Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
The heart’s pacemaker cells residing in the sinoatrial node (SAN) normally generate spontaneous rhythmic changes of their membrane potential, producing relatively periodic spontaneous action potentials (APs), that is, normal automaticity. The essence of cardiac pacemaker cell automaticity is diastolic depolarization (DD), that is, a slow spontaneous decrease in membrane polarization until it reaches the membrane excitation threshold culminating in generation of an AP. Initial focus in pacemaker cell research had been on the behaviors of sarcolemmal ion currents generating the DD; hence, the knowledge base was limited to surface membrane proteins. The ensemble of these currents recreated in silico from experimental voltage-clamp data can generate spontaneous APs, and a plethora of in silico models were developed based on this principle and termed the membrane oscillator or membrane clock . Subsequently, the important role for intracellular Ca cycling in normal automaticity had been discovered. The sarcoplasmic reticulum (SR) generates roughly periodic diastolic Ca releases, termed a Ca oscillator or Ca clock, that can affect the membrane clock and accelerate DD via activating electrogenic Na/Ca exchanger (NCX) and ion channels. Further studies suggested that the Ca clock depends on the membrane clock and the functions of both clocks are tightly and synergistically integrated, which was formulated as the coupled-oscillator (or coupled-clock) theory of normal automaticity. , Thus within individual pacemaker cells, clock coupling, conserved during evolution of the animal kingdom, ensures robustness, that is, “fail safe” operation, and flexibility (i.e., the ability to react to demands for faster or slower AP firing rates). The present concept of the coupled-clock system driving automaticity of individual pacemaker cells has been numerically modeled , and extensively reviewed. , , This chapter reviews key and new aspects of this concept.
On the higher level of signal processing in SAN tissue, that is, within a complex network of SAN cells, the present perspective is that the output impulse emanating from the SAN is generated by a collective behavior of loosely coupled cells and not dominated by the DD of a single cell. The interactive network of mechanisms intrinsic to SAN cells must also interpret and react to signals arising extrinsic to the cell (e.g., stretch, electrotonic impulses, or neurotransmitter or hormonal stimulation of surface membrane receptors). This results from the timely integration of signaling events at multiple levels within the SAN, including subcellular, cellular, and tissue architecture. The current and future frontier of SAN research is aimed to clarify how the coupled-clock approach is applied across multiple scales, that is, how individual numerous heterogeneous local Ca and electrical oscillatory events interact to give rise to the robust and flexible signals at the SAN output. Thus this chapter also briefly reviews recent progress in this direction.
In each pacemaker cycle the Ca and membrane clocks interact via multiple time, voltage, and Ca dependencies of proteins comprising the system. The membrane clock’s key molecules include L-type Ca channels (LCCh) and T-type Ca channels (generating I CaL and I CaT , respectively); K channels; nonselective hyperpolarization-activated cation channels (HCN4); generating I f ; and NCX, generating I NCX ( Fig. 25.1 , top ). SAN cells express two types of LCCh : Ca V 1.3 generating I CaV1.3 with a low activation threshold of approximately −55 mV and Ca V 1.2, generating I CaV1.2 at voltages above −40 mV, so that I CaL = I CaV1.3 + I CaV1.2 . NCX exchanges 3 Na for 1 Ca, thereby generating a membrane current. This operation is both Ca dependent and voltage dependent, executing an efficient coupling mechanism discussed later. The Ca clock (see Fig. 25.1 , bottom ) features a Ca pump, SERCA2 and Ca release channels, that is, ryanodine receptors (RyRs) that generate rhythmic, diastolic local Ca releases (LCRs).
