Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
It is well established that ethnic minorities have a low likelihood of matching with an unrelated hematopoietic stem cell donor. In recent years, there has been increasing interest in alternative stem cell donors given the genetic heterogeneity of our population coupled with diminishing family size. Cord blood (CB) represents an alternative donor type for hematopoietic cell transplantation (HCT) and yields outcomes similar to matched donors. In addition, CB has several unique characteristics. CB units are readily available for procurement from CB banks when urgent transplants are needed. Properties of the unit are also known ahead of time including any evidence of microbial contamination, total nucleated cell (TNC) dose, and in many instances, the CD34+ cell dose and viability. Given the immaturity of stem cells and accompanying T cells, the risks of acute and chronic graft-versus-host-disease are relatively low, requiring less stringent HLA (human leukocyte antigen) matching. Cord blood transplantation (CBT) has also been associated with a decreased risk of relapse, especially in the setting of minimal residual disease.
Just as optimal donor selection is key to the success of a stem cell transplant (SCT), a successful CBT depends on ideal unit selection. Experience in CB unit selection requires time and understanding of multiple variables that represent CB unit quality and affect outcomes. Both the American Society of Transplant and Cellular Therapy and the National Marrow Donor Program have resources to support graft selection ( https://network.bethematchclinical.org/education/education-catalog/cord-blood-unit-selection/ ). Consultation with a transplant center experienced with CB transplantation may also be considered. There are several characteristics that are typically considered in the selection process, and transplant centers may have different CB unit selection algorithms where they may give priority to one CB characteristic over another, as recently outlined. The main CB unit characteristics to be considered in graft selection are discussed in the following sections.
One of the main determinants of transplant-related mortality (TRM) in CBT is the infused TNC and CD34+ cell doses as they correlate with incidence and time to hematopoietic recovery. Historically, TNC was the only cell dose variable considered at the time of CB unit selection. The TNC dose is still relevant today, and most centers consider a TNC of 2.5 to 3.0 × 10 7 /kg suitable as a single CB unit graft. However, the underlying diagnosis must be taken into consideration as those with nonmalignant disease require higher cell doses to achieve reproducible engraftment, estimated between 4 to 5 × 10 7 /kg. Cell doses below this threshold result in delayed hematopoietic recovery, higher risk of graft failure, and increased treatment-related mortality (TRM). The limited cell dose in each individual unit can be in part overcome by the utilization of double CB unit grafts, as outlined in the following sections. More recently, greater emphasis has been given to the importance of considering the CD34+ cell dose as a measure of CB unit quality and potency. A minimum pre-cryopreservation CD34+ cell dose of 0.9 to 1.1 × 10 7 /kg is typically considered. Recently stored units will have both pre-cryopreservation TNC and CD34+ cell counts available for graft selection, especially with red cell depletion and modern banking procedures. While TNC and CD34+ cell counts were expected to be correlated, a recent study found that the correlation between TNC and CD34+ was in fact poor ( r = .024). The ratio of CD34+ to TNC was 0.34% with an interquartile range of 0.23% to 0.48%. Note that when the TNC/CD34+ ratio is significantly outside this range, other unit characteristics, delivery conditions, and experience with the CB bank should be considered in unit selection. In summary, current recommendations are to take both the TNC and CD34+ cell counts into consideration in CB unit selection ( Fig. 108.1 ).
