a myopathy. The pattern of weakness may also be diffuse or predominantly distal. Babinski sign may be present.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here