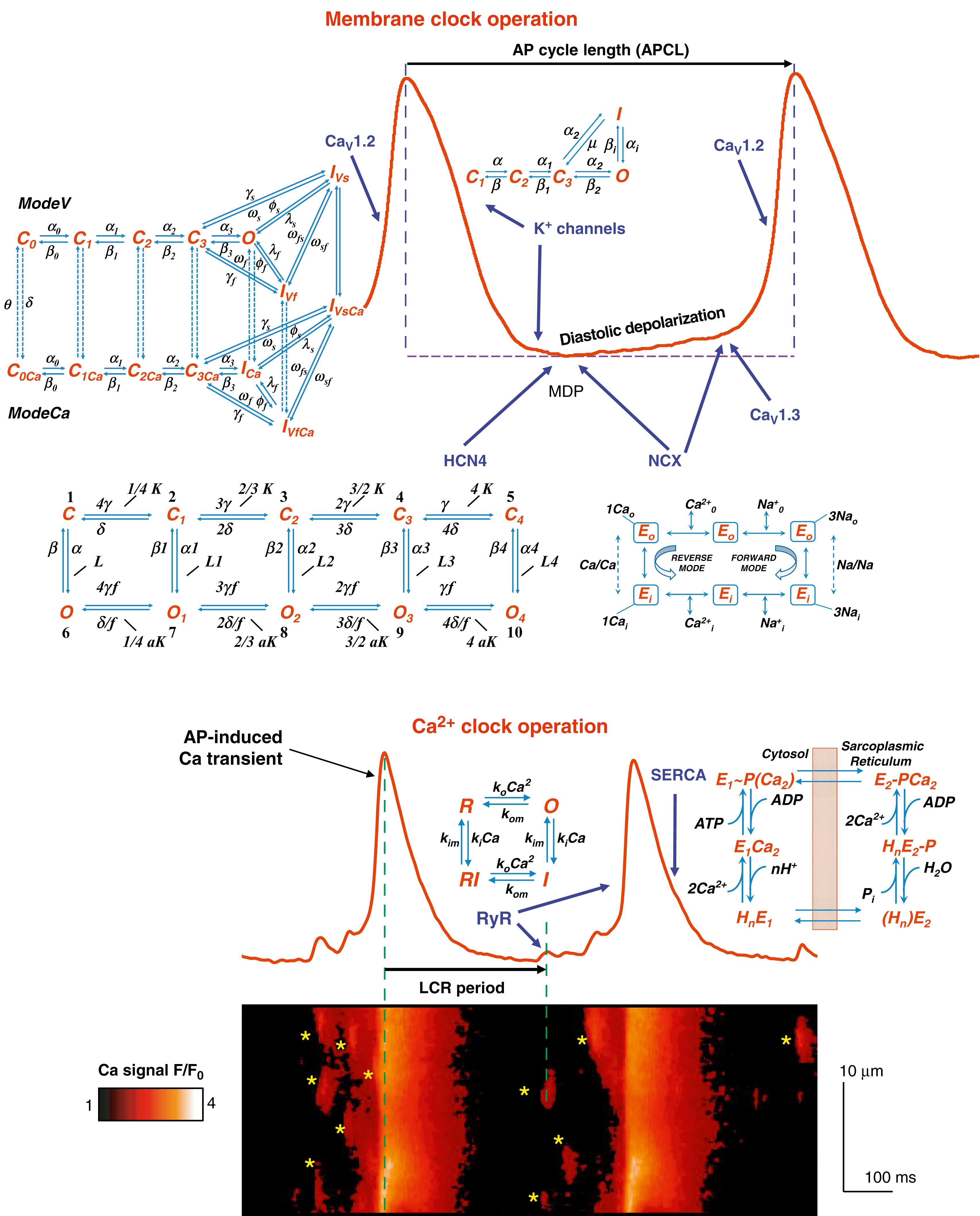
Each spontaneous AP is followed by the reestablishment of maximum diastolic potential (MDP), and the membrane potential begins to slowly depolarize because of activation of I f and I CaT , removal of K channel activation, and spontaneous local RyR activation that generates multiple submembrane LCRs during DD (see Fig. 25.1 ). The LCRs first appear as small, Ca spark-like events at about the time of the MDP. The LCR period is the delay between the AP-induced cytosolic Ca transient and LCR emergence. LCR periods inform not only on the RyR activation but also on the kinetics of recharging the SR Ca capacitor. , , These local Ca signals increase in size over time during DD appearing as locally propagating wavelets (aborted waves) and, finally, in the late DD, the propagating LCRs merge into AP-induced Ca transient (APCT) ( Fig. 25.1 , bottom ). Summation of individual LCRs regulated, in part, by Ca-induced Ca-release (CICR), , produces an LCR ensemble Ca signal that activates an inward I NCX . Further explosive growth of the LCR ensemble Ca signal, accelerating I NCX activation, initiates a respective DD acceleration (nonlinear DD component), activating I CaL . Thus the time to the nonlinear DD informs on the kinetics of LCR generation and synchronization to generate the LCR ensemble Ca signal that increases I NCX activation. In turn, I f decreases during late DD as it gets closer to its reversal potential (approximately −30 mV).
