Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Abnormalities in the carbohydrate, lipid, and lipoprotein metabolism can significantly affect the structure and function of the cornea.
Treatments addressing the mechanisms of disease offer promising treatment for diabetic patients through recovery of corneal sensation and tear production.
The ocular manifestations of dyslipoproteinemias might not affect vision significantly, but rather provide the first clue for a genetic systemic disease and act as an alert to potential risk for coronary disease that warrants medical and laboratory investigation.
Hyperlipoproteinemias, in general, are associated with accelerated bilateral corneal arcus and xanthelasma, whereas diffuse bilateral opacification with or without corneal arcus is a feature of hypolipoproteinemias.
The corneal manifestations of drug-induced lipidosis are important, as they are often dose related and may reflect the potential risk for retinal changes.
The introduction of oral and topical cysteamine has revolutionized the treatment and prognosis of cystinosis, but new therapeutic options are being investigated.
Genetic therapy and stem cell research shed light onto promising therapeutic options in many of the metabolic diseases.
The cornea together with the sclera forms the outer shell of the eyeball. The cornea not only serves as the major refracting structure for the light entering the eye, but with its epithelial barrier, plays a major role in the ocular biodefense system. Maintenance of the corneal shape and transparency is of paramount importance. The optical properties of the cornea are determined by its transparency, surface smoothness, contour, and refractive index. The absence of corneal blood vessels and the regular arrangement of the stromal collagen fibers play an important role in the transparency of the cornea. The uniform arrangement and the continuous slow production and degradation of the stromal collagen fibers are essential for corneal transparency.
The corneal stroma is made of extracellular matrix, keratocytes, and nerve fiber bundles. The extracellular matrix, made up of collagen (type I) and glycosaminoglycans (GAGs), makes up the bulk of the corneal stroma with the cellular components contributing to only 2%–3% of the total stromal volume. Not only are the collagen fibers in the corneal stroma highly uniform, but also the distance between them is highly uniform. Several GAG are present among the stromal collagen fibers. The most abundant GAG is keratan sulfate, which constitutes about 65% of the total GAG content. The remaining GAG includes chondroitin sulfate and dermatan sulfate. Although the corneal hydration is predominantly controlled by the endothelial pump, it is also influenced by the intraocular pressure, stromal swelling pressure, and the epithelial barrier function. The GAG content of the stroma plays a substantial role in this homeostatic process. Metabolic changes intrinsic to the cornea or from secondary involvement due to a systemic disorder can lead to abnormal accumulation of storage products within the cornea, which can interfere with both the clarity and the function of the cornea. Abnormalities in the carbohydrate, lipid, and lipoprotein metabolism can significantly affect the structure and function of the cornea. Lysosomal storage disorders, of which over 40 are now known, are caused by the defective activity of lysosomal proteins that result in the intralysosomal accumulation of undegraded metabolites. In this chapter, we summarize the major systemic metabolic diseases that affect the cornea.
Diabetes mellitus (DM) is a syndrome of chronic hyperglycemia due to relative insulin deficiency, resistance, or both. An estimate of the World Health Organization, found that by 2014 there were 422 million adults with this condition worldwide, compared to 108 million in 1980. This corresponds to a prevalence increase from 4.7% to 8.5%. This prevalence increase has been faster in low and middle-income countries than in high-income countries.
As the overall prevalence of diabetes is expected to continue increasing, so are the volume of patients who will present ocular involvement. Diabetic keratopathy (DK) is the second most common ocular pathology after diabetic retinopathy. Several ultrastructural and functional changes occur in a diabetic cornea resulting in DK, which encompasses a clinical spectrum including superficial punctate epitheliopathy, persistent epithelial erosions, corneal hypoesthesia, nonhealing persistent epithelial defects (PEDs), and corneal edema ( Fig. 58.1 ). The reported prevalence of DK is around 70% among diabetic patients.
The multiple ultrastructural changes in the cornea caused by DM that occur before DK manifests clinically are outlined next. Epithelial abnormalities: delayed wound healing, compromised barrier function, , abnormalities in shape of the epithelial cells, PEDs, recurrent erosions, epithelial fragility, edema, ulceration, low cell density, stem cell dysfunction, increased autofluorescence, and basement membrane thickening. Immunologic abnormalities: dendritic cell accumulation, increased polymorphonuclear cells and proinflammatory cytokines with increased inflammatory response. Stromal abnormalities: abnormal collagen bundles, increased corneal thickness and optical density, stromal edema. Endothelial abnormalities: decreased cell density, , cell pleomorphism. Tear film abnormalities include low tear secretion, decreased tear film thickness and stability, and increased tear osmolarity. Biomechanical: increased corneal response factor.
