Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Skin infections occur at a great frequency in solid organ and bone marrow transplant recipients largely as a consequence of immunosuppression.
Neoplasms, particularly squamous cell carcinomas, occur at greater frequency, are more difficult to manage, and are more likely to metastasize than in the nontransplant population.
Graft-versus-host disease, particularly the chronic variant, remains a problem, particularly among patients with bone marrow or stem cell transplantation.
The drugs used for immune suppression have potential mucocutaneous adverse reactions, which must be managed.
The use of immunosuppressive medications has allowed the long-term survival of transplanted solid organs. As a direct result of the required immunosuppression, there is an increase in the incidence and prevalence of both skin infections and neoplasms. Furthermore, the immunosuppressive agents themselves can produce a wide range of cutaneous side effects.
The clinical diagnosis of cutaneous disorders in transplant recipients can prove challenging because the clinical presentation, including the morphology of the lesions, is often altered by the ongoing immunosuppression. This immunosuppression can lead to an exaggeration of clinical findings, as in the case of verrucae vulgaris, or more aggressive behavior, as in the case of cutaneous squamous cell carcinoma or the attenuation of clinical findings such as the diminished inflammatory response seen in deep fungal infections.
The early years of renal transplantation were marked by frequent life-threatening infections, with over half of patients dying of infections during the first 3 years after transplantation. Although much progress has been made in the past decade with regard to prophylaxis (antibacterial, antifungal, and antiviral), early diagnosis, and effective treatment, infections remain a major cause of morbidity and mortality in transplant recipients.
Organ transplant recipients are susceptible to bacterial, viral, and fungal skin infections. Infections during the first month after transplantation are usually caused by nosocomial pathogens or reactivation of latent disease. Up to 6 months after transplantation, reactivation of latent disease or opportunistic infections predominate. Continuation or initiation of moderate- to high-dose corticosteroids increases the risk of developing the latter. Late infections may be caused by opportunistic or conventional pathogens, with the former seen more commonly in those with heightened immunosuppression because of the need to treat graft rejection or active graft-versus-host disease (GVHD).
Infections with herpesviruses (in particular herpes simplex virus-1 [HSV-1], HSV-2, varicella-zoster virus [VZV], and cytomegalovirus [CMV]) were once a common cause of morbidity and mortality during the first year following transplantation. For example, in one series, 97% of patients had one or more herpesvirus infections. However, with the routine use of prophylactic antivirals and the preemptive use of valganciclovir or ganciclovir (e.g., based on weekly blood CMV antigen or polymerase chain reaction [PCR] assays in allogeneic hematopoietic stem cell transplant patients), this situation has improved dramatically. Chronic human papillomavirus infections, however, still remain a significant problem in patients who have received solid organ transplants, because of their need for lifelong immunosuppression. In contrast, an attempt is made to taper and then discontinue immunosuppressive therapy in allogeneic hematopoietic stem cell transplant patients over 12 to 18 months—that is, unless there is active GVHD.
Herpes simplex virus (HSV) was a common cause of infection in transplant recipients until the use of prophylactic acyclovir became commonplace. Although active infections with this virus can occur at any time, they were observed most frequently during the first month after transplantation. In the absence of antiviral therapy, viral shedding rates were found to be as high as 70% in renal transplant patients, 20% in heart–lung recipients, and 60% in bone marrow recipients. The vast majority of HSV infections represent reactivation of a latent infection; occasionally, transplant patients can develop primary disease, and rarely, HSV transmission via transplanted organs has been reported. Reactivation of HSV appears to be a consequence of impaired cellular immunity, as it occurs despite high titers of antibodies directed against the virus.
In mucocutaneous sites, the initial primary lesions of HSV are similar to those seen in immunocompetent hosts, i.e., grouped vesicles, crusts, or erosions on an erythematous base. However, in addition to sites such as the nasal mucosa, vermilion border of the lip, oral mucosa that overlies bone, fingers, buttocks, and genitalia, HSV infections in immunocompromised hosts often involve the “soft” oral mucosa, the perianal region, and the esophagus. In addition, rather than being self-limited infections that last for 7 to 10 days even in the absence of antiviral therapy (as in immunocompetent hosts), these herpetic ulcers tend to be chronic and expansive ( Fig. 39-1 ).