There are two schools of thought regarding the importance of specific events within the coupled-clock system occurring during early DD in the AP cycle. One school considers LCRs as the key events that initiate the AP cycle, based on experimental results that (1) spontaneous LCRs occur under voltage clamp, that is, independent of membrane depolarization; (2) LCRs occur as early as AP repolarization reaches the MDP ; and (3) oscillatory LCRs are observed in membrane-free (i.e., a detergent-permeabilized) SAN cells bathed in a physiologic [Ca] of 100 nM. , These membrane-independent LCRs are generated at rates of 1 to 5 Hz in rabbit SAN cells (i.e., encompassing rates of spontaneous AP firing in this species).
Another school of thought considers low voltage-activated Ca currents, such as I CaT and I caV1.3 as primary initiating events, with their respective Ca influxes coupling the clocks. Indeed, loss of Ca V 1.3 function in a human channelopathy is associated with bradycardia and congenital deafness. A recent study by Torrente and colleagues in Ca V 1.3 knockout mice showed that Ca V 1.3 deficiency substantially reduced Ca dynamics, including the rate of LCRs and their synchronization that slowed spontaneous activity. Thus Ca V 1.3 channels play an important role not only in DD, but also in Ca clock regulation via triggering LCRs.
A recently developed concept of the AP ignition process reconciles the apparent disparate views on the importance of early events occurring during the DD (around the time of MDP and subsequent to MDP). According to this concept, pacemaker APs are ignited during DD via a feed-forward control mechanism that includes membrane potential, LCRs, NCX, I CaT , and I CaL ( Fig. 25.2 ). In short, the ignition phase begins when the magnitude of inward I NCX begins to increase because of its activation by LCRs. The I NCX , together with I f and I CaT , brings the membrane potential to I Cav1.3 activation threshold of approximately −55 mV. During the ignition phase, I CaV1.3 depolarizes cell membrane, and concurrent depolarization-dependent Ca influx generates more LCRs via CICR that further activates inward I NCX , implementing a feed-forward control that drives membrane potential to the threshold potential of −40 mV required for activation of I CaV1.2 , which, in turn, generates the next AP upstroke.
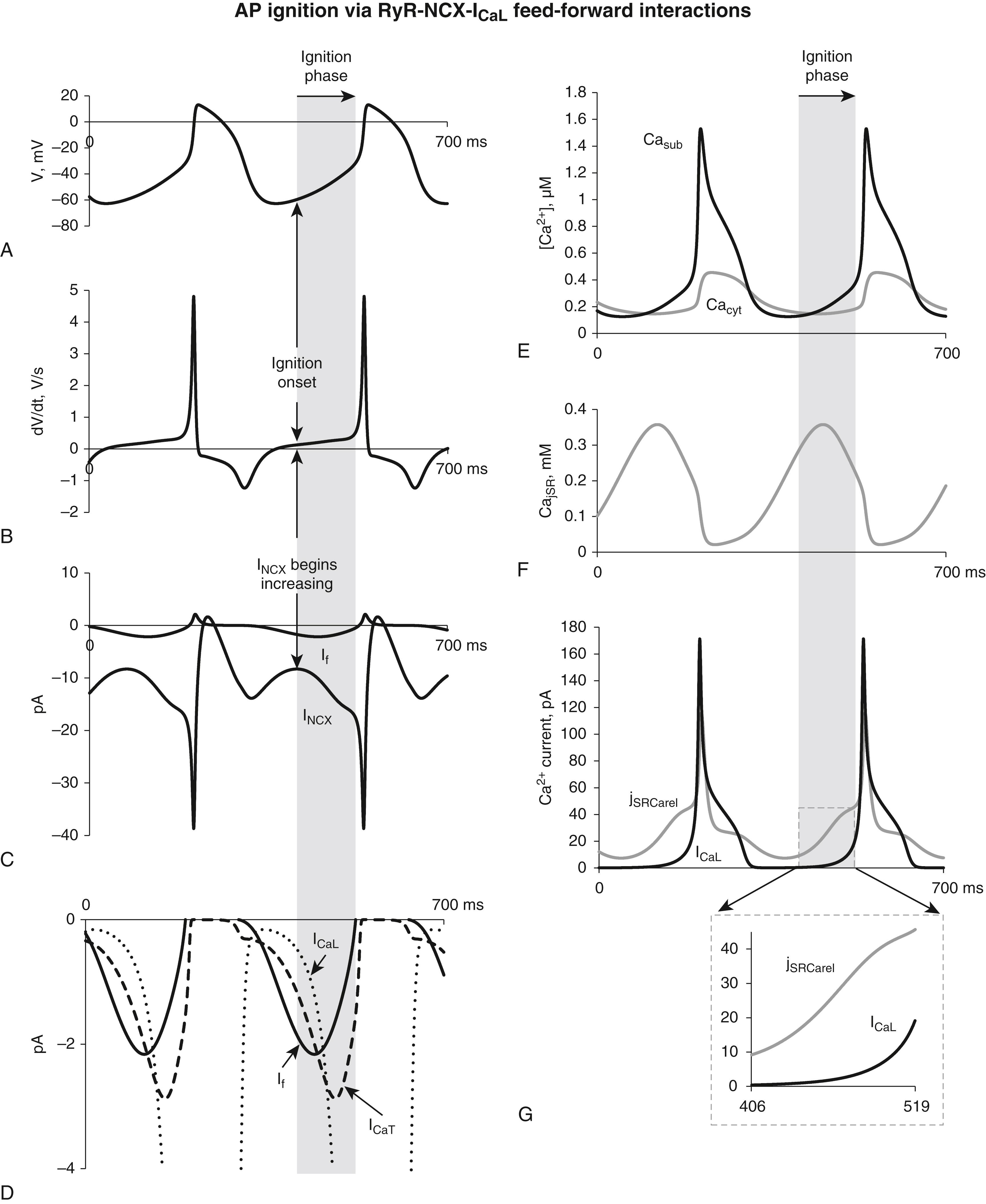
