Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
One of the most challenging aspects of contact lens practice is the diagnosis and management of potentially sight-threatening microbial keratitis (MK). When involved in any such form of clinical decision-making, practitioners rely on their own clinical experience and draw upon guidance that can be found in the relevant literature. This latter aspect has become problematic. Notwithstanding the evolution of a solid body of basic and clinical research into this topic over the past four decades, attempts at defining contact lens–associated keratitis have resulted in the adoption of various terms, definitions and classification systems which are ambiguous and difficult to apply in a clinical setting.
Two terms emerging from the contemporary literature which are used to describe inflammatory reactions of the cornea are ‘keratitis’ and ‘corneal infiltrative event’ (CIE). The latter entity has also been referred to as a ‘corneal inflammatory event’. Dorland’s Medical Dictionary defines ‘keratitis’ as ‘inflammation of the cornea’ and defines ‘inflammation’ as being characterised by ‘ … leukocytic migration into the inflammatory focus’ ( Fig. 26.1 ). The terms ‘keratitis’, ‘corneal infiltrative event’ and ‘corneal inflammatory event’, therefore, can be considered as being synonymous because all these terms imply an infiltration or migration of cellular elements into the cornea.
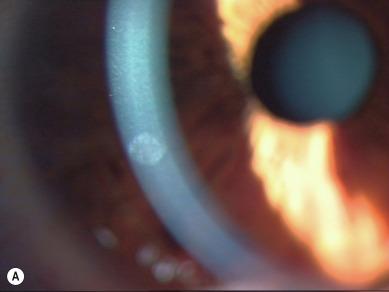
Considerable confusion has emerged because some authors have used these terms to denote severity. The term ‘CIE’ has been taken to mean low-grade, innocuous corneal infiltration/inflammation ( Fig. 26.2 ), whereas ‘keratitis’ has been taken to imply a severe event (e.g. ‘severe microbial keratitis’) ( Fig. 26.3 ).
Keratitis may be infectious, suggesting that replicating microorganisms are responsible. This is referred to as ‘microbial keratitis’. As will be discussed in detail in this chapter, it is not possible to distinguish whether or not a low-grade, early-stage CIE is MK. The relationship between these conditions is demonstrated as a Venn diagram in Figure 26.4 . Simply put, all cases of MK are also CIEs, but not all cases of CIEs are MK. As well as being caused by microorganisms, a CIE might be caused by such factors as toxicity, immunological reaction, metabolic disturbance or trauma.
These considerations created some difficulty in terms of how the discussion of such conditions could be organised in this book. Given that these two conditions are generally treated as separate entities in the literature, separate chapters are presented on CIEs (this chapter) and MK ( Chapter 27 ). This chapter will cover the full spectrum of keratitis more from the standpoint of definitions and epidemiology and will outline general principles of management. Chapter 27 will consider MK from a clinical standpoint. In considering the information in these chapters, readers must remain mindful of the overlap of these conditions as defined in Figure 26.4 .
This chapter will consider the various approaches that have been advocated in respect of the clinical diagnosis of keratitis and will highlight the difficulties that each of these pose. It will be demonstrated that problems often relate to the adoption of arbitrary terminology that is self-contradictory and/or inconsistent with classic, universally accepted definitions enshrined in the medical literature. These semantic problems have been compounded by a failure to challenge or depart from traditional concepts. This chapter will challenge the ‘conventional wisdom’ and present a rational, logical and coherent basis for underpinning clinical decision-making in this area. Unless otherwise stated, it should be assumed that throughout this chapter, reference is being made to contact lens–associated keratitis (so the descriptor ‘contact lens–associated’ will not always be used).
To test and challenge the various approaches that have been used to define and classify keratitis, a substantial body of data was generated to document the clinical characteristics of all forms of this disease, from mild, self-limiting and clinically innocuous manifestations to the most severe and painful forms of MK. This was achieved by conducting a prospective survey between January 25, 2003, and January 24, 2004, of all patients who were wearing contact lenses (irrespective of the primary reason for presentation) and attending the acute service of the Royal Eye Hospital, Manchester, United Kingdom.