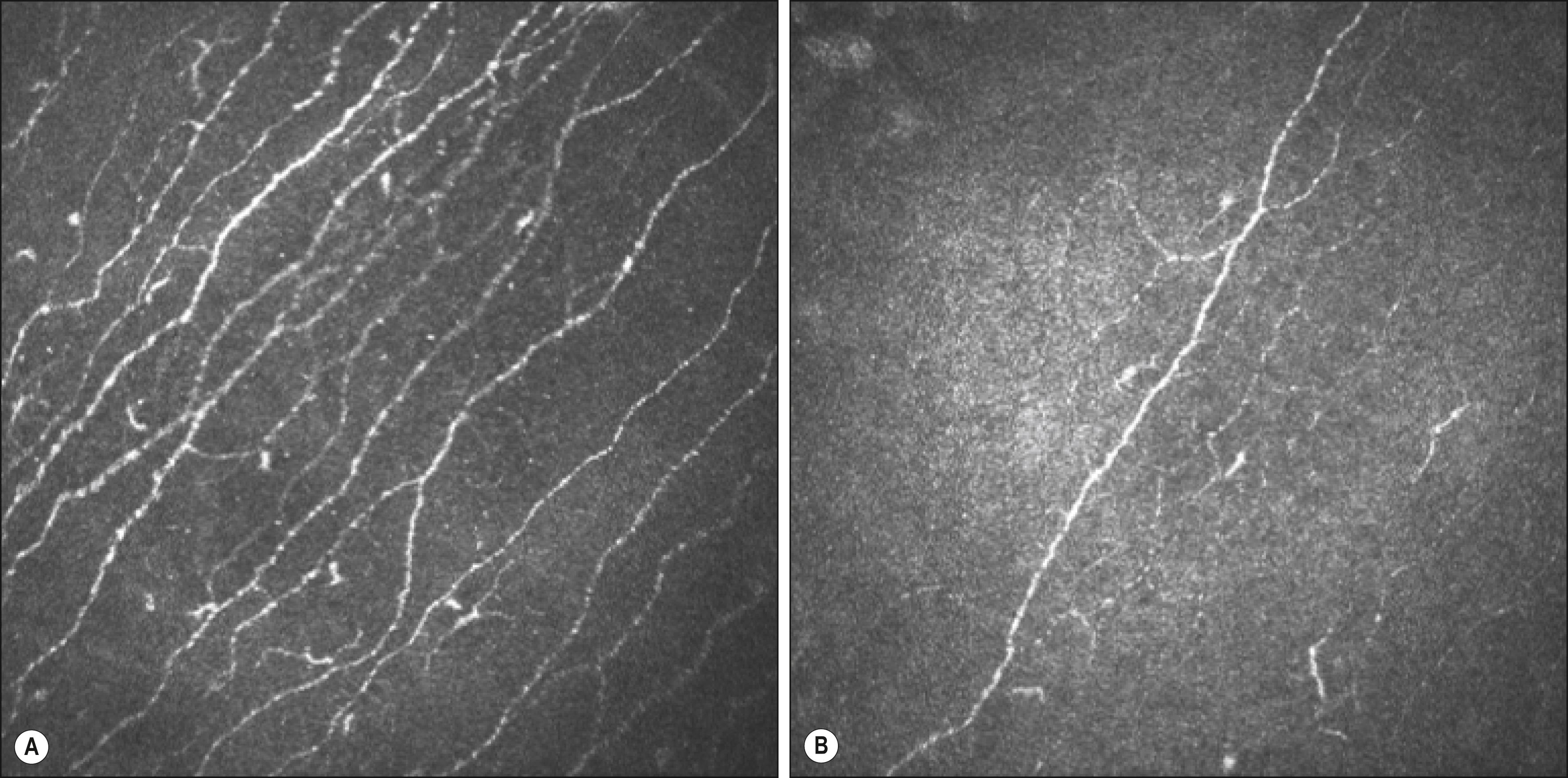
Neuropathic changes include decreased sensitivity, , decreased subbasal nerve fiber and branch density, , delayed nerve regeneration after injury, increased stromal nerve thickness and tortuosity. , Confocal studies show reduction in the number of corneal nerve bundles, reduction in the density of nerve fibers suggestive of enhanced degeneration, and reduction in the branching of nerve bundles suggestive of limited regeneration ( Fig. 58.2 ). , These confocal changes seem to precede the impairment of corneal sensitivity when measured clinically, and they also relate to the measures of somatic neuropathy in diabetic patients. A decreased supply of trophic neuropeptides, secondary to corneal nerve loss, is followed by a prolonged healing time. Alterations in corneal innervation affecting its sensitivity can lead to dry eye and neurotrophic keratitis, and can potentially have serious visual consequences.
The epithelium has been widely studied regarding underlying molecular diabetic corneal changes. The epithelial basal membrane has been found to have increased fragility, a decreased number of hemidesmosomes, altered adhesion to the epithelial layer, and delayed reassembly after injury. , , , This correlates with the reduced immunostaining of some of its molecular components (laminins and integrins). , The higher level of the proteolytic enzymes matris metalloproteinase-10 (MMP-10) and cathepsin F suggests that this fragility could also be due to tissue degradation. ,
The role of different growth factors has also been studied in this scenario. Findings suggest high levels of insulin-like growth factor (IGF-1) that mediate epithelial cell migration and survival, hepatic growth factor involved in proliferation migration and apoptosis, opioid growth factor—a negative mediator of epithelial proliferation —and Thymosin β4, a mediator of cell migration and regeneration that promotes reepithelialization.
DK has long been underdiagnosed, and most patients that live with this condition are treated for dry eye with symptomatic management (artificial tears, autologous serum, bandage contact lenses, tarsorrhaphy). Recent therapeutic approaches include silicone hydrogel CLs, which provide an epalrestat (aldose reductase inhibitor) release platform.
Insulin drops are one of the proposed therapeutic approaches, since they have been shown experimentally to accelerate corneal wound healing and prevent loss of subbasal nerves. , Several clinical studies also support its positive effect in corneal wound healing. IGF-1 and substance P, a neuropeptide present in sensory nerves, have been shown to accelerate corneal epithelial wound healing as well. Nerve growth factor (NGF) has been proven to reduce inflammation and apoptosis in the diabetic cornea. A recombinant form of NGF, cenegermin (Oxervate) was recently approved for neurotrophic keratitis treatment and is available as eye drops and was granted approval by the European Commission. Other factors under investigation are glial cell-derived neurotrophic factor (GDNF) and ciliary neurotrophic factor (CNTF). These have also demonstrated the ability to stimulate corneal epithelial cell proliferation and migration, and to enhance their immune-protective function.