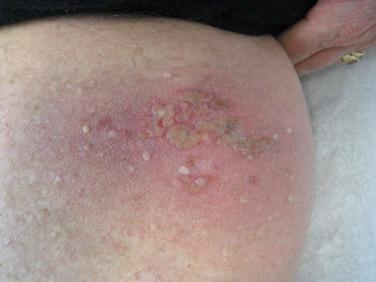
In transplant patients, systemic HSV infections are rather uncommon and are often not accompanied by cutaneous lesions. As a result, the clinical diagnosis requires a high index of suspicion. Reported sites of systemic involvement include the central nervous system (CNS), lungs and liver, as well as the gastrointestinal tract. Occasionally, in the setting of immunosuppression, cutaneous lesions of HSV are “disseminated” on the trunk or extremities.
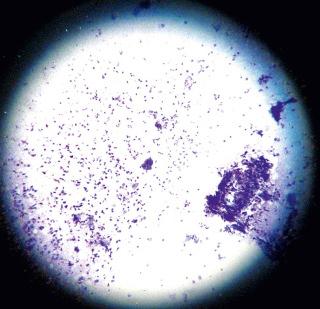
Because of its accuracy and rapid turnaround time of a few hours, the most helpful diagnostic procedure for HSV infections is the PCR assay. In the case of mucocutaneous lesions, it is important to provide the laboratory with an ample supply of keratinocytes. A viral culture should also be done on any tissue sample, including skin, submitted for the PCR assay. Dermatologists still do an office-based test known as a Tzanck preparation ( Fig. 39-2 ) to look for virally infected multinucleated giant cells (seen in HSV-1, HSV-2, VZV, and occasionally CMV infections), but this test is less sensitive than the PCR assay and requires more expertise to interpret.
Depending on the severity, treatment options for active HSV infection in an immunocompromised host include oral agents with enhanced bioavailability (e.g., famciclovir, valacyclovir), or intravenous acyclovir. The antivirals should be continued until there is complete clinical clearing (rather than for a preset number of days), and treatment is usually successful if the diagnosis is made promptly. As in patients with HIV infection who have received multiple or prolonged courses of acyclovir, acyclovir-resistant strains of HSV have been reported, and in these patients intravenous foscarnet (and less often intravenous cidofovir) is employed; topical cidofovir (1% gel) may be tried in milder cases.
Herpes zoster is a common infection in transplant recipients, occurring in up to 15% of cases. As in immunocompetent hosts, it is a result of the reactivation of the varicella-zoster virus (VZV) that resides in dorsal root or cranial nerve ganglia. Clinical disease is usually characterized by a unilateral distribution of grouped vesicles or crusts on an erythematous base, with lesions usually being confined to one or a few adjacent dermatomes ( Fig. 39-3 A ). However, both chronic VZV and cutaneous dissemination of VZV can occur in this patient population ( Fig. 39-3 B ); in the latter situation, at least 20 lesions reminiscent of varicella are widely scattered on the trunk and extremities. Less often there is associated meningoencephalitis, pneumonitis, or hepatitis. Prolonged infection is associated with reduced specific cellular immune responsiveness.
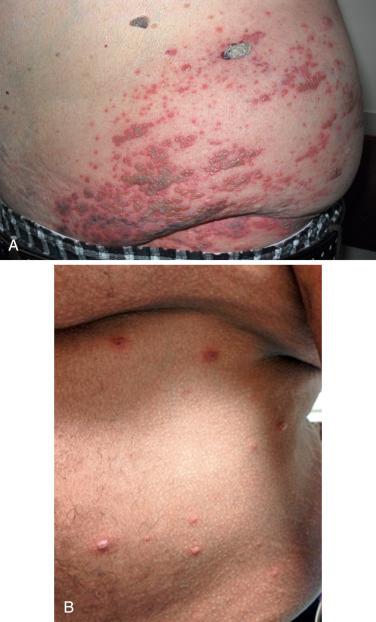
As is the case with HSV infections, the diagnosis of herpes zoster is established via the PCR assay, Tzanck smears, and/or PCR; viral cultures of VZV require a longer incubation period than those of HSV. In patients with severe disease, cutaneous dissemination, or suspected systemic involvement, the treatment of choice is intravenous acyclovir. If the disease is mild and there is no evidence of cutaneous dissemination, then oral agents with greater bioavailability (e.g., famciclovir, valacyclovir) can be prescribed.