The details of this work – collectively referred to as ‘The Manchester Keratitis Study’ – have been published previously and will be referred to extensively throughout this chapter. The design of this experiment is represented schematically in Figure 26.5 . In essence, all presenting patients who habitually wore contact lenses completed a patient survey questionnaire which gathered information about whether or not they slept wearing lenses (i.e. wearing modality), the type of lenses they wore (rigid, hydrogel daily disposable, all other hydrogel or silicone hydrogel) and other personal, health and lifestyle factors.
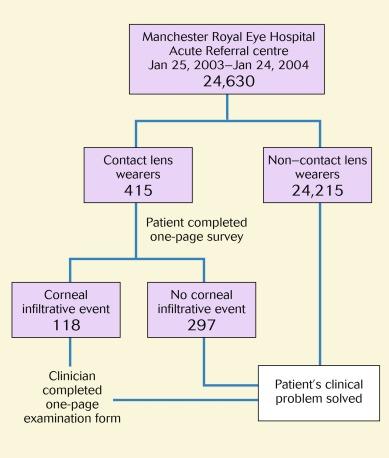
If a CIE was noted upon ocular examination, the attending clinician completed a survey form that detailed clinical information relevant to this study. An important feature of this form was a ‘clinical severity matrix’ – a schema described by Aasuri et al. and advocated by other authors. This involved the clinician scoring the severity of each of 10 signs and symptoms on a 0-to-3 scale, as shown in Table 26.1 . The cumulative score for each event – the ‘clinical severity score’ – was between 2 (the minimum score relating to the criterion for this study of only including patients exhibiting an infiltrative response) and 22, and thus, the higher the score, the more clinically severe was the event. A total of 118 CIEs were observed during the survey period; the severity distribution of these events is shown in Figure 26.6 .
| Parameter | Severity score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Symptoms | None | Mild | Moderate | Severe |
| Lid swelling | Absent | Present | ||
| Conjunctival redness | Absent | Localised | Generalised | |
| Infiltrate shape | Round | Irregular | ||
| Infiltrate size | <1 mm | 1–2 mm | >2 mm | |
| Fluorescein staining | Absent | Present | ||
| Surrounding cornea | Clear | Slight haze | Severe haze | |
| Endothelial debris | Absent | Present | ||
| Hypopyon | Absent | Present | ||
| Effect of lens discontinuation | Resolving | No change | Slight worsening | Significant worsening |
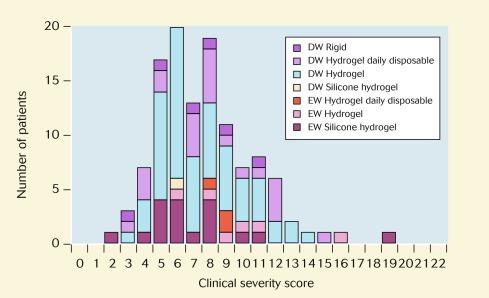
An important feature of the Manchester Keratitis Study is that no attempt was made to distinguish between MK and sterile (non-infectious) keratitis, in view of the difficulties in making this clinical distinction, as will be described later in this chapter. However, to relate the results of the Manchester Keratitis Study to the existing literature and to form a basis for testing different characteristics of the various binomial and polynomial classification schemes that have been advocated previously, the criterion of Aasuri et al. was adopted. According to this scheme, CIEs with clinical severity scores greater than 8 could be considered ‘severe keratitis’, which would be analogous to the traditional definition of ‘presumed microbial keratitis’, and CIEs with clinical severity scores of 8 or less could be considered ‘non-severe keratitis’, which would be analogous to the traditional definition ‘sterile keratitis’.