Gene therapy using adenovirus RNA can accelerate epithelial wound healing. 45,63–65 Adenovirus RNA targets over expression of c-met proto-oncogene and silences MMP-10 and cathespin F, increasing integrin expression in the epithelial basal membrane.
Stem cell approach is another appealing treatment option. The limbal epithelial stem cells in diabetic corneas are known to be dysfunctional. Gene therapy targeting these cells can potentially restore their function once the treated cells are transplanted. , Bone marrow-derived mesenchymal cell transplantation may also suppress excessive inflammatory responses, activating endogenous corneal progenitor cells, promoting alternative polarization of infiltrated macrophages, and enhancing repair of diabetic corneal wounds.
Recently a new type of matrix therapy agent (ReGeneraTing Agent [RGTA]) has provided encouraging results in the treatment of neurotrophic ulcers and PEDs. A topical RGTA agent (Cacicol, Thea, France) has recently obtained CE marking for the treatment of neurotrophic ulcers and PED. Cacicol is a structural analog of GAGs such as heparan sulfate (HS). Topical application replaces the degraded HS, thereby protecting the matrix proteins from further proteolysis and producing a suitable environment for the recruitment of the necessary growth factors for corneal stromal repair.
There are at least 50 different lysosomal storage disorders (LSD), which include different genetic conditions that occur secondary to the accumulation of specific substrates, usually due to deficiency of a lysosomal enzyme. Lysosomes are membrane-bound organelles that consist of limiting external membrane and intralysosomal vesicles. The different lysosomal enzymes break down all types of biologic molecules including proteins, nucleic acids, lipids, and carbohydrates. Mutations in the genes that encode any of these proteins can lead to LSDs ( Fig. 58.3 ).
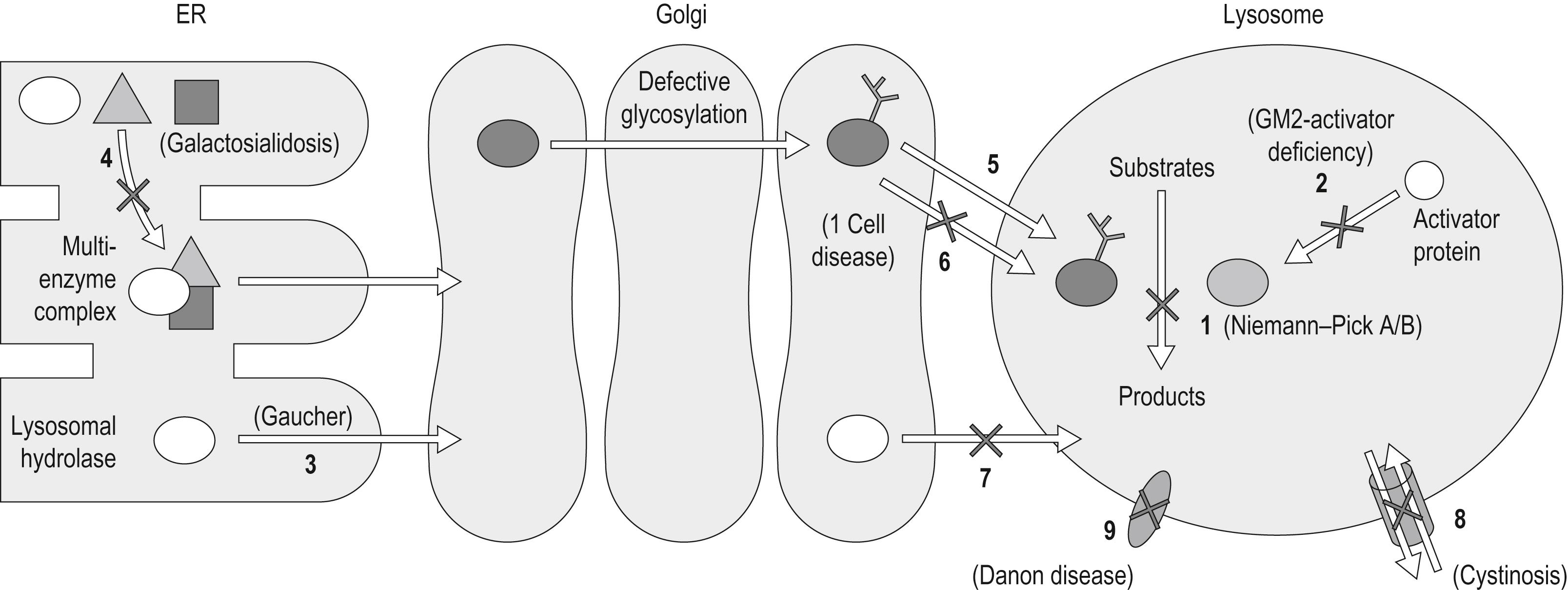
LSDs can be grouped according to various classifications, but perhaps the most useful is based on the characterization of the defective enzymes or protein, rather than of the nature of the accumulated substrate(s) ( Table 58.1 ). Classification of many LSDs can still be made based on the nature of the substrate that accumulates. For example, in mucopolysaccharidoses (MPS), GAGs accumulate due to the impaired function of the lysosomal enzymes, which are required for the sequential degradation of GAGs. In sphingolipidoses, unmetabolized sphingolipids accumulate due to defective enzyme activity, and in oligosaccharidosis, oligosaccharides accumulate. In some cases, because of a common determinant, the deficiency of a single enzyme can result in the accumulation of different substrates.