Because Ca and membrane mechanisms tightly interact within the coupled-clock system, a selective perturbation of either clock indirectly affects the other clock and the resultant AP firing rate of the system. The LCR period and AP cycle length (APCL) shift toward longer times almost equally by either direct perturbations of membrane clock (by ivabradine, a selective I f inhibitor) or Ca clock (by cyclopiazonic acid, a selective SERCA inhibitor), indicating that the LCR period reports the crosstalk between the clocks. In the case of ivabradine-induced bradycardia, the initial effect on the membrane clock reduces the rate of I CaL activation, and respective Ca influx and SR Ca loading. Within the novel concept of AP ignition, in which LCRs interact with NCX and I CaL , this important result can be recast in the new term of the time-to-ignition-phase (when I NCX becomes activated by LCRs), which is determined by clock interactions and plays a key role in determining the cycle length. Thus any selective perturbation of either clock indirectly (but inevitably) affects the other clock, leading to respective shifts in the time-to-ignition phase and the resultant AP firing rate of the system (tested with ivabradine, cyclopiazonic acid, and ryanodine ).
The complex feed-forward control of AP ignition features fail-safe, robust operation with substantial reserve margin. For example, a severe knock down of 80% of NCX molecules in genetically modified mice does not affect the resting heart rate. This result was initially interpreted to indicate that NCX is not important for resting heart automaticity. Further numeric analysis of the coupled-clock system revealed, however, that I NCX is crucial for resting heart automaticity, and even 20% of the remaining NCX molecules is sufficient to keep the diastolic I NCX amplitude almost unchanged, with the ignition mechanism remaining intact. This regulation is based on the local nature of the diastolic Ca release and CICR. In short, LCRs occur only in a part of the cell under normal conditions, and a substantial portion of NCX molecules are not activated during DD; some remain in reserve. NCX-deficient cells exhibit a compensatory increase in the spatial extent of the LCR ensemble that, in turn, activates almost all remaining NCX molecules. The I NCX preservation, however, comes at a cost: NCX molecules become fully activated under resting conditions, leaving no reserve to support a further I NCX increase and hence AP firing rate increase, thus explaining the lack of fight-or-flight SAN activity in the NCX-deficient mice.