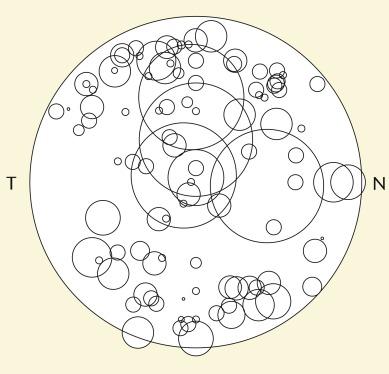
The size and position of the infiltrate or ulcer on the cornea in 111 of these patients were also carefully documented. For analytical purposes, this information is presented in the form of a cartogram whereby each CIE is depicted by a circle, the diameter of which is equivalent to the largest dimension of the infiltrate as observed on the cornea of the patient ( Fig. 26.7 ). The cartogram is generated as a ‘right eye only’ representation whereby the location of any event occurring in the left eye is horizontally mirror-transposed; this form of analysis facilitates consideration of possible nasal/temporal effects.
A number of binomial classification systems (defining two categories of CIE) and polynomial classification systems (defining three or more categories of CIE) have been advocated. It appears that a primary aim of these systems has been to separate out those cases that do or do not require therapeutic intervention. Conceptually, this makes sense because the fundamental clinical decision that must be made with respect to any presenting case of keratitis is whether or not to prescribe anti-microbial drugs (if the practitioner is therapeutically endorsed) or refer the patient for medical attention.
The binomial classification ‘microbial versus sterile’ keratitis attempts to address directly the question of whether or not to prescribe anti-microbial drugs. Other binomial systems, such as ‘ulcerative versus non-ulcerative’, ‘suppurative versus non-suppurative’ and ‘central versus peripheral’, can be considered as surrogate measures, in that specific ocular signs and symptoms are assessed so as to infer whether the condition is microbial or sterile as the basis for deciding whether or not to prescribe. As will be demonstrated, all of these binomial classification systems suffer, to a greater or lesser degree, from definition overlap and ambiguity.
The adoption of criteria that classify a condition as being MK versus sterile keratitis is problematic from a microbiological perspective, because of the difficulties in identifying definitively whether or not a given CIE is infective in aetiology, especially those of mild to moderate severity. True confirmation of MK is dependent upon proof of microbial infection, which is sought by scraping the cornea and culturing for evidence of pathogenic microorganisms. However, in the Manchester Keratitis Study, 57% of cases of severe keratitis (i.e. presumed MK) were found to be culture negative. This finding is consistent with previous studies, which have found that on approximately 50% of occasions, when a corneal scrape is taken from a patient with presumed MK (based on clinical signs and symptoms), the result turns out to be culture-negative. It is difficult to interpret a culture-negative result because it is not possible to determine whether this indicates that microorganisms were truly absent (a true-negative result) or whether microorganisms were present but simply not picked up in the course of the scraping procedure (a false-negative result). A culture-negative result can also occur as a result of the patient having used antimicrobial medication prior to corneal scraping.
A positive culture is generally presumed to be a true finding (a true-positive result); however, it may indicate the presence of potentially pathogenic microorganisms that were part of the normal ocular flora, which were cultured coincidentally and therefore were unrelated to the CIE under investigation. Such a false-positive result, which was observed in 10.3% of cases scraped for bacterial keratitis by Sharma et al., can occur as a result of the vast array of potentially pathogenic bacteria that constitute the normal flora of asymptomatic contact lens wearers (and non–lens wearers). However, microbiology laboratories employ protocols involving the use of multiple cultures that guard against false-positive reporting.
Notwithstanding the difficulties outlined earlier, it must be recognised that although corneal scraping and culturing may be of limited assistance in discerning the difference between MK and sterile keratitis, performing such procedures may be of benefit for a different reason. The results of scraping might help identify the causative bacteria in a case of severe keratitis and, in conjunction with antibiotic sensitivity testing, can form the basis for choosing an appropriate antibiotic therapy.
Because it is only possible to presume a difference between microbial and sterile keratitis, many clinicians have chosen to use clinical definitions instead of microbiological definitions as the basis for deciding whether or not to prescribe anti-microbial medication. However, there is little agreement with respect to the various criteria adopted, which are all problematic in various respects. Poggio et al. defined MK as ‘…a corneal stromal infiltrate with an overlying epithelial abnormality (ulceration) clinically diagnosed as microbial keratitis … [and the patient] … received antibiotic treatment for presumed microbial keratitis…’. To diagnose a condition as MK because it was ‘clinically diagnosed as MK’ is tautological and of little value to others attempting to use this definition. The problems in defining MK in terms of the presence or absence of ulceration will be discussed later.
