Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hemorrhagic shock is the leading cause of preventable death following injury. Historically, major advances in the management of hemorrhagic shock have come during periods of armed conflict, when hemorrhage occurs at higher rates and in situations where the military is able to rapidly institute new protocols and measure effectiveness in a defined population. Among the most recent of these advances is the concept of damage control resuscitation (limited crystalloid, whole blood or high ratio of plasma and platelets to red blood cells, permissive hypotension) for hemorrhagic shock.
Hemorrhage causes greater than 60,000 deaths yearly in the United States, with 80% of deaths from hemorrhage attributable to injury. Annually, trauma deaths represent 2 million years of life lost domestically and 75 million years of life lost worldwide. Hemorrhagic shock carries significant risk of mortality, with almost one in four patients arriving at role 3 military facilities with systolic blood pressure under 90 mm Hg dying, similar to civilian populations. As many as 85% of preventable military deaths may be due to bleeding. Thirty to 50% of civilian trauma-related deaths due to hemorrhage are preventable, with one-third occurring prior to arrival at a hospital. Preventable death due to hemorrhage occurs due to noncompressible truncal hemorrhage in approximately three quarters of patients, over one third of those being due to multicompartment hemorrhage in both the chest and abdomen. These patients generally require rapid transport to the hospital for hemorrhage control in the operating room.
Shock is the state that occurs when the body can no longer maintain adequate oxygen delivery to the peripheral tissues, resulting in anaerobic metabolism. Mortality from hemorrhagic shock occurs due to distinct pathophysiology at different time points: near-immediate death due to uncontrolled exsanguination, early death due to unrecoverable oxygen debt, and late death due to multisystem organ dysfunction. When significant hemorrhagic insult is sustained, immediate catecholamine release leads to vasoconstriction in an attempt to maintain normal blood pressure. Hemorrhagic shock occurs when this mechanism is inadequate to sustain blood pressure, and the magnitude of blood loss and compensatory vasoconstriction causes inadequate delivery of oxygen to the tissues to support aerobic metabolism. The resultant conversion to anaerobic metabolism leads to production of lactate, inorganic phosphates, and oxygen radicals, which cause further damage through both direct effects on tissues, and further altering the oxygen-carrying capacity of blood by causing acidemia. Early hypoperfusion and decreased oxygen delivery to the endothelium and blood itself set the stage for the reperfusion injury that weakens fibrin clots and leads to acute traumatic coagulopathy, characterized by fibrinolysis. Following correction of the initial hemorrhagic insult, refractory vasodilatory hypotension may occur as a result of exuberant activation of the innate immune system and simultaneous impairment of the adaptive immune system. This is due to a combination of mechanical damage to tissues and metabolic derangement caused by tissue ischemia. A variety of immunomodulatory small molecules, including cytokines, leukotrienes, interleukins, thromboxane, prostaglandin, prostacyclin, tumor necrosis factor, and complement proteins, contributes to the ongoing pathophysiology of this severe distributive shock. The uncontrolled propagation of these immunologic signaling cascades contributes considerably to multisystem organ dysfunction and late death.
Traditionally, shock has been thought of in macrocirculatory terms, utilizing measures and resuscitation end points such as blood pressure, heart rate, and cardiac output to guide therapy. These measures are readily available and easily recognizable by clinicians and make sense in the early phases of resuscitation for the patient in hemorrhagic shock. Blood volume lost must be replaced, ideally with blood, and persistent hypotension then corrected with vasopressors. Increasingly, however, traumatic and hemorrhagic shock are now studied on the level of the microcirculation and inflammasome, which play a larger role in the care of the hemorrhagic shock patient who survives the early acute posttrauma period.
Following traumatic injury to tissues and subsequent cell necrosis, damage-associated molecular patterns (DAMPs) such as high-mobility group box 1 are released, activating responses meant to deal with the range of insults associated with traumatic injury from hemorrhage and direct tissue damage to entry of pathogens through breached tissues. This is accomplished via activation of the serine protease system, including kinin, and the coagulation cascade, as well as inflammatory and innate immunity agents including the complement cascade. Other innate immune responses ( Fig. 1 ) include production of reactive oxygen species and neutrophil extracellular traps, targeting bacteria that may enter via damaged tissue barriers. DAMPs also activate a leukocyte response to injury via TLRs (TLR4, specifically), NLRs, RAGE, and purinergic receptors.
