Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Peter Gilbert, PhD
Professor
Biostatistics
Fred Hutchinson Cancer Center, Seattle
Washington
United States
Research Professor
Biostatistics
University of Washington, Seattle
Washington
United States
The determination of a correlate of protection (CoP) by a vaccine is often critical to its development, as its measurement permits a number of both use Peter B. Gilbert theoretical and practical extrapolations. Aside from the intellectual interest in identifying the immune response that is protective, knowledge of a CoP permits measurement of consistent lot potency of a vaccine and differentiation of susceptible from immune individuals. If an infection is uncommon or deadly, such that an efficacy trial is not feasible or ethical, a CoP may yet enable licensure of a vaccine, and once established, a CoP will allow bridging from one vaccine preparation to another.
The terminology in this field is confusing, as various definitions have been published, but semantics are important and confusion will be avoided if the definitions used are understood. Table 4.1 gives the terminology used in this chapter and is crucial to its understanding. Whereas a CoP is simply a variable immune response that is statistically associated with protection, the important distinction is between a mechanistic correlate of protection (mCoP) that is directly responsible for protection and a nonmechanistic correlate of protection (nCoP) that is easy to measure but which may only be a substitute for a mCoP that is unknown or difficult to measure. In addition, a CoP may be absolute, in the sense that there is a threshold value above which protection is certain, or relative in the sense that higher values are quantitatively more protective than lower values but there are occasional failures even at high levels and occasional protection at lower levels. Last, and importantly, there are cocorrelates, meaning that more than one immune function induced by a vaccine correlates with protection in an additive or synergistic way. The reader who wishes to learn more on this subject is referred to previously published articles.
| Term | Definition |
|---|---|
| Correlate of protection (CoP) | An immune response that is statistically correlated with protection |
| Mechanistic correlate of protection (mCoP) | The immune response that is responsible for protection |
| Nonmechanistic correlate of protection (nCoP) | An immune response that substitutes for the true mechanistic correlate of protection, which may be unknown or not easily measurable |
| Absolute correlate | A specific level of response highly correlated with protection; a threshold |
The determination of a CoP is sometimes established early in vaccine development, which is the ideal circumstance in that it makes later vaccine development easier, but sometimes Phase III trial analyses are necessary to identify the CoP. In the latter case, immune responses of vaccine failures are compared with a sample of subjects who did not become infected. Ideally, the immune responses would be measured at the time of exposure to the infection, but most often samples are available only shortily after vaccination. Nevertheless, a useful CoP is often extracted from those data. Other ways in which CoP is inferred include meta-analysis of a series of Phase III trials correlating average immune responses with overall vaccine efficacies, the definition of protective levels of passive antibody given parenterally or acquired by an infant from its mother through the placenta, observations made on vaccinated immunodeficient or immunosuppressed individuals exposed to the infection, challenge of vaccinated volunteers by the agent in question, and rarely by extrapolation from the challenge of vaccinated animals. Human challenge models have often given critical information about CoP and should be employed if the challenge is sufficiently attenuated to be ethically acceptable and if a broad range of immune responses is studied. Examples of human challenges that have yielded important information include influenza, cholera, dengue, and cytomegalovirus.
Chapter 2 of this book describes the immunology surrounding vaccination, but the point to bear in mind regarding CoP is that many different functions may serve as a CoP. Serum and mucosal antibodies come in different isotypes and functionality, and T cells, whether CD4 + or CD8 + , can act in a variety of ways, including direct action on infected cells, help to B or other T cells, secretion of cytokines, and even downregulation of immune responses by T regulatory cells. This chapter seeks to simplify immunology in order to identify principles of protection, although the reader should keep in mind that the immune system is complex and redundant. In addition, the reader is directed to chapters on specific vaccines for more extended analysis of each case.
Protection against infection may relate to different immune markers than protection against disease. Polio is an example in which paralysis can be prevented by serum antibodies, as the virus must pass from the pharynx or intestines to the central nervous system via blood, whereas infection is prevented by antibodies at the mucosal level, either locally produced immunoglobulin (Ig) A antibodies or diffusion of IgG antibodies onto the mucosal surfaces of the nasopharynx and intestine. Another instructive example is pneumococcal infections. Prevention of bacteremia can be mediated by antibodies in the range of 0.20–0.35 µg of antibody as measured by enzyme-linked immunosorbent assay (ELISA). However, prevention of pneumonia, otitis media, and nasopharyngeal carriage may require levels 10 times higher. ,
The level of antibodies that protect against measles has been defined. A level of 120 mIU of antibody measured by ELISA indicates protection against clinical measles and infection. A level between 200 and 1000 mIU confers protection against measles disease, but not always against infection as indicated by antibody rises; a level of 1000 mIU does provide protection even against subclinical infection. However, vaccinated B-cell–deficient humans do recover from disease, whereas T-cell–deficient humans may suffer continued viremia leading to serious and fatal measles. It has been shown in monkeys that the CD8 + T cells specific for measles are required to suppress viremia.