The definition of Lam et al. that MK is characterised as ‘…a corneal stromal infiltrate more than 1 mm 2 but not necessarily with an overlying epithelial defect’ is over-simplistic because, as Weissman and Mondino noted, MK begins as a minor epithelial anomaly, which progressively increases in size. To discount any CIE less than 1 mm is to ignore the natural history of the condition; that is, the size of an infiltrate in a case of MK will self-evidently be less than 1 mm during the very early phase of the natural history of the disease. Indeed, it must start with an area of 0 mm . The question of diagnosing CIEs without regard to the natural history of the disease process is highly problematic, as discussed later in this chapter.
Stapleton et al. adopted a complex definition for ‘moderate to severe MK’, which is a based on a combination of microbiological and clinical criteria. This condition is said to occur if the following criteria are met:
the CIE is culture proven, or
the CIE is culture negative with at least one of the following:
the lesion is within the central 4 mm of the cornea;
there is an anterior chamber reaction and the infiltrate is 2 mm or greater in diameter;
the patient is suffering from significant pain and the infiltrate is 2 mm or greater in diameter.
This definition is problematic because it appears to rely largely upon the unfounded assumption (for reasons outlined later) that a CIE occurring in the central cornea is MK and a CIE occurring in the peripheral cornea is a sterile, non-infectious entity referred to as ‘contact lens–induced peripheral ulcer’ (CLPU) (also discussed later).
The Manchester Keratitis Study revealed that the vast majority of CIEs are less than 2 mm in diameter; therefore, in effect, according to the definition of Stapleton et al., such CIEs cannot be classified as MK if they occur outside the central 4 mm of the cornea. Of the severe CIEs in the corneal periphery in the Manchester Keratitis Study (i.e. those that would generally be considered to be ‘presumed microbial keratitis’), only 18% were culture positive and 3% were greater than 2 mm in diameter. Thus, only a minority of cases of severe keratitis seen in the Manchester Keratitis Study would have been classified as moderate to severe MK if the criteria of Stapleton et al. had been applied. This indicates that the criteria of Stapleton et al. are likely to misrepresent what others would call ‘severe’ or ‘microbial’ keratitis, and to substantially under-estimate the incidence of such events.
A ‘high probability of MK’ was defined in the study of Schein et al. as one or more corneal stromal infiltrates greater than 1 mm in size with more than mild pain and one or more of the following: anterior chamber reaction more than minimal, mucopurulent discharge or positive corneal culture. This definition suffers from the same difficulties as that of Lam et al. in that it ignores the natural history of severe CIE. The criteria that Schein et al. set for the classification of MK also appear to be far more stringent than those adopted by others and are likely to substantially under-estimate the incidence of such events.
To illustrate the effect of adopting different criteria for the diagnosis of MK, the criteria of Poggio et al., Lam et al., Stapleton et al. and Schein et al. were each applied separately to the data set of 111 CIEs observed in the Manchester Keratitis Study. Those CIEs that would have been classified as MK according to each definition are shown in the cartograms in Figure 26.8 . Also included in this figure is a cartogram showing the CIEs that were classified as severe keratitis as a result of a clinical severity score of greater than 8 in the Manchester Keratitis Study. As can be seen from this figure, the use of different criteria results in vastly different sets of CIEs being identified as severe or MK, thus highlighting the problems in deriving a meaningful clinical definition for this condition. The criteria of Lam et al. and Schein et al. can be seen to select out larger CIEs; the criteria of Stapleton et al. tend to identify central CIEs; and the criteria of Stapleton et al. and especially Schein et al. select out fewer CIEs. The results of this analysis are entirely consistent with predictions adduced from the descriptions of these various criteria, as outlined earlier.