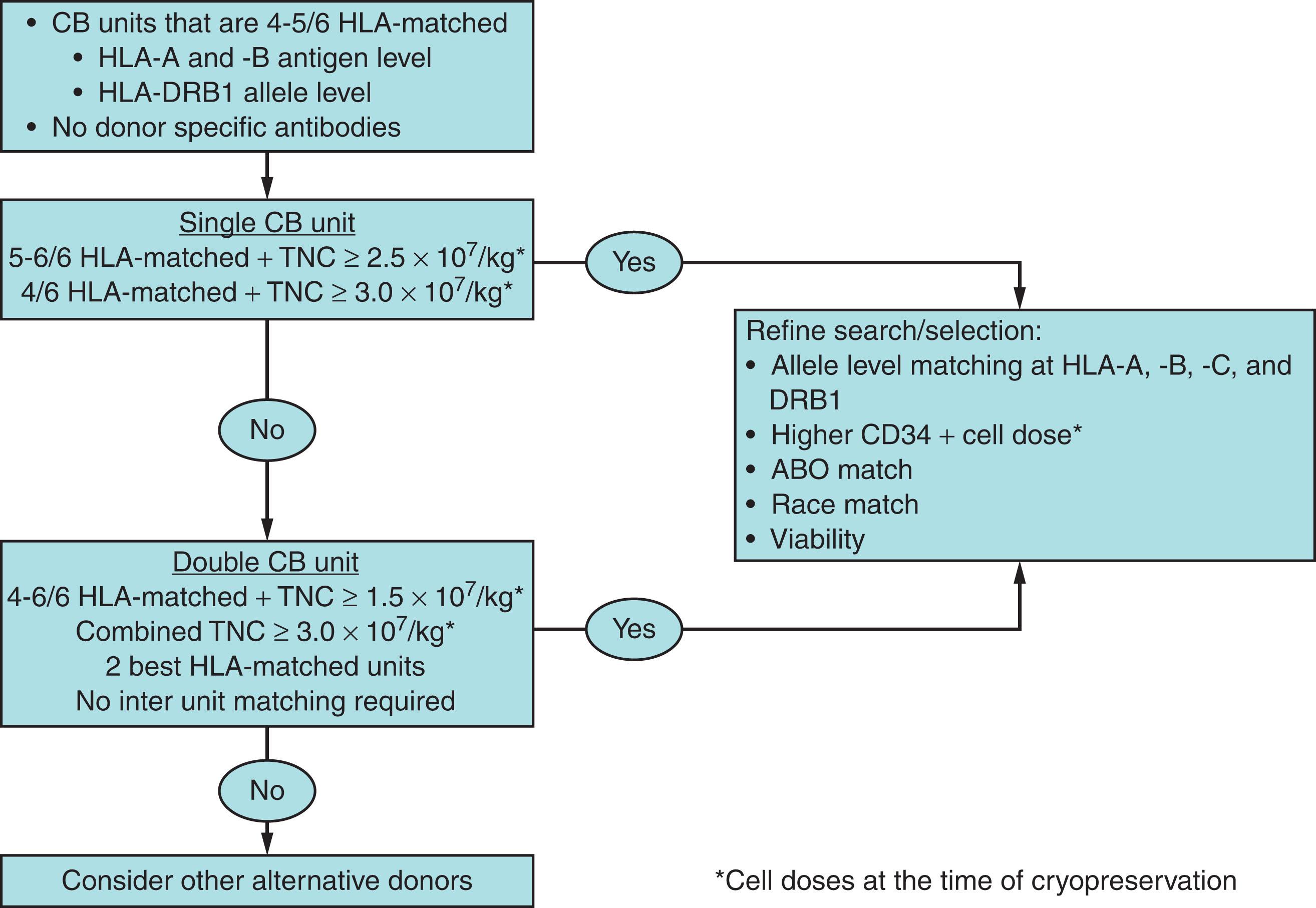
The use of CB for allogeneic HCT started in a pediatric setting, where younger and smaller patients may receive a TNC dose of 5 to 10 × 10 7 /kg. In part due to TNC dose, CB grafts in children achieve consistent engraftment, with outcomes similar to unrelated adult donors. As investigators attempted to extend CB to adult patients, it became evident that cell dose was a barrier to the efficacy of this donor type for larger and adult patients. Initially developed at the University of Minnesota as a platform to study novel graft manipulations, the use of two CB units as a graft (double CB graft) extended this donor type to a physically larger patient population. Double CB transplantation rapidly became an approach commonly used to overcome the cell dose limitation of a single unit. While the principles of quality and HLA-matching apply to the selection of each unit composing the double CB graft, the most commonly used TNC cell dose threshold for each of the two units is a minimum of 1.5 × 10 7 TNC/kg. There is no widely defined CD34+ cell dose for each individual unit in double CB transplantation. Prospective randomized data in children and retrospective registry-based data in adults have shown that the outcomes of transplantation with single or double units result in similar outcomes once the pre-cryopreservation TNC threshold ≥2.5 × 10 7 TNC/kg is met. However, children are still more likely to find suitable single units than adults. Thus, the use of double CB graft is still important in extending this donor type to older and larger patients.