| Disease | Defective Protein | Storage Material | Screening Test | Diagnostic Test |
|---|---|---|---|---|
| Mucopolysaccharidoses (MPS) | ||||
| MPS I (Hurler, Scheie, Hurler/Scheie) | α-iduronidase | Dermatan and heparan sulfate | Urine GAGs | WBC enzyme assay |
| MPS II (Hunter) | Iduronate-2-sulfatase | Dermatan and heparan sulfate | Urine GAGs | Plasma enzyme assay |
| MPS IIIA (Sanfilippo) | Heparan N -sulfatase (sulfamidase) | Heparan sulfate | Urine GAGs | WBC enzyme assay |
| MPS IIIB (Sanfilippo) | N -Acetyl-α-glucosaminidase | Heparan sulfate | Urine GAGs | Plasma enzyme assay |
| MPS IIIC (Sanfilippo) | Acetyl-CoA, α-glucosamide N -acetyltransferase | Heparan sulfate | Urine GAGs | WBC enzyme assay |
| MPS IIID (Sanfilippo) | N -acetylglucosamine-6-sulfatase | Heparan sulfate | Urine GAGs | WBC enzyme assay |
| MPS IV (Morquio) IVA | N -acetyl galactosamine-6-sulfatase | Keratan sulfate | Urine GAGs | WBC enzyme assay |
| MPS IV (Morquio) IVB | β-galactosidase | Keratan sulfate | Urine GAGs | WBC enzyme assay |
| MPS VI (Maroteaux–Lamy) | N -acetylgalactosamine-4-sulfatase | Dermatan sulfate | Urine GAGs | WBC enzyme assay |
| MPS VII (Sly) | β-glucuronidase | Heparan, dermatan, and chondroitin sulfate | Urine GAGs | WBC enzyme assay |
| MPS IX (Natowicz) | Hyaluronidase | Hyaluronic acid | None | Cultured cells |
| Sphingolipidoses | ||||
| Fabry disease | α-galactosidase A | Globotriaosylceramide and blood group B substances | None | WBC enzyme assay |
| Farber lipogranulomatosis | Ceramidase | Ceramide | None | WBC enzyme assay |
| Gaucher disease | β-glucosidase, saposin C activator | Glucosylceramide | None | WBC enzyme assay |
| Niemann–Pick A and B | Sphingomyelinase | Sphingomyelin | None | WBC enzyme assay |
| GM1 gangliosidosis | β-glucosidase | GM1 ganglioside | Urine oligosaccharides | WBC enzyme assay |
| GM2 gangliosidosis (Tay–Sachs) | β-hexosaminidase A | GM2 ganglioside and related glycolipids | None | WBC enzyme assay |
| GM2 gangliosidosis (Sandhoff) | β-hexosaminidase A and B | GM2 ganglioside and related glycolipids | None | WBC enzyme assay |
| GM2 gangliosidosis (Gm2 activator deficiency) | GM2-activator protein | GM2 ganglioside and related glycolipids | None | Cultured cells and natural substrate |
| Globoid cell leucodystrophy Krabbe | Galactocerebrosidase | Galactosylceramides | None | WBC enzyme assay |
| Mucolipidoses | ||||
| ML I (sialidosis I) | Neuraminidase | Sialic acid | Urine sialic acid | Cultured cells |
| MLII (I-cell) | Transferase | Many | Urine oligosaccharides | Plasma enzyme assay |
| ML III (pseudo-Hurler) | Transferase | Many | Urine oligosaccharides | Plasma enzyme assay |
| ML IV | Mucolipin-1 | Lipids and mucopolysaccharides | Urine oligosaccharides | Histology |
| Glycoproteinoses | ||||
| α-mannosidosis | α-mannosidase | α-mannosides | Urine oligosaccharides | WBC enzyme assay |
| β-mannosidosis | β-mannosidase | β-mannosides | Urine oligosaccharides | WBC enzyme assay |
| Fucosidosis | Fucosidase | Fucosides glycolipids | Urine oligosaccharides | WBC enzyme assay |
| Aspartylglycosaminuria | Aspartylglucosaminidase | Aspartylglucosamine | Urine oligosaccharides | WBC enzyme assay |
| Schindler disease | α-galactosidase B | N -acetyl-galactosamide glycoprotein | Urine oligosaccharides | WBC enzyme assay |
| Glycogen | ||||
| Pompe disease | α-glucosidase | Glycogen | ECG characteristic | Lymphocyte enzyme assay |
| Lipid | ||||
| Wolman disease and cholesterol ester storage disease (CESD) | Acid lipase | Cholesterol ester | None | WBC enzyme assay |
| Niemann–Pick C (NPC) | NPC 1 and 2 | Cholesterol and sphingolipids | Filipin staining of cultured cells | Cholesterol esterification studies |
| Diseases Caused by Defects in Integral Membrane Proteins | ||||
| Cystinosis | Cystinosin | Cystine | Renal function | WBC cystine |
| Danon disease | Lysosome associated membrane protein 2 (LAMP 2) | Cytoplasmic debris and glycogen | Unknown | Unknown |
| Infantile sialic acid storage disease and Salla disease | Sialin | Sialic acid | Urine oligosaccharides | Cultured cells |
| Others | ||||
| Galactosialidosis | Cathepsin | Sialyloligosaccharides | Urine oligosaccharides | Cultured cells |
| Multiple sulfatase deficiency | Cα-formylglycine-generating enzyme | Sulfatides | Urine GAGs | WBC and plasma enzyme assay |
| Neuron ceroid lipofuscinosis (Batten disease) | ||||
| NCL 1 | CLN-1 (protein palmitoylthioesterase-1) | Lipidated thioesters | Histology | Cultured cells and DNA |
| NCL 2 | CLN 2 (tripeptidyl amino peptidase-1) | Subunit C of mitochondrial ATP synthase | Histology | Cultured cells and DNA |
| NCL 3 | Arginine transporter | Subunit C of mitochondrial ATP synthase | Histology | DNA |
The MPS are the second most frequent type of LSDs, after sphingolipidoses. They encompass 12 types of genetic disorders, each of which is produced by an inherited deficiency of an enzyme involved in the degradation of GAGs. This condition is clinically progressive and has many common features that result from the accumulation of partially degraded GAGs in various tissues. They have a prevalence that ranges between 2.2 and 5.2 per10,000. , Their current nomenclature, biochemistry, screening, and diagnostic tests are summarized in Table 58.1 .