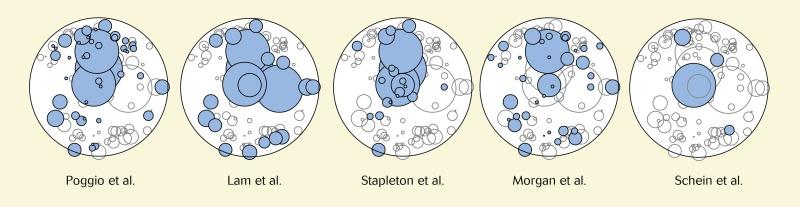
The confounding influence of the use of different criteria for MK can be illustrated by comparing the criteria adopted in separate studies published in 1989 and 2005 by the same author (Oliver Schein, who was third author of the 1989 study of Poggio et al. and first author of the 2005 study ). It can be inferred from Figure 26.8 that the criteria adopted in Schein’s 2005 study generate a lower incidence figure compared with the criteria adopted in his 1989 study ; indeed, this difference is 9.8-fold. Thus, had Schein adopted the criteria used in his 1989 study when determining the incidence of MK in patients wearing Focus Night & Day silicone hydrogel lenses (CIBA Vision Corp, Duluth, Georgia, USA) in his 2005 study, he might have reported an incidence of MK of 176, rather than 18, cases per 10,000 wearers per year. This analysis does not imply a criticism of Schein’s work; indeed, both the 1989 and 2005 studies are internally consistent. However, this analysis does highlight that caution that must be exercised when comparing incidence figures from different studies – even by the same author – that have adopted different criteria for distinguishing between microbial versus sterile keratitis. Further consideration of the incidence figures derived from such studies can be found later in this chapter (see ‘Incidence of Keratitis’ ).
The use of the clinical case definition ‘ulcerative’ keratitis versus ‘non-ulcerative’ keratitis is similarly problematic as this distinction depends upon the criterion for corneal ulceration. Dorland’s Medical Dictionary defines ‘ulcer’ as ‘a local defect, or excavation, of the surface of an organ or tissue, which is produced by the sloughing of inflammatory necrotic tissue’. Therefore, strict confirmation of the presence of an ulcer would require evidence of inflammation and a local tissue defect, which, in the case of the cornea, would mean the presence of infiltrates with overlying corneal staining.
Stein et al. defined a corneal ulcer in terms of its size and shape and the presence or absence of corneal staining overlying stromal infiltrates. They distinguished between ‘epithelial defects’ and ‘superficial punctate keratitis’; however, they did not define either of these conditions, which would generally be considered as being synonymous. Although Stein et al. attempted to provide broad guidelines for the classification of ulcerative keratitis versus non-ulcerative keratitis, they documented considerable overlap in the features of these two conditions. Furthermore, diagnoses were made in relation to whether or not the condition was culture positive, which introduces further uncertainties as discussed previously.
In essence, the definition of Stein et al. implies that a CIE with overlying corneal staining is ‘ulcerative keratitis’ (a surrogate for ‘MK’), whereas a CIE without overlying corneal staining is ‘non-ulcerative keratitis’ (a surrogate for ‘sterile keratitis’). In the Manchester Keratitis Study, 68% and 89% of cases of non-severe and severe keratitis, respectively, displayed fluorescein staining in the region of the infiltrates. This suggests that the presence of corneal staining overlying a CIE (and, therefore, a corneal ulcer according to Stein et al. ) is not a reliable method of classifying keratitis with a view to determining which patients require anti-microbial treatment.
Classification of contact lens–associated keratitis on the basis of the presence of suppuration (indicating ‘MK’) or absence of suppuration (indicating ‘sterile keratitis’) is problematic because this sign may be observed in cases of sterile keratitis and may be absent in cases of MK. For example, Stein et al. reported four cases of severe keratitis (three bacterial and one Acanthamoeba ) in which there was no discharge, whereas Sweeney et al. reported that a purulent or mucopurulent discharge was evident in 18% of non-infectious CIEs.
There is a sound physiological basis for assuming that CIEs occurring in the peripheral cornea are less clinically severe than those occurring in the central cornea. If the site of insult (e.g. a localised infection) is close to the limbus, a relatively rapid host immune response might be expected to limit the extent of tissue compromise as a result of the relatively short distance that polymorphonuclear leucocytes and other defensive cellular elements need to migrate from the limbal vessels to the site of tissue insult. It follows that disturbances in the central cornea will be less well protected and any progressive form of pathology in this region is likely to advance further before the host immune response can dampen the reaction.