The poxviruses provide another example. An individual given vaccinia virus requires both B and T cells to overcome the replicating virus and to become immune. However, in a previously vaccinated person, only neutralizing antibodies at levels between 1/20 and 1/32 are required for protection, although CD8 + T-cell responses are helpful in the absence of antibodies. Inasmuch as antibody titers decline by 20 years postvaccination, the presence of antipoxvirus T cells enables the vaccinee to have a mild secondary infection in case of exposure.
Vaccination against poliomyelitis is usually highly effective in the prevention of paralysis, as stated above, because of the induction of serum antibody. However, gut immunity is imperfect and can be overcome by large infectious doses. A study challenged subjects previously given live vaccine and killed vaccine with two different doses of the oral live vaccine: 800 or 600,000 TCID 50 (median tissue culture infective dose). The live vaccine recipients were infected by the low-dose challenge 3% of the time and by the high-dose challenge 15% of the time, whereas 30% of the killed vaccine recipients could be infected by a low dose and 70% by a high dose, indicating that high challenge doses can overcome intestinal immunity.
Another challenge study involved an experimental cytomegalovirus vaccine. Seronegative, naturally seropositive, and previously vaccinated subjects were challenged parenterally with 10, 100, or 1000 PFU of a low-passage natural strain of cytomegalovirus. Seronegative subjects were infected with the lowest dose, whereas naturally seropositive subjects were resistant to 10 or 100 PFU; however, some could be infected by 1000 PFU. The vaccine prevented infection by 100 PFU of the challenge virus, but not by 1000 PFU.
Table 4.2 lists the commonly used licensed vaccines and their predominant mechanisms for causing disease. Many viral and bacterial agents reach target organs through viremia or bacteremia; consequently, it is easy to understand that antibodies can prevent that passage. Some agents replicate only on the mucosa, but there, too, the local presence of antibody is preventive. The pathogenesis of toxin-producing bacteria such as diphtheria and tetanus can be restricted by antitoxic antibodies. In the case of rabies, replication occurs in the subcutaneous tissue before