Each lysosomal enzyme is specific for a linkage. An inherited deficiency of any enzyme involved disrupts the sequential degradative process and leads to the accumulation of partially degraded GAGs within the lysosomes that eventually disrupts the cellular architecture and function. The tissue and organs affected, as well as the severity, will depend on the degree of enzyme deficiency (i.e., partial or total). Deposition of these substances in the connective tissue leads to connective tissue laxity manifest by inguinal and umbilical hernias. Connective tissue thickening secondary to GAG accumulation and collagen deposition leads to coarse facial features, peripheral nerve entrapments, thickened meninges, spinal cord compression, and hydrocephalus. Deposition of GAGs in the heart valve leaflets, endocardium, and myocardium leads to symptomatic heart disease, which is the common cause of death in these patients. Most of these disorders produce short stature, secondary to impaired long bone growth and vertebral abnormalities. These and other changes in the bony skeleton are referred to as dysostosis multiplex . Central nervous system (CNS) storage may produce progressive mental retardation, especially in disorders involving the impaired degradation of HS (MPS I, II, and III).
Regarding ocular findings, corneal clouding and visual handicap result from the storage of the partially degraded GAGs within the corneal stroma, especially in disorders involving impaired degradation of dermatan sulfate and keratan sulfate (MPS I, IV, and VI). The corneal stroma, which constitutes more than 90% of the corneal thickness, is composed of extracellular matrix, keratocytes, and stromal nerve fibers. Over 97% of the stroma is made up of collagen (type I) and GAGs. Various GAGs are present between the collagen fibers in the corneal stroma. With the exception of hyaluronan, all of these GAGs bind to core proteins to form proteoglycans. The most abundant GAG in the cornea is keratan sulfate, constituting about 65% of the total GAG content. The remaining GAG content is made up of dermatan sulfate and chondroitin sulfate. Excessive accumulation of GAGs within the corneal stroma in MPS interferes with the regular arrangement of the collagen–GAG matrix, thus causing corneal clouding. Except for Hunter syndrome (MPS II), in which the missing enzyme is specified by a gene on the X chromosome and the inheritance is X-linked, all of the other MPS are inherited as autosomal recessive. In most cases the affected offspring have heterozygous carrier parents, both of whom have about half normal level of the enzyme for which the affected individual is deficient.
Other reported ocular findings are open angle glaucoma secondary to GAG deposition in iris trabecular meshwork and peripheral cornea, , photoreceptor loss and retinal degeneration due to GAG deposits in the retinal pigment epithelium, , optic nerve atrophy, and strabismus.
MPS type I includes 90 mutations in the gene coding for the lysosomal enzyme α-L-iduronidase. It has three different presentations: MPS IH (Hurler) syndrome, MPS IS (Scheie) syndrome, and MPS IH/S (Hurler-Scheie) syndrome. The pathophysiology includes intracellular and extracellular accumulation of the GAGs dermatan and HS, causing cell death, tissue damage, and excessive excretion of GAGs in the urine. There is a wide phenotypic variation of MPS I with the severe form being described as Hurler syndrome, the milder form Scheie syndrome, and the intermediate form Hurler-Scheie syndrome. Ocular features are common in all MPS I subtypes. Corneal clouding is found in approximately 70% of cases.
MPS I manifests at around 6–12 months of age, with coarsening of facial features, skeletal deformities, and other systemic manifestations. Skeletal problems include dysostosis multiplex, odontoid dysplasia, spondylolisthesis, thoracolumbar gibbus, and joint contractures. Other manifestations include large head circumference, communicating hydrocephalus, mental retardation, hepatosplenomegaly, and cardiac complications (cardiomyopathy, coronary artery disease, and heart valvular disease). Intellectual impairment is a prominent feature of MPS IH. Untreated children usually die during the first decade of life. Bone marrow transplantation in MPS IH has a significant impact on the life span and the systemic health of the patient.