Cytomegalovirus (CMV) is one of the more common opportunistic infections in transplant recipients and is potentially a major source of morbidity and mortality. Until the development of more effective anti-CMV medications, this virus accounted for up to 25% of deaths, 20% of graft failures, 30% of febrile episodes, and 35% of leukopenic episodes in renal transplant patients. One of the major advances, especially in allogeneic hematopoietic stem cell transplant patients, has been the use of weekly antibody staining of circulating neutrophils or weekly PCR-based assays of peripheral blood as a means of detecting CMV antigen or CMV DNA, respectively, and hence early infections. The latter assays are particularly helpful in neutropenic patients. In the setting of positive results, “preemptive” oral valganciclovir or intravenous ganciclovir therapy can be instituted prior to the development of clinical symptoms such as fever, pneumonitis, or enterocolitis.
Infections in transplant recipients may be primary, i.e., due to the presence of CMV in the transplanted organ or cells, or they may represent reactivation of a latent infection. In the past, clinical infections were most common during the first 6 months after transplantation; e.g., in one series of kidney transplant patients the median time of onset was 46 days after transplantation. However, with more solid organ transplant patients receiving prophylactic therapy during the first 3 months after transplantation, the time of onset is often delayed until after discontinuation of antiviral therapy.
Clinical manifestations of CMV infection include leukopenia, pneumonitis, gastroenteritis, retinitis, hepatitis, and encephalitis, as well as a mononucleosis-type syndrome. Skin involvement is present in a small percentage of patients with systemic CMV infection; cutaneous presentations include morbilliform eruptions, verrucous plaques, petechiae and purpura, and ulcerations. It can also be seen incidentally in cutaneous biopsies in patients with no active eruption. In mucocutaneous ulcers CMV often coexists with other viruses such as HSV or VZV, and as a result its true pathogenicity has been questioned.
The diagnosis of CMV infection is suggested histopathologically by the presence of intranuclear inclusions surrounded by a halo within endothelial cells. Viral culture or a PCR-based assay of involved tissues, including the skin, is required for confirmation. It is important not to confuse active infection with viral shedding, as is commonly seen in the urine or throat washings of transplant patients.
In addition to causing exanthema subitum (roseola infantum) in healthy young children, human herpesvirus 6 (HHV-6) has been associated with fever, pancytopenia, pneumonitis, gastroenteritis, and encephalitis in transplant patients. There are also a few scattered reports of an associated morbilliform eruption at the time of reactivation. Viral DNA can be detected in the skin as well as peripheral blood mononuclear cells.
Infections with two other herpesviruses, Epstein–Barr virus (EBV) and human herpesvirus 8 (HHV-8), are associated with the development of neoplasms in transplant patients: post-transplant lymphoproliferative disorder and Kaposi’s sarcoma (KS), respectively. The term post-transplant lymphoproliferative disorder is used rather than lymphoma because the histologic findings range from a polyclonal B-cell infiltrate to a monoclonal B-cell lymphoma. Rarely, T-cell lymphomas are seen.
Because this lymphoproliferative disorder is reflective of “over”-immunosuppression, a reduction in immunosuppressive therapy is a component of the initial therapy. Clearly, the extent to which the immunosuppressive agents can be reduced depends on the type of transplant. Renal transplant patients have the option of being placed back on dialysis, but patients with heart, lung, or liver transplants cannot afford graft rejection. For localized disease, surgical excision or radiation therapy combined with a reduction in immunosuppression may prove sufficient. However, extensive or rapidly progressive disease requires systemic therapy with rituximab, often in combination with multidrug chemotherapy. Discontinuation of standard immunosuppression in conjunction with administration of chemotherapy usually does not lead to graft rejection, given the immunosuppressive effects of the chemotherapy.