The growth of the inventory of CB over the years has allowed for more frequent identification of single unit grafts with the adequate cell dose for children and certain adult patient populations. Once these larger units become available in the national and international donor search platforms, they tend to be in the CB banks inventory for a short period of time. Moreover, to meet HLA-matching requirements, minorities continue to find only smaller units and require double CB drafts. Current efforts in overcoming the cell dose limitation and making a larger proportion of the existing inventory available to more patients are focused on ex vivo expansion, as discussed later in this chapter.
While methods used to collect and cryopreserve a CB unit may impact its quality, conditions of storage and thaw of the CB unit may also have an impact on the final quality of the CB product. Thus, beyond using proper established and validated thaw methodology, it is necessary to monitor post-thaw cell counts as these counts also play a crucial part in optimizing outcomes. A recent report in the double CB setting described the effect of post-thaw cell count and viability on outcomes and found that a higher CD34+ cell dose and a CD34+ cell dose viability >75% resulted in a higher likelihood of engraftment and CB unit predominance. While this study was in the double CB transplant setting, these findings must also be considered in the context of single CB transplantation; if a single CB unit graft has poor TNC or CD34+ recovery and/or viability, close monitoring and early intervention for graft failure should be considered.
The HLA-matching of CB units to the patient historically required 4–6/6 matching at HLA-A and HLA-B at the antigen level and HLA-DRB1 at the allele level. Despite this low level of HLA-matching in most cases, the incidence of graft-versus-host disease (GVHD) with CBT has been relatively low compared to bone marrow (BM) transplants using other stem cell sources. There is growing consensus in the literature regarding the importance of better HLA-matching in CB transplantation. The data in single CB in children suggest that 6/6 allele level HLA-matched CB results in the best outcomes. In contrast, in adult patients closer HLA-matching considering 6 to 10 loci does not seem to affect survival in either single or double CB settings. However, in the double CB setting when the dominant unit is better HLA-matched to the recipient, it seems to lead to improved outcomes. In the adult population, in particular in ethnic minorities, selected CB units tend to be more HLA-mismatched in order to meet minimum TNC criteria. A lower level of HLA-match may result in a lower risk of relapse, but this is offset by an increased risk of TRM, resulting in similar survival. The novel ex vivo expansion platforms have the potential to shift this balance by allowing the selection of initially smaller but better HLA-matched CB units that can then be expanded to suitable cell doses for most patients. When widely available, this type of innovation will make a larger proportion of the CB inventory available for transplantation of larger and adult patients. Currently, HLA-matching in CB graft selection still starts with the historical standards described. Once this initial search identifies numerous potential CB units, experienced CB transplant centers will further refine the selection by selecting units with the closest allele level HLA-matching considering HLA-A, -B, -C, and -DRB1. In the setting of double CB transplantation, the two units should be selected based on each individual unit’s characteristics aiming for the best conventional and, if possible, allele HLA-matching to the recipient, with no need to match the units to each other.
CB banks are a key element for successful CB transplantation. Under appropriate CB unit selection for banking, quality control, cryopreservation, and storage standards, CB units can be stored and available for treatment for many years. For example, the storage of CB units that have had their volume reduced to around 25 mL have better cell recovery as compared to those that have larger volumes. It is also important that units are red cell depleted as units not depleted of red cells have been associated with a high risk of serious infusion reactions. The familiarity of the transplant center with the CB banks and their practices is another factor to be considered in CB graft selection. The CB bank community is self-regulated with most banks seeking accreditation by NetCord-Foundation for the Accreditation for Cellular Therapy (FACT), which has helped make CB Bank practice more consistent. Notably, CB banks that were NetCord-FACT accredited had higher and more consistent post-thaw cell viability in one study. In addition, CB banks may also undergo licensure by the US Federal Drug Administration (FDA). CB units collected by licensed CB banks are labeled, licensed, and dispensed as a drug, whereas units collected prior to licensure or in unlicensed CB banks and labeled as unlicensed have to be dispensed and used for transplantation under an Investigational New Drug (IND) application. Engraftment and survival are similar whether using a licensed or unlicensed CB unit. Please refer to papers on the topic of CB banking for a more complete discussion of this topic.