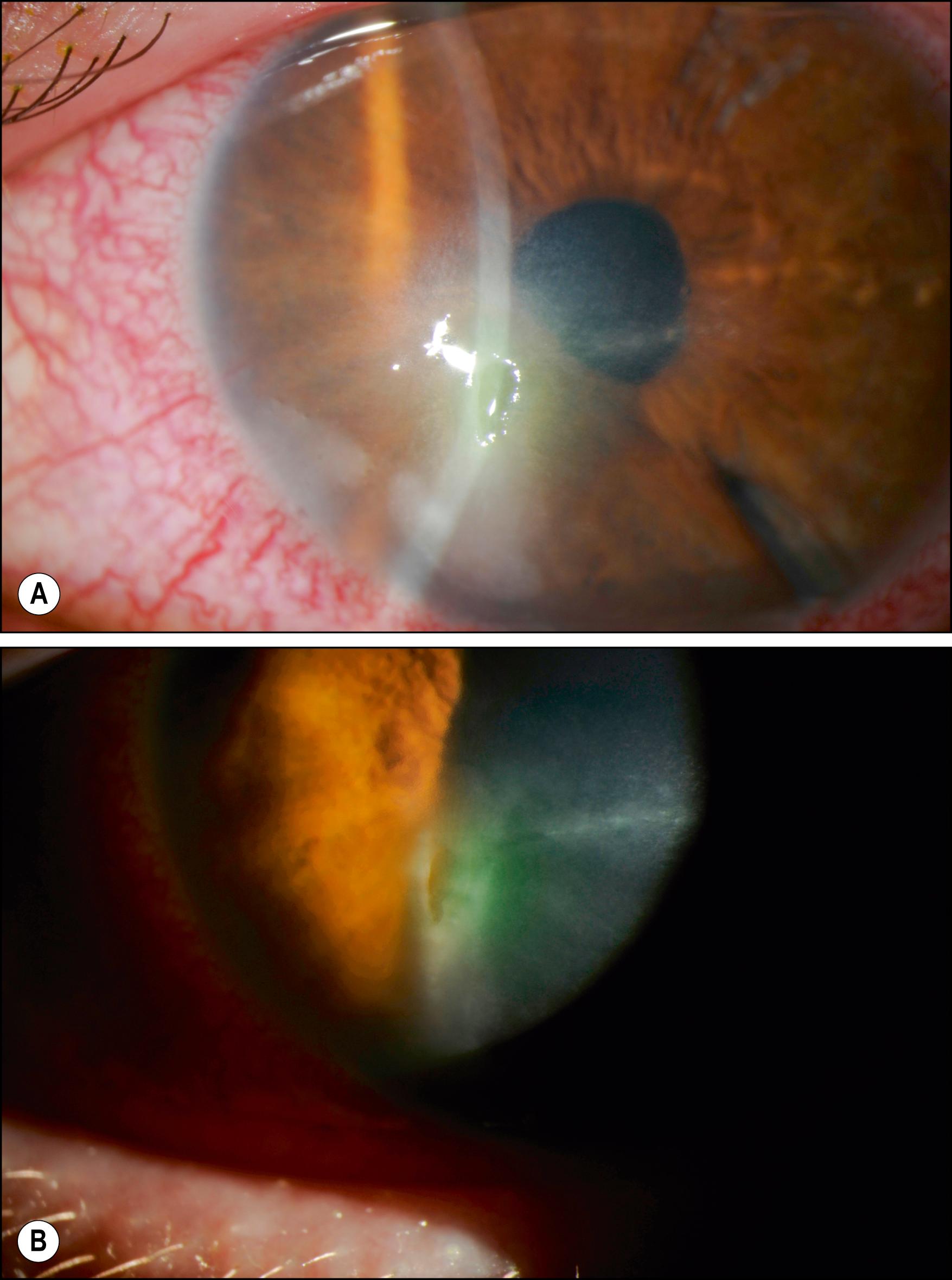
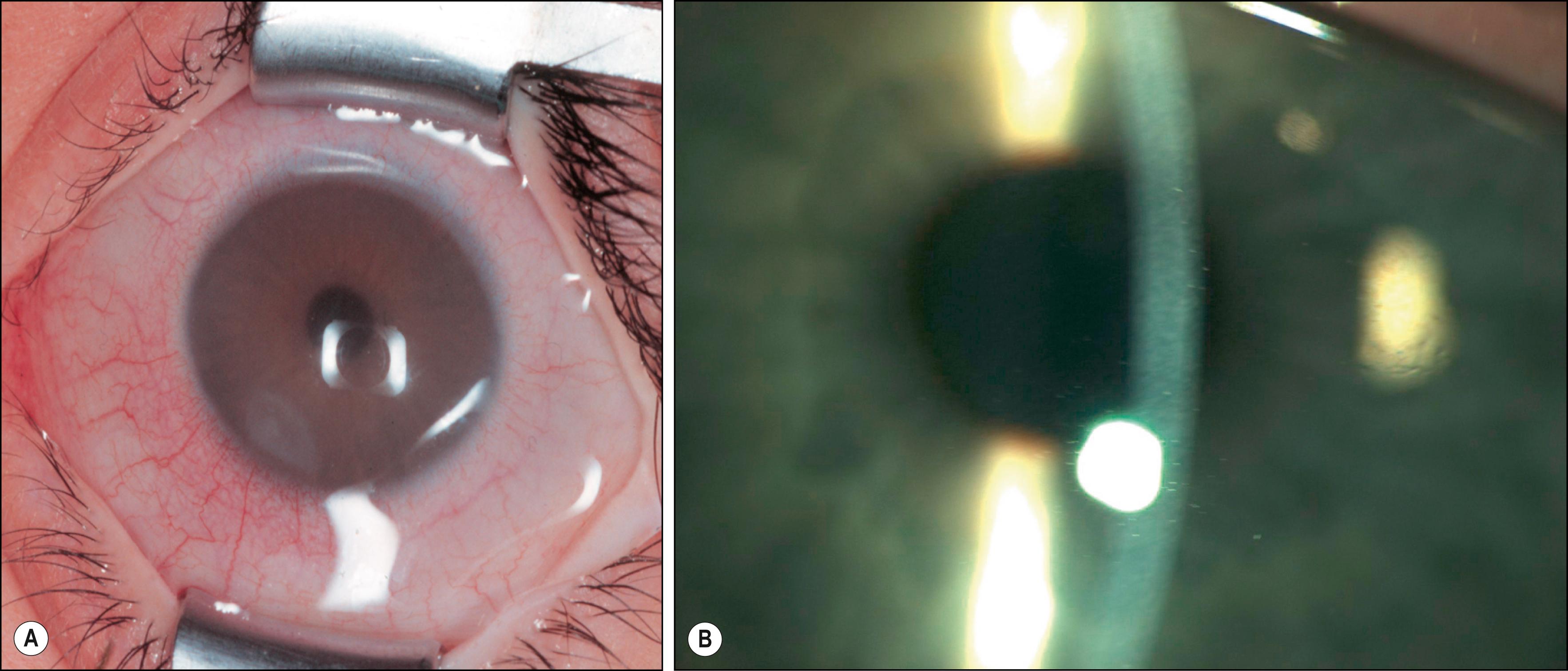
The primary ocular manifestations of MPS I include severe corneal clouding ( Figs. 58.4–58.6 ), retinal pigmentary degeneration, glaucoma and optic nerve head swelling, and optic atrophy. Prominent, wide-set eyes (shallow orbits, hypertelorism) may be seen in severely affected patients. The retinal and optic nerve involvement may limit the success of corneal transplantation in these patients. In addition to posterior segment pathology open angle glaucoma has also been reported.
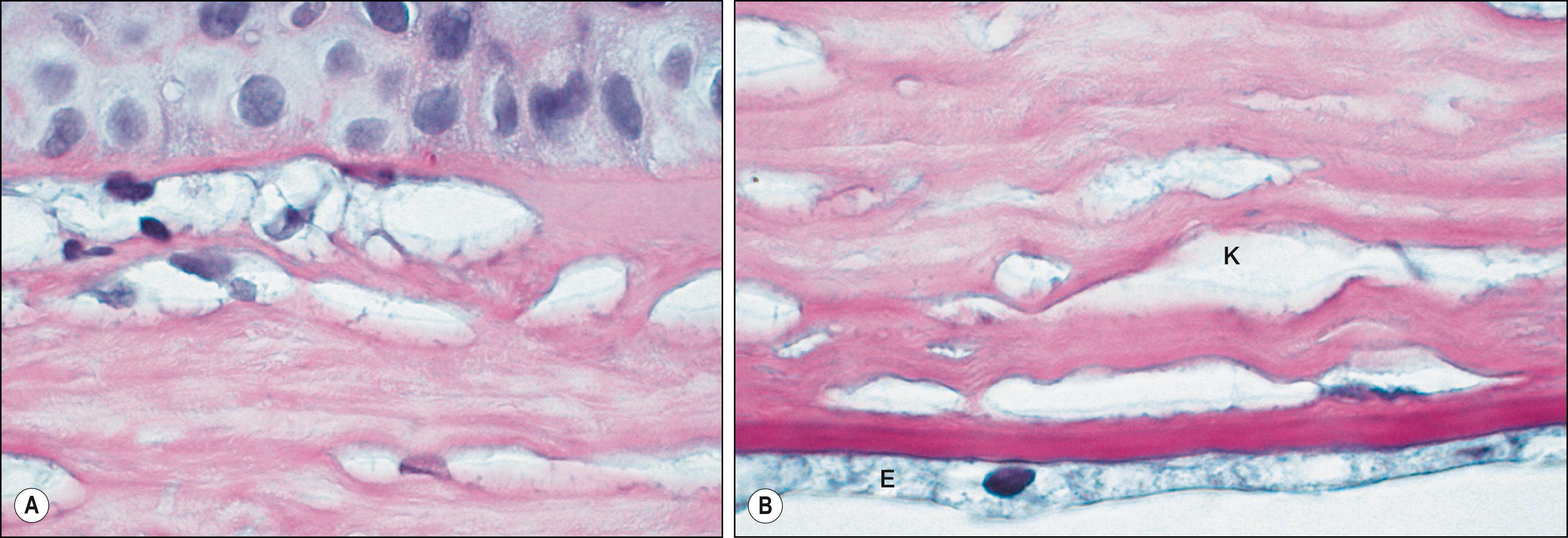
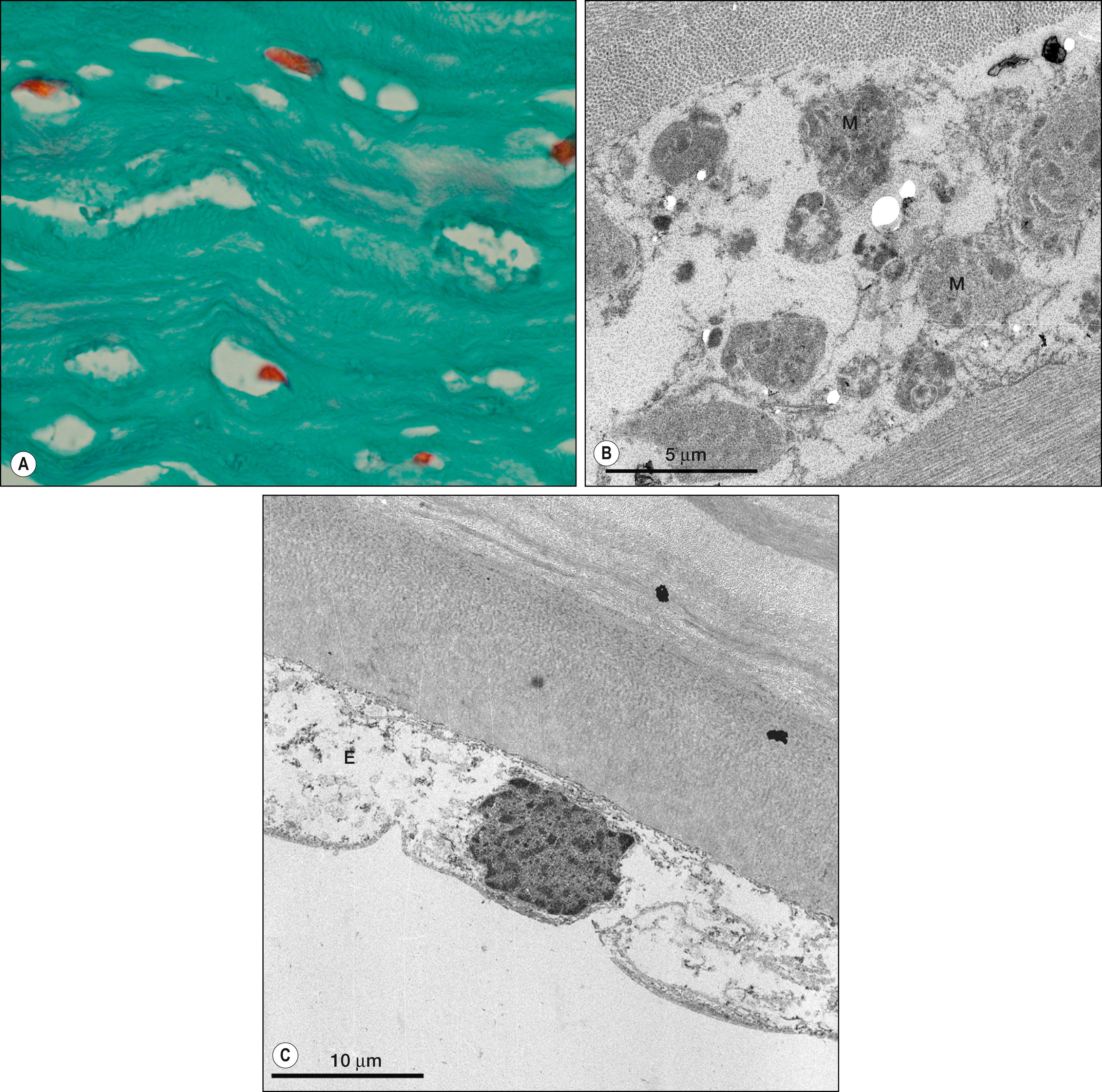
This rare disorder is also due to the deficiency of α-L-iduronidase and presents as severe corneal clouding, deformity of the hands, and aortic valvular disease. Symptoms usually appear between the ages of 5 and 15. These subgroups of patients have normal height and intelligence. Life expectancy may be nearly normal. Although the corneal clouding is severe and affects the entire cornea, there are reports of dense peripheral corneal clouding with almost clear central corneas in subgroups of