Many clinicians have anecdotally observed that CIEs, which occur more towards the corneal periphery, are less likely to be clinically severe than those sited further from the limbus. Indeed, this is implicit in the polynomial classification schema of Sweeney et al. in which ‘contact lens-induced peripheral ulcer’ is considered to be a non-serious event ( Fig. 26.9 ). This concept has been extended further, and it has been suggested that because a CLPU is non-serious, it must be non-infectious. Sweeney et al. defined a CLPU as being an event in the mid-peripheral or peripheral cornea, characterised by focal excavation of the epithelium, infiltration and necrosis of the anterior chamber. Symptoms include limbal and bulbar redness and tearing, and patients with this condition are said to experience any level of discomfort ranging from asymptomatic to severe. Besides ‘location’ (itself a flawed criterion), there is nothing in this description to differentiate a case of so-called CLPU from MK.
Holden et al. attempted to draw a distinction between CLPU and MK by suggesting that Bowman’s membrane remains intact in CLPU but is destroyed in MK. However, of three of the patients who were suffering from what was diagnosed as a CLPU and had a biopsy specimen taken from their cornea, one actually displayed a ruptured Bowman’s membrane. Wu et al. attempted to induce CLPU-like lesions in rabbit eyes. These authors reported that such lesions could only be induced by a combination of three factors: (a) the presence of Staphylococcus aureus , (b) a scratch in the cornea and (c) contact lens wear. However, scratches were only induced in the peripheral cornea, so the obvious question as to whether CLPU-like lesions can also be observed in the central cornea was not tested.
On the basis of observations of histological samples taken from rabbit CLPUs, Wu et al. suggested that their failure to observe replicating bacteria within CLPU-like lesions indicated that these lesions were probably caused by toxins released from bacteria present remotely from the lesion. Their failure to identify bacteria in a selected number of histological sections could be a function of the sampling methodology employed; however, assuming that some CIEs can be caused by bacterial endotoxins, it is not possible to clinically differentiate such events from those caused by replicating bacteria.
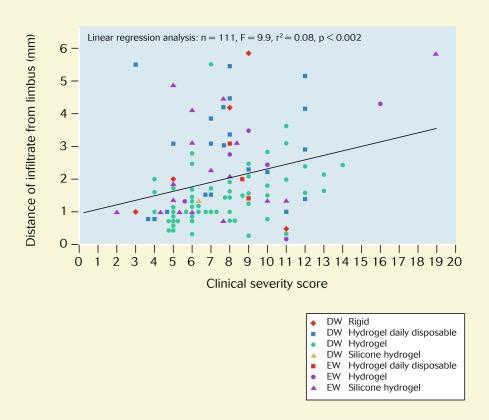
The Manchester Keratitis Study has provided evidence, for the first time, of a significant correlation between the distance of the infiltrate from the limbus and the clinical severity score (F = 9.9; p = 0.002; r 2 = 0.08) ( Fig. 26.10 ). This correlation also holds for the grouped data relating to daily wear (F = 4.3; p = 0.04; r 2 = 0.05) and extended wear (F = 5.7; p = 0.03; r 2 = 0.19); that is, this relationship applies irrespective of lens wearing modality. Notwithstanding these statistical associations, the data are of limited predictive value with respect to an individual patient; that is, a significant proportion of clinically severe CIEs also occur at the corneal periphery and a significant proportion of clinically non-severe CIEs are often observed in the central cornea.
Anecdotal observations purporting to support the concept of non-infectious CLPUs, with respect to the diagnostic indicator that the condition occurs in the peripheral cornea, have been further confounded by the fact that there is much more ‘peripheral cornea’ than ‘central cornea’ by way of area. The cornea can be considered as having central, mid-peripheral (sometimes referred to as ‘paracentral’) and peripheral zones, as shown in Figure 26.11 , and CLPUs are said to occur ‘in the periphery to mid-periphery’. A distinction is not generally drawn between the mid-peripheral and peripheral zones, and the term ‘peripheral’ usually implies both.