attachment to neurons, and elicitation of antibodies before that attachment prevents rabies. Only in the cases of zoster and tuberculosis vaccines does it appear that antibodies are not the primary mechanism of control, but that stimulation of specific T-cell subsets correlate with protection. Aside from these theoretical arguments, the fact is that passive administration of antibody works for many infections also preventable by vaccination. Indeed, the efficacy of hepatitis A vaccine was predictable from the fact that induced antibody levels are a thousand times higher than those shown to protect after administration of gamma globulin. As mentioned under Principle 1, higher levels of antibody are necessary to prevent mucosal colonization than disease, for example, in the case of pharyngeal colonization after Haemophilus influenzae type b vaccine.
| Viral | |
|---|---|
| Viremia | Smallpox, yellow fever, measles Mumps, rubella, polio, varicella Hepatitis A, hepatitis B Japanese encephalitis, tickborne encephalitis |
| Mucosal replication | Influenza, rotavirus, human papillomavirus |
| Neuronal invasion | Rabies |
| Neuronal reactivation | Zoster |
| Bacterial | |
| Bacteremia | Haemophilus influenzae type b, Meningococcal, Pneumococcal, Typhoid (Vi) |
| Mucosal replication | Pertussis, typhoid (Ty 21a) |
| Toxin production | Diphtheria, tetanus, pertussis Cholera, anthrax |
| Macrophage replication | Tuberculosis |
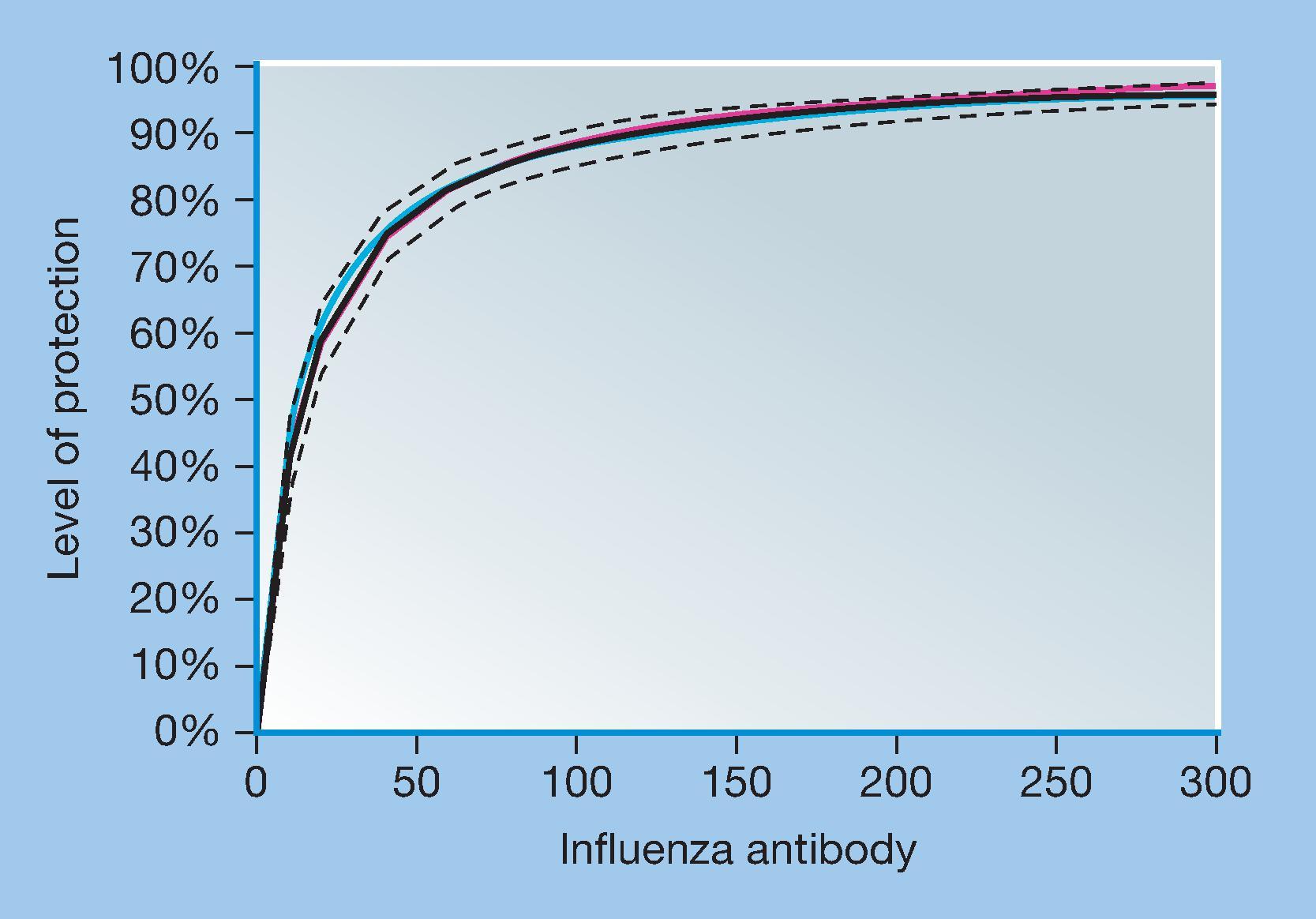
Although high levels of antibodies are more protective than lower levels, after some vaccines breakthroughs may occur at high levels, even if less frequently than at lower levels. Fig. 4.1 shows a retrospective analysis of the relationship between antibody responses to influenza hemagglutinin and protection against disease. At an anti-HA (hemagglutinin) titer of 1/40, generally thought to be an acceptable response, only approximately 50% of vaccine recipients are protected; even at titers four times higher, breakthrough infections occur. Similarly, high levels of pertussis antitoxin after immunization was related to protection both in household exposures and nonhousehold exposures, but mild disease occurred at intermediate levels of antibody. Owing to greater exposure, more antibody was required to prevent infection within households than outside households ( Table 4.3 ).