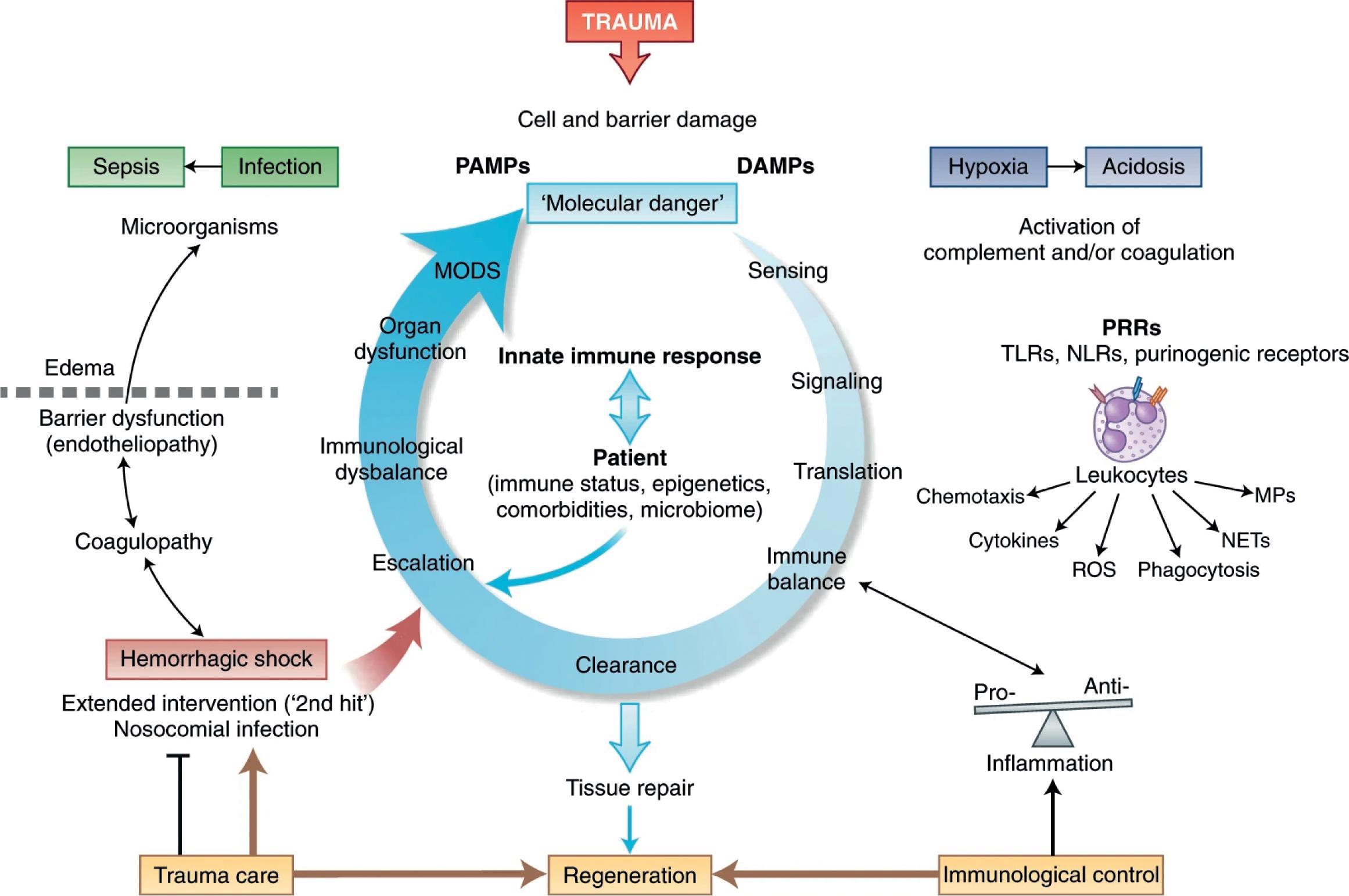
After minor traumatic injury, these responses are balanced and lead to appropriate inflammatory and healing processes. However, following severe polytrauma or hemorrhagic shock, robust inflammatory responses may propagate further cell damage and more release of DAMPS, leading to a self-sustaining and increasingly destructive inflammatory cascade. Tissue hypoxia and acidemia resulting from hemorrhagic shock also further exacerbate the inflammatory and innate immune response to trauma, as demonstrated by Halgebauer et al in a study comparing polytrauma patients with and without hemorrhagic shock. They found that interleukin-6, MMP-13, claudin-5, angiopoietin-2, and syndecan-1 were significantly elevated in patients with hemorrhagic shock. Others have also identified increased levels of interleukin-6 as a likely early mediator of the systemic inflammatory response syndrome after trauma and hemorrhagic shock. Interestingly, different immune inflammatory profiles have been noted on admission in blunt trauma patients who go on to develop nosocomial infections, versus those who do not, independent of injury mechanism or severity or patient sex. While this study found inflammatory differences to be independent of age, others reported that cytokine and chemokine concentration changes are attenuated in elderly patients and may account for increased mortality in older patients with similar injury severity scores.
This inflammatory response manifests as systemic inflammatory response syndrome, starting as early as 30 minutes after major injury, and leads to a host of downstream complications including multiple-organ dysfunction syndrome (MODS) and postinjury cardiomyopathy and immune dysregulation, the last of which may persist long after the initial traumatic insult as persistent inflammation-immunosuppressive catabolism syndrome. The robust, nonspecific immune response to trauma, as well as the stress release of cortisol after trauma, may also render the immune system (particularly neutrophils) less able to defend against pathogens, leading to increased susceptibility to sepsis and further exacerbation of the immune response and MODS.
MODS is a common cause of late death after traumatic hemorrhagic shock. Blood lactate concentration and base deficit, indirect measures of anaerobic metabolism, are typically used to identify patients at highest risk of MODS, which is caused in part by changes in microcirculatory dynamics. This microcirculatory shock continues, even in the face of normalized systemic hemodynamics, if microcirculatory dysfunction prevents oxygen delivery to peripheral tissues. Over the past decades, sublingual