The testing for donor-specific HLA-antibodies (DSA) is an important selection criterion in unrelated and mismatched HCT. The data in single CBT have consistently shown that the presence of DSA with mean fluorescence intensity (MFI) of 1000 is associated with a significantly higher incidence of graft failure that ranges from 20% to 68%. The presence of HLA-antibodies, even if not directed against the graft, may be associated with a higher risk of graft failure. This is further suggested by the presence of higher titer antibodies resulting in non-engraftment with either CB unit in double CB transplantation. When comparing patients with DSA who were engrafted to those who had graft failure, the latter had a higher median MFI. There are also some data suggesting that DSA against HLA-C, DP, DQ, and DRB3/4/5 may result in a higher risk of graft failure. The data in double CB transplantation were less clear in part because of the varying MFI thresholds used in different reports. Nonetheless, owing to the strong impact seen in single CB transplantation, testing for DSA has also been incorporated in double CB graft selection by most centers. In summary, testing for DSAs against high expression HLA loci and using virtual cross-matching to assist with the selection of CB units is highly recommended. While HLA A-, B-, C-, -DQB1, and -DRB1 are typically available in current confirmatory typing reports, some cases may require additional HLA typing for determinating HLA-DP and -DRB3/4/5, adding time and complexity to the selection process. While most centers will use an MFI greater than 1000 as positive, higher-risk DSAs are those with MFI greater than 3000. Consultation with the immunology laboratory to determine the relevance of DSAs is also valuable. Administration of rituximab, intravenous immunoglobulin, and bortezomib with or without plasma exchange has been used immediately prior to conditioning to reduce the DSA titer and proceed with the transplant with a specific CB unit. DSA debulking requires further evaluation before it can be widely applied in CB transplantation care, and selecting an alternative CB unit with no DSA may be the best course of action, if possible.