| Symptoms | Exposure | |||
|---|---|---|---|---|
| Household | Nonhousehold | |||
| Severe | 79 U/mL | 99 U/mL | ||
| Mild | 156 U/mL | 124 U/mL | ||
| None | 246 U/mL | 155 U/mL | ||
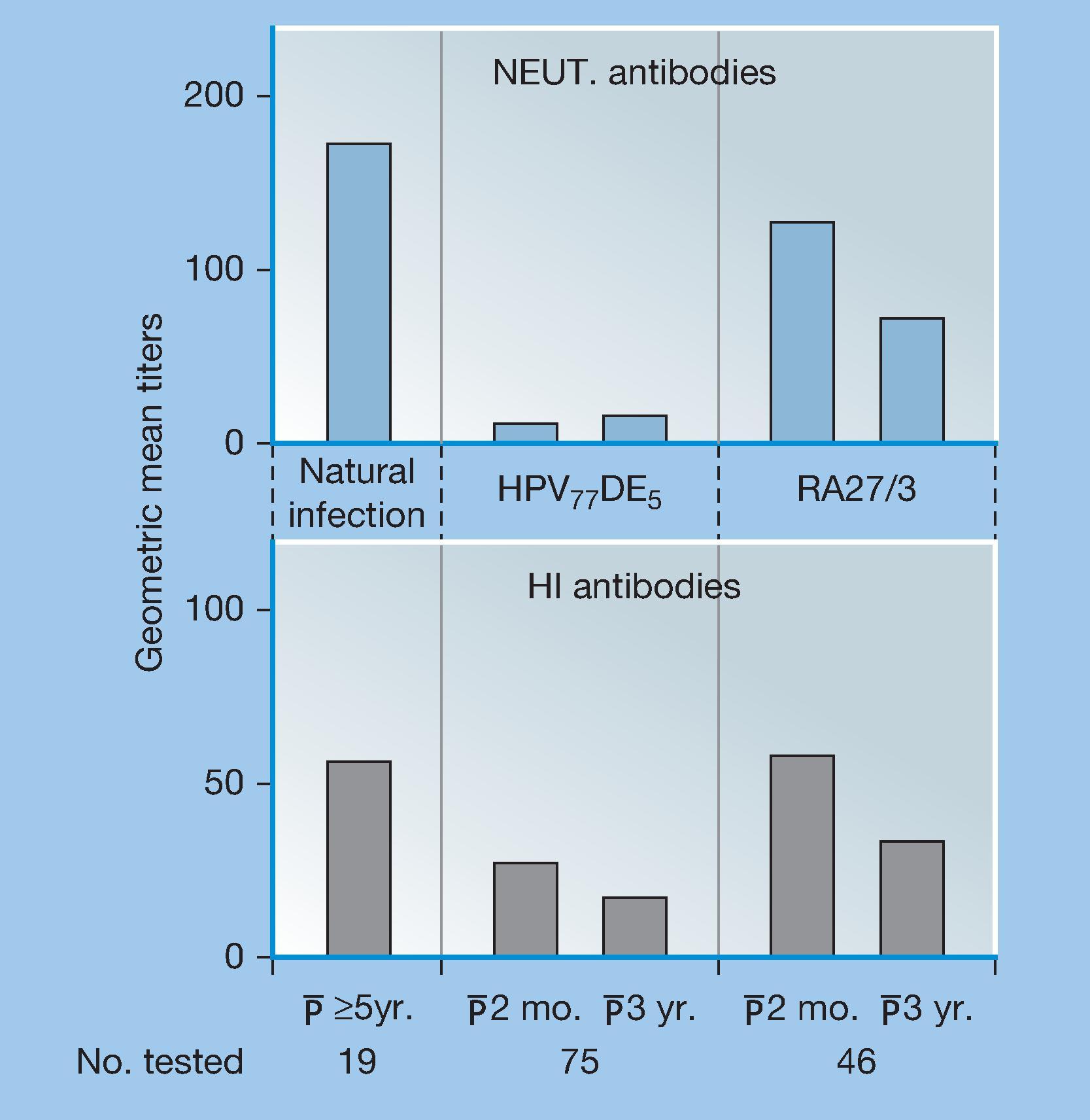
Although neutralization of viruses and killing of bacteria are often mCoP, it has been increasingly realized that there are other important antibody functions. First, Fig. 4.2 shows a case in which neutralization was definitely important, where titers were measured by neutralization or by hemagglutination-inhibition after vaccination with two different strains of rubella vaccines. As can be seen, both strains induced hemagglutination inhibiting antibodies, but RA27/3 stimulated considerably more neutralizing antibodies and proved to be the more protective vaccine.
A study of meningococcal Group C polysaccharide vaccine conducted in Quebec showed the difference in function between antibodies measured by ELISA or by bacterial killing. Adults produced both types of antibodies and were highly protected; children 2 to 5 years old produced more antibodies and had moderate protection; and 1-year-old infants generated ELISA but little bactericidal antibody and had no protection.
A striking example of the importance of antibody function has been reported after an HIV vaccine trial in Thailand. A regimen of priming with a canarypox vector presenting the viral envelope followed by a boost with envelope protein generated little neutralizing antibody but did generate significant levels of antibody-dependent cellular cytotoxic antibody that potentiates natural killer cell activity. IgG3 antibody-dependent cell-mediated cytotoxicity (ADCC) antibodies directed against the V1-V2 loops of virus were shown to correlate with protection. This illustrated that there are many mechanisms by which antibody may protect.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here