The rapid depolarization during the AP upstroke mainly informs on the availability of Ca V 1.2 channels for activation by the acute depolarization (see Fig. 25.1 , top ). The greater the availability of channels to respond to a membrane voltage change prompt is, the greater dV/dt of the AP upstroke. The resultant Ca influx, via activated I CaL , partially binds to RyRs and synchronizes their activation via CICR, resulting in synchronous RyR Ca release that generates the APCT (see Fig. 25.1 , bottom ), depleting Ca in the SR. LCCh begins to inactivate with time at the depolarized membrane potential. The APCT and Ca influx via LCCh activate SERCA2 to pump Ca into the SR, and the kinetics of pumping allow efficient and timely recharging of Ca store.
Membrane depolarization activates K channels and inactivates forward Na-Ca exchange. AP repolarization kinetics inform on the combined actions of K channels, cytosolic [Ca], I NCX , and I f . APCT also activates Ca-dependent K channels, assisting in cell membrane repolarization, even prior to achieving the full surface membrane repolarization (i.e., MDP) and full I f activation. Time from AP upstroke to MDP is regulated by the aforementioned ensemble of molecular activation and inactivation.
Interestingly, membrane ion channels and transporters regulate the Ca balance of the coupled system not only directly, via impact on Ca influx/efflux of LCCh/NCX, but also indirectly, such as by I K activation. This repolarizes the AP, limiting Ca influx of LCCh and simultaneously increasing Ca efflux via voltage-dependent activation of the NCX forward mode, which assists in removing the Ca of APCT. Even I f activation regulates cell Ca balance by limiting the MDP, limiting voltage-dependent Ca efflux via NCX.
Previous studies of abnormal automaticity had demonstrated an important role of Na in pacemaker cell function. , Based on the coupled-clock concept, and especially the key role of NCX, the coupling of Na and Ca electrochemical driving forces (V m -E Na and V m -E Ca , respectively) can be envisioned as the major regulator of clock coupling. This idea has been recently tested in SAN cells perturbed by digitonin, which blocks Na/K ATPase and leads to Na accumulation in the cell. Initial increases in [Na] i and [Ca] i and respective reductions in E Na and E Ca in response to digitonin led to a small reduction in MDP, enhanced LCRs, and reduced average APCL. As [Na] i and [Ca] i continued to increase, MDP, E Na , and E Ca were further reduced; LCR signals were reduced, and APCL was progressively prolonged. This was accompanied by increased APCL variability. Numeric modeling of this process revealed that as [Na] i increased, the E Na and E Ca became monotonically reduced and ion currents (I CaL , I CaT , I f , I Kr , and I bNa ) decreased. In parallel with the biphasic APCL changes, the diastolic I NCX also manifested biphasic changes in response to Na/K ATPase inhibition: An initial I NCX increase attributable to enhanced LCR ensemble Ca signal was followed by I NCX reduction as E NCX (E NCX = 3E Na − 2E Ca ) decreased. Thus Na plays a key modulatory role in clock coupling via modulation of LCRs, NCX, and basically all membrane ion currents.
RyRs are organized and operate in clusters, known as Ca release units in cardiac cells, including SAN cells. Biophysical measurements of LCRs and numeric modeling of their collective behavior have demonstrated that LCRs emerge via synchronization of activation of Ca release units leading to oscillatory, phase-like transitions in SAN cells. More recent theoretical studies , of collective firing of individual RyRs within Ca release units, have also demonstrated phase transitions (including order-disorder transitions), pointing to fractal-like functional and structural (hierarchical) organization of RyR assemblies in SAN cells (i.e., within and among Ca release units).
Based on these results and the importance of Na (described earlier), the ignition theory and local interactions during DD can now be recast in the new terms of phase transition, criticality, and electrochemical gradient oscillations. As we mentioned, LCRs begin to occur prior or around the time of the MDP. These early release events, however, are small and stochastic (i.e., disordered) in nature, like sparks in ventricular myocytes at rest. Subsequent simultaneous growth of the diastolic ensemble LCR signal and DD during AP ignition can be envisioned as manifestations of a self-organization of the system of local oscillators interacting with excitable cell membrane that reaches criticality in late diastole. This is followed by a phase transition manifest as the rapid AP upstroke that feeds forward Ca to trigger an APCT.