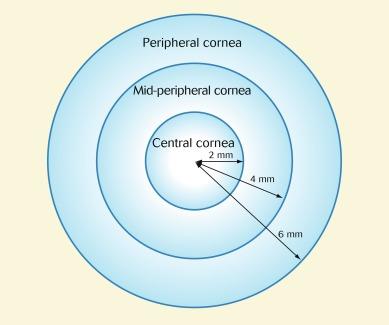
According to the model in Figure 26.11 , the ‘peripheral’ cornea (i.e. mid-peripheral and peripheral cornea combined) represents 89% of the total area of the cornea, when treating the cornea as a flat surface viewed from the front, which is the way the cornea is viewed clinically and the context in which CIEs are noted on a record card. Thus, if infiltrative events were to occur at random locations across the cornea, 89% of such events would be characterised as occurring in the ‘peripheral’ cornea. Clearly, therefore, the criterion that an event occurs in the ‘peripheral’ cornea is of limited diagnostic value (i.e. most of the cornea can be described as ‘peripheral’). Accordingly, the term ‘CLPU’ should discontinued, and such events should simply be referred to as CIEs.
A classification scheme that differentiates between central versus peripheral CIEs would be useful for the purposes of clinically differentiating between microbial versus sterile keratitis if it were true that all CIEs occurring in the peripheral cornea were non-infectious. However, this is not the case. A small CIE in the ‘peripheral’ cornea could be an early-stage MK, or a late-stage MK caused by an infective agent of low virulence. Of course, such an event could also be a true sterile keratitis that has occurred as a result of a mechanical, toxic or allergic insult. Such an event could also be caused by bacterial endotoxins (rather than replicating microorganisms at the site of inflammation). Although the Manchester Keratitis Study documented a variance in the clinical severity of CIEs with respect to corneal location – whereby those occurring in the peripheral cornea were less severe than those occurring in the central cornea – there is no evidence to support the notion that a CIE located in the corneal periphery is most likely to be sterile or that a CIE located in the central cornea is most likely to be infectious.
Attempts have been made to classify CIEs into a number of categories or sub-types. Josephson and Caffery used aetiology as the basis of their classification scheme, whereas Sweeney et al. classified CIEs in terms of clinical severity. Both these works represented significant contributions, but for different reasons. The study of Josephson and Caffery, published over 30 years ago, was a pioneering effort that first drew attention to the complexity and diversity of clinical presentation of CIEs. The work of Sweeney et al. is a substantial analysis of over 900 cases of CIEs observed over a 10-year period in two leading research clinics. However, there are considerable difficulties in applying the classification schemes devised by these authors – again, for different reasons.
Josephson and Caffery were probably the first to undertake a detailed analysis of contact lens–associated CIEs, which they categorised into the following eight types according to aetiology: (a) traumatic, (b) viral, (c) allergic, (d) preserved solutions, (e) lens fit, (f) coated lenses, (g)toxic vapours and (h) idiopathic. The various signs and symptoms associated with the each category were also documented.
The system proposed by Josephson and Caffery was published in 1979 and is of reduced relevance to contact lens practice today for the following reasons: Modern-generation contact lens solutions do not contain potentially toxic preservatives, such as chlorhexidine and thimerosal, which Josephson and Caffery attributed as being the cause of CIEs associated with preserved solutions. ‘Coated lenses’ ceased being a significant clinical problem after the introduction of disposable lenses in the late 1980s.
Lens fit was an important issue in the 1970s, when virtually all lenses on the market were made from relatively thick, low-water-content hydroxyethyl methacrylate. Furthermore, such lenses were provided in a wide range of base curves, and soft lenses were fitted along the lines of rigid lens fitting – relating base curves to central corneal curvature. With modern, thin, hydrogel and silicone hydrogel materials, lenses are more ‘forgiving’, and lens fit is a less critical consideration. It would appear that Josephson and Caffery did not include MK as one of their categories because they probably considered ‘CIE’ to mean ‘non-severe’ or sterile keratitis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here