The association between HHV-8 and KS is seen in both immunocompetent and immunosuppressed patients (see Chapter 23, Chapter 35 ). In solid organ transplant recipients, the prevalence of KS is 50 times that of the general population in endemic areas, and increases to 400 to 500 times in nonendemic areas. Regression of the mucocutaneous lesions of KS has been observed in transplant patients following a reduction in the level of immunosuppression or a switch from a systemic calcineurin inhibitor to mTOR inhibitor therapies such as sirolimus (rapamycin).
Verrucae vulgaris (viral warts) are very common in patients who have received solid organ transplants and reflect their requirement for lifelong immunosuppression. The prevalence of warts increases with the duration of graft survival, such that the majority of renal transplant recipients with graft durations of more than 5 years have verrucae. There are over 90 different types of human papillomavirus (HPV), and specific types or groupings are associated with particular clinical forms of the disease. For example, HPV types 1, 2, and 4 are associated with banal warts, often on the hands and feet ( Fig. 39-4 ); HPV types 3 and 10 are associated with flat warts (verruca plana); and HPV types 6 and 11 (nononcogenic) as well as 16 and 18 (oncogenic) are associated with condylomata acuminata (venereal warts) ( Fig. 39-5 ).
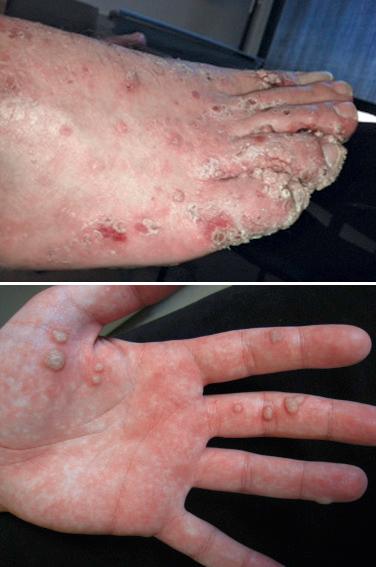
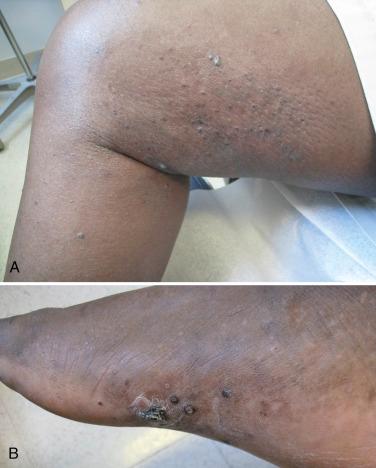
Not only can transplant patients have all of the HPV types just mentioned, but they have been found to harbor unusual types that were once thought to be uniquely associated with a rare disease known as epidermodysplasia verruciformis (e.g., HPV 5, 8, 19 to 25). In addition, the warts are frequently extensive and even more difficult to treat than in the general population. The oncogenic potential of some of these HPV types combined with the chronic immunosuppression creates a difficult situation in a number of patients. The potential for developing squamous cell carcinomas of the cervix, vulva, and anus in addition to the skin (see below) represents a real threat to these patients, and an ongoing screening program that includes close inspection and biopsy of any suspicious lesion is required. Treatment of HPV infections is discussed in Chapter 33, Chapter 35 .
Mollusca contagiosum (MC) are 2- to 10-mm dome-shaped skin-colored to pink papules that have a central umbilication. They are caused by a poxvirus. Infections with MC are more common in patients with atopic dermatitis and in immunocompromised hosts (see Chapter 33, Chapter 35 ). In the latter group, lesions can prove difficult to treat. Cutaneous lesions of cryptococcosis and systemic dimorphic fungal infections can resemble MC, and therefore dermal scrapings or a skin biopsy specimen should be examined if the diagnosis is uncertain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here