microcirculatory monitoring has been used as a means to directly examine the effects of shock and critical illness states on the microcirculation. Alterations in the microcirculation have previously been identified as predictive of in-hospital mortality in critically ill patients. Similarly, microcirculatory perfusion has been shown to be important in both animal models and human cohorts of traumatic hemorrhagic shock. In a study utilizing sublingual video microscopy in intubated patients with traumatic hemorrhage, microcirculatory parameters (perfused vessel density and microcirculatory flow index) better predicted the development of MODS than traditional measurements, such as systolic blood pressure and lactate levels.
In the early postinjury period, it appears that microcirculation and macrohemodynamics are correlated. However, at an unknown time point these two appear to diverge. Although microcirculatory parameters may improve following restoration of blood volume, they do not return to normal, even in the presence of normalized systemic measures such as cardiac output. In fact, microcirculation may become altered in shock independently of systemic hemodynamic alterations, presenting a more sensitive method to identify patients sustaining the inflammatory and metabolomic insults resulting from shock states. Interestingly, in a swine model of nontraumatic hemorrhagic shock with early restoration of circulating blood volume, this dissociation between the microcirculatory parameters and systemic hemodynamics did not occur, indicating that the response to trauma is responsible for the divergence of the microcirculation from macrohemodynamics.
Of particular interest is gut microcirculation, as damage to the gut via impaired microcirculation leads to disruption of the gut barrier and bacterial translocation, a contributing factor in MODS, shown to be worse in models of combined polytrauma and hemorrhagic shock than either alone. It should be noted, however, in spite of the prognostic link between the sublingual microcirculation and outcomes, there are conflicting data on correlations between sublingual microcirculation and gut perfusion. While in a single sheep model of hemorrhagic shock the sublingual and gut microperfusion do appear to correlate, septic shock and other models have not demonstrated the same relationship reliably. It remains to be seen whether resuscitation guided by microcirculatory parameters will result in significantly different patient management or change outcomes. Presently, examination of the sublingual microcirculation in shock is an investigative technique and does not have validated clinical monitoring applications.