Spontaneous AP cycles result from spontaneous electrochemical gradient oscillations. In other terms, emergence and self-organization of LCRs at a critical time of an AP cycle (diastole) entrains a membrane depolarization, initiating an electrochemical gradient oscillation within the system that accelerates to achieve criticality in late diastole, culminating in the rapid AP upstroke. In this regard, late diastole might be considered to be an intrinsic entrainment zone or time window in which Ca and membrane oscillators critically interact each cycle and become mutually entrained over many cycles, controlling the rate and rhythm of AP firing of that cell.
Although Ca is mainly entered in SAN cells via Ca influx through I CaL , store-operated Ca entry (SOCE) via store-operated Ca channels may represent an additional pathway for Ca entry on Ca store depletion. Studies in mouse SAN discovered store-operated activity that may be attributable to TRPC expression and suggested that store-operated Ca channels are involved in regulating pacemaker firing rate. A study by Liu and coworkers investigated the role of two new proteins involved in SOCE, stromal interacting molecule (STIM), which is an endoplasmic reticulum Ca sensor, and the surface membrane channel Orai, a prototypic gene encoding for SOCE. They found that both STIM and Orai are expressed in pacemaker cells and that after store depletion, STIM1 is redistributed to the cell periphery and colocalized with surface membrane-linked Orai1, suggesting involvement of these proteins in SOCE activity and cardiac pacemaker function. Transient receptor potential canonical-3 (TRPC3) channel is involved in SOCE mechanism, and recent evidence obtained in TRPC3(−/−) mice suggests that Ca entry through this channel can be linked to sinoatrial arrhythmias.
In addition to RyR, another type of SR Ca release channel, the inositol 1,4,5-trisphosphate receptor (IP3R), can also contribute to automaticity of SAN cells. Pharmacologic perturbations of these channels were effective in wild-type mice, but not in transgenic IP3R2 knockout mice. Stimulation of IP3Rs accelerates the spontaneous beating rate in isolated mouse SAN cells, and their inhibition slows the rate. In NCX knockout mice, in which the Ca clock and the membrane clock are uncoupled, IP3R agonists and antagonists modulated the rate of spontaneous Ca waves, suggesting that IP3R-mediated Ca release modulates the Ca clock operation.
Other new players include Ca-activated K channels. In spontaneously beating cells derived from human embryonic stem cells, IK(Ca) inhibition of small K′′SK4 Ca-activated K channels, generating IK(Ca), resulted in MDP depolarization and pacemaker suppression. All three SK isoforms (SK1, SK2, and SK3) were identified in mouse SAN. Inhibition of SK channels with apamin prolonged APs in isolated SAN cells, slowed DD, and reduced pacemaker rate in isolated SAN cells and intact SAN tissue. It was proposed that (1) these channels modulate pacemaking via activation of a repolarizing current activated by Ca, and (2) this modulation could be used to treat SAN dysfunctions, particularly those linked to Ca overload. Intraperitoneal injection of the SK4 channel blockers greatly reduced the arrhythmic features of CASQ2-D307H knock-in mice and CASQ2 knockout mice at rest and during exercise, demonstrating a role of SK4 Ca-activated K channels in pacemaker function and making these channels promising therapeutic targets for the treatment of cardiac arrhythmias, such as catecholaminergic polymorphic ventricular tachycardia. Because SR Ca release is both necessary and sufficient for SK channel activation, it is possible that SK channels directly interact with the Ca clock.
Large-conductance Ca- and voltage-activated K channels (BK channels, also known as maxi-K channels) also seem to play a prominent role in pacemaker function. Although the heart rate was substantially reduced by BK channel inhibitors in wild-type mice, it remained unchanged in BK channel knockout (Kcnma1[−/−]) mice. AP firing rate in isolated mouse SAN cells was substantially reduced (from 28% to 55% by different BK channel inhibitors). Baseline AP firing rates in SAN cells isolated from Kcnma1(−/−) mice were 33% slower than that in wild-type SAN cells, and this effect was caused by prolongation of the DD phase.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here