MPS. Although the deposition of proteoglycan material among the stromal lamella is characteristic of all MPS, histologic appearance of epithelial breaks with peg-like undulations, marked attenuation of the Bowman layer, and the appearance of fibrous long-spacing collagen may present histopathologic changes unique to Scheie syndrome.
Some patients with a phenotype that is intermediate between that of Hurler and Scheie syndrome are thought to represent compound heterozygotes, having inherited one Hurler and one Scheie gene from each parent. They usually have normal intelligence and only mild facial changes and die in their 20s or later of associated cardiorespiratory disease. Diffuse corneal opacification and retinopathy occur.
This disorder results from defective function of iduronate-2-sulfatase resulting in deposition of the GAG dermatan and HS. It is characterized by: (1) slow progression with longer survival ; (2) lack of corneal clouding; and (3) X-linked rather than autosomal recessive inheritance. Unlike MPS I, seizures are more common in MPS II and patients develop learning difficulties with progressive neurodegeneration.
Ocular abnormalities in MPS II Hunter syndrome include exophthalmos, hypertelorism, disk swelling, optic atrophy, and retinopathy. In contrast to MPS I, the corneal changes in MPS II do not usually significantly impair vision.
MPS III (Sanfilippo) results from deficiency of at least four different enzymes that lead to the deposition and accumulation of HS (see Table 58.1 ). Clinically the four types of MPS III A–D are indistinguishable. Patients with MPS III (Sanfilippo) present with learning difficulties and severe behavioral disturbance but have mild systemic manifestations. Early development is normal but slows or halts between the ages of 2 and 6 years, after which mental deterioration is often rapid. They often have a large head, coarse hair, and hirsutism. The clinical diagnosis may be suspected from severe mental retardation, which appears disproportionate to the relatively mild somatic and radiologic abnormalities. Moderate to severe retinopathy with pigmentary retinal degeneration and associated electroretinogram changes are prominent features of MPS III.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here