Intimately associated with the innate immune response in traumatic and hemorrhagic shock, the endothelium also responds swiftly to hypotensive and inflammatory insults. After trauma, the endothelium becomes prothrombotic and proinflammatory, in contrast to its normal antithrombotic, anti-inflammatory state. In response to the rapid vasoconstriction found with hypovolemia, the endothelium releases nitric oxide leading in part to the aforementioned microcirculatory dysregulation. When thrombin is then activated by tissue factor released from damaged cells, the endothelium releases cytokines, further promoting the immune inflammatory response, and expresses adhesion molecules, promoting both clot formation and leukocyte extravasation. Tight junctions and glycocalyx are also disrupted by ischemia and inflammation, as they are in the gut after polytrauma and hemorrhagic shock, leaving the endothelium leaky and leading to tissue edema. Similar to other immune responses to polytrauma and hemorrhagic shock, these responses may become overactive and maladaptive.
Animal models are useful for studying both the pathophysiology and treatment of hemorrhagic shock; however, the bulk of studies are performed in lower-order species, which have significant genetic, physiologic, and immunologic discrepancies from humans. While the consistency in genetics between specimens in lower-order species lends itself to experimental reproducibility, it also results in a poor representation of the variation in age, comorbidities, and other physiologic parameters in the trauma patient population.
Mouse models offer the advantage of being inexpensive and easily maintained, with good interspecimen consistency and ready availability of knockouts and reagents. However, their small size and blood volume makes them unsuitable for complex surgery or study of hemodynamics. Mice also share only approximately 80% of their genome with humans, thus conclusions regarding the molecular underpinnings of the hemorrhagic shock response may not be translatable to humans. Canine, porcine, and ovine models present better fidelity in terms of hemodynamic parameters; however, they are more expensive to maintain and have greater interspecimen variability than rodents, without being significantly genetically closer to humans. Further, data from animal studies of vasopressor use may not be transferable to humans due to differences in receptors and their ligands; thus, results from these models should be interpreted with caution. And, while primate models are clearly the closest approximation to humans genetically and physiologically, use is limited by cost and ethical issues. In general, nonhuman primate studies are reserved for questions that cannot be answered adequately in lower-order animal models.
The three basic models of hemorrhagic shock utilized in animal models are fixed volume, fixed pressure, and uncontrolled hemorrhage. Each has utility in studying specific aspects of hemorrhagic shock, but none is a good mimic of traumatic injury in humans, which may vary in characteristics between these three models at different points in time and different injury types. Further, these models do not account for the natural history of some bleeding, which may include cessation of hemorrhage as blood pressure drops and hemostatic mechanisms take effect. Innovation in this field continues.
Identification of patients with early hemorrhagic shock can be difficult, as young healthy patients may lose up to 30% of their blood volume prior to manifesting obvious hemodynamic changes or other signs ( Table 1 ). While some patients present with hypotension, tachycardia, and obvious end-organ hypoperfusion (i.e., altered mental status), the young healthy patient with robust cardiovascular compensation may only be identified in a delayed fashion at the time of total cardiovascular collapse. While readily available physical data such as vital signs or assessment of distal pulses can be helpful, they do not reliably identify the patient who has ongoing tissue and cellular hypoperfusion in the setting of normal vital signs, termed compensated or subclinical shock. Patients in severe (class III–IV) hemorrhagic shock, however, will be easily identified even before vital signs are obtained by their lethargy, pallor, and clammy extremities.
| Class | Volume Lost (mL) | Volume Lost (%) | Heart Rate | Blood Pressure | Pulse Pressure | Urine Output | Examination Findings |
|---|---|---|---|---|---|---|---|
| I | <500–750 | <15 | ↔ | ↔ | ↔ | ↔ | Normal or mild anxiety |
| II | 750–1500 | 15–30 | ↑ | ↔ | ↓ | ↔ | Anxiety |
| III | 1500–2000 | 30–40 | ↑↑ | ↓ | ↓↓ | ↓ | Pallor, cold/clammy extremities, dry mucous membranes |
| IV | >2000 | >40 | ↑↑↑ | ↓↓ | ↓↓ | ↓↓ | Lethargy |
Tissue perfusion can be assessed crudely during transport or in the trauma bay by noting altered mental status, absence of distal pulses, and poor capillary refill. Lactate level is the best readily available indicator of tissue hypoperfusion and correlates well with base deficit. It can be obtained rapidly in the trauma bay or even en route by some emergency medical service providers using portable point-of-care lactate devices. Convenient and timely assessment of lactate levels and trends helps assess the trend in oxygen debt accumulation and therefore guides the use of blood product resuscitation.
Patients with hemodynamically significant hemorrhage (approximately 6%–9% of trauma patients) can be divided into three similarly sized groups based on the natural history of their hemorrhage and response to volume resuscitation. The first group are those with exsanguinating hemorrhage who do not respond to volume resuscitation. These patients have significant cardiac or vascular injury in the chest, abdomen, or retroperitoneum and only survive to hospital care when transported rapidly by a well-organized and efficient trauma care system. To survive, these actively bleeding patients require prompt control of hemorrhage, generally in the operating room. The patients in the second group have moderate to severe hemorrhage and may respond transiently to volume resuscitation. They require timely bleeding control and resuscitation and will likely die within 6 to 12 hours otherwise. This group is still at risk of MODS and other sequelae of polytrauma and hemorrhagic shock, even when managed appropriately. The final group of patients are those with transient hypotension who experience stabilization of vital signs, either spontaneously or after initial fluid resuscitation, due to adequate compensatory mechanisms (responders). While these patients tend to do better than those in the former two groups, they are still at risk of organ and immune dysfunction due to hypoperfusion during the transient hypotensive period.
Early hemorrhage control and replacement of lost blood volume are the two primary components of the management of hemorrhagic shock. Several civilian mass casualty incidents in recent years, specifically, the Sandy Hook Elementary School shooting, led to a multiorganizational collaborative led by the American College of Surgeons to empower bystanders to achieve hemorrhage control (Stop the Bleed). To date this initiative has trained over 1 million civilians in basic bystander bleeding control techniques, including application of direct pressure, wound packing, and tourniquet use. While effectiveness of this training is difficult to ascertain in real-life scenarios, prevention of early blood loss prior to the arrival of trained emergency medical technicians helps mitigate the effects of excess blood loss and clearly has saved lives.
For truncal (noncompressible) hemorrhage, the American emergency medical services (EMS) model uses a “scoop and go” strategy to rapidly transport injured patients to nearby hospitals for definitive care, with the minimum necessary stabilization performed on scene. On the other hand, compressible bleeding with active hemorrhage from extremity or junctional areas (neck, groin, axilla) can be initially addressed in the field. En route, EMS will attempt to establish intravenous (IV) access via IV catheter or intraosseous line. While most EMS only have crystalloid available, some may have packed red blood cells (pRBCs) or whole blood available for transfusion in hypotensive patients.
Based on expert panel review of the literature, on arrival to the emergency department several actions should be taken immediately and during the initial evaluation: activation of massive transfusion protocol, measurement of lactate or base deficit, transfusion of blood products in a 1:1:1 ratio, viscoelastographic measurement of coagulopathy, and minimization of crystalloid resuscitation. While not yet demonstrated for this particular bundle, implementation of trauma care as a bundle has been shown in other cases to improve patient outcomes and helps ensure adherence to evidence-based practices. Optimizing care with elimination of delay to hemorrhage control is vital to avoid preventable deaths—minutes matter.
Vascular access is essential to restore circulatory volume rapidly. The most important factor in considering the procedure for procuring vascular access is the anatomical location and magnitude of the injuries and the level of skill and expertise of the care provider. Venous access should be avoided in an injured limb. In patients with injuries below the diaphragm, at least one IV line should be placed in a tributary of the superior vena cava, as there may be vascular disruption of the inferior vena cava. For rapid administration of large amounts of IV fluids, short, large-bore catheters should be used. This principle is based on Poiseuille’s Law:
Thus, doubling the internal diameter of the venous cannula increases the flow through the catheter 16-fold. A 14-gauge, 5-cm catheter in a peripheral vein will infuse fluid twice as fast as a 16-gauge, 20-cm catheter passed centrally. When using 8.5-French pulmonary catheter introducers, the side port should be removed, as this increases the resistance roughly fourfold.
Advanced Trauma Life Support guidelines recommend rapid placement of two large-bore (16-gauge or larger) IV catheters in the patient with serious injuries and hemorrhagic shock. The first choice for IV insertion is a peripheral extremity vein. The most suitable veins are at the wrist, the dorsum of the hand, the antecubital fossa in the arm, and the saphenous vein in the leg. These sites can be followed by the external jugular and femoral vein. The complication rate of properly placed IV catheters is low. Intravascular placement of a large-bore IV should be verified by checking for backflow of blood. An IV site should infuse easily without added pressure. IV fluids can extravasate into soft tissues when pumped under pressure through an infiltrated IV line and may create a compartment syndrome.
Rapid peripheral percutaneous IV access may be difficult to achieve in patients with hypovolemia and venous collapse, edema, obesity, scar tissue, history of IV drug abuse, or burns. Under these circumstances, intraosseus access is the next choice and may be obtained in the field by trained EMS or on arrival to the emergency department. Intraosseus access infuses directly into the bone marrow and may be obtained more rapidly and reliably than central access, particularly in pediatric patients or under conditions of circulatory collapse. Access may be obtained at several locations, including the proximal anteromedial tibia (preferred in children), proximal humerus (preferred in adults), distal medial tibia, and distal anterolateral femur. Insertion should be performed under sterile conditions, and the catheter used only until other IV access can be obtained. Resuscitative fluids including blood and crystalloid, as well as medication, including vasopressors, may be infused. Pitfalls of intraosseus insertion include through-and-through penetration of the bone, particularly in patients with less soft tissue overlying the bone or when a longer needle is used, while subcutaneous or periosteal infiltration is possible with shorter needles or patients with more soft tissue. Physeal plate injury is also of concern, particularly in children, thus anatomic landmarks should be carefully observed.
Subclavian and internal jugular catheterization should not be used routinely in hypovolemic trauma patients. The incidence of complications is higher and the rate of success is lower due to venous collapse. However, when femoral access is not feasible or significant inferior caval injury is suspected, subclavian catheterization provides rapid venous access in experienced hands. The most frequent complication of subclavian venipuncture is pneumothorax. Pneumothorax is more likely to occur on the left side because the left pleural dome is anatomically higher. Subclavian and internal jugular catheters should be inserted on the side of injury in patients with chest wounds, reducing the chances of collapse of the uninjured lung, especially if a thoracostomy tube is already in place. A simple pneumothorax may result in respiratory compromise in individuals with pulmonary contusions or a pneumothorax in the contralateral hemithorax. Venous air embolism is another complication of central line insertion. Although percutaneous placement of internal jugular catheters is an excellent means of attaining rapid large-bore catheter access, this is an unusual site for IV insertion in trauma patients because of the possibility of cervical trauma and the need for cervical immobilization.
Femoral vein cannulation is another alternative for line placement, associated with fewer acute complications. Bowel perforation may occur, especially in patients with femoral hernia. Penetration of the hip may result in septic arthritis. Thrombophlebitis occurs more often with femoral than with internal jugular or subclavian catheters; however, this is most likely with prolonged use.
Venous cutdowns can be performed when rapid, secure, large-bore venous cannulation is desirable, such as a patient in extremis who loses pulses in the trauma bay, when percutaneous peripheral or central access is either contraindicated or impossible to achieve. As with all central access, strict aseptic technique should be used. Surgical masks and caps should be worn. Venous cutdown has a low potential for anatomic damage. Cutaneous nerve injury is the most common problem. The infection rate is relatively low when used acutely but increases over time. Therefore, it is recommended that venous cutdown catheters be removed as soon as possible. In addition, any lines placed during resuscitation of a trauma patient without strict aseptic technique should be removed as soon as the patient’s condition allows.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here