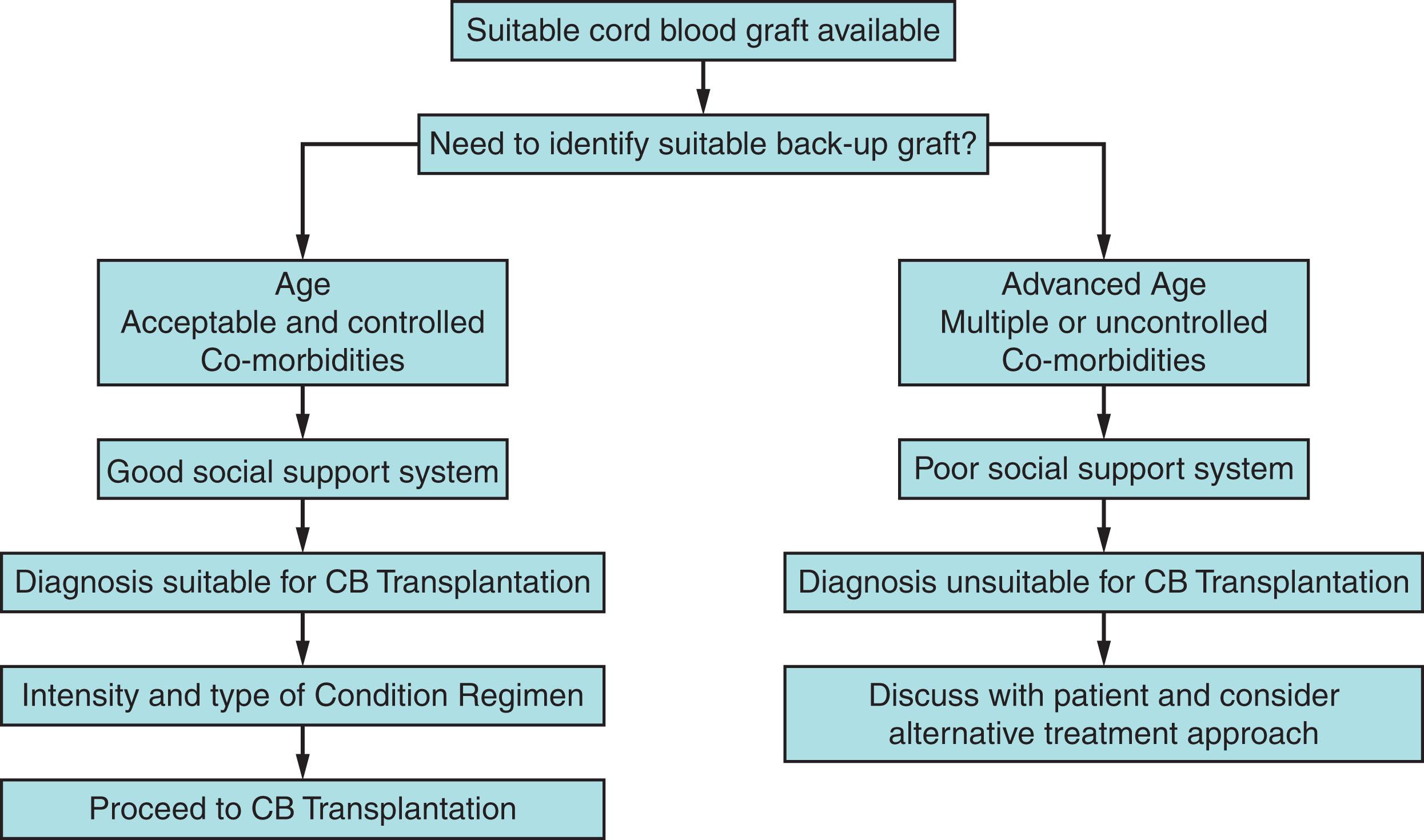
Initial CBT conditioning regimens were designed to provide additional immunosuppression to facilitate engraftment in the context of HLA-mismatching. Early regimens included anti-thymocyte globulin (ATG) and a short course of steroids, which resulted in successful engraftment in children but had limited success in adult patients. The introduction of fludarabine (FLU) to the conditioning regimens for CBT significantly improved engraftment in general and was key in extending this donor type to adults in the myeloablative and reduced intensity settings. Table 108.1 summarizes some of the conditioning regimens used in CBT. It is important to keep in mind that in CBT, the conditioning regimen is part of the platform and that success using this donor type is a full package that includes graft selection, patient evaluation, and supportive care, which depends heavily on center experience ( Fig. 108.2 ).
| Reference | Center | Conditioning Regimen |
|---|---|---|
| Myeloablative Conditioning Regimens | ||
| Barker et al. Blood 2005 | University of Minnesota, USA |
|
| Anand et al. BBMT 2017 | Duke University, USA |
|
| Sanz et al. BMT 2012 | University of Valencia, Spain |
|
| Atsuta et al. Blood 2009 | University of Tokyo, Japan |
|
| Reduced Intensity and Non-Myeloablative Regimens | ||
| Brunstein et al. Blood 2007 | University of Minnesota, USA a |
|
| Ponce et al. Blood 2013 | Memorial Sloan Kettering Cancer Center, USA |
|
| DeFilipp et al. BMT 2020 | Dana Farber Cancer Institute and Massachusetts General Hospital, USA |
|
| Ballen et al. BBMT 2007 | Dana Farber Cancer Institute and Massachusetts General Hospital, USA |
|
| Ciurea et al. Leuk Lymphoma 2012 | MD Anderson Cancer Center, USA |
|
| Yamamoto et al. BBMT 2016 | Toranomon Hospital, Japan |
|
| Deeg et al. BBMT 2018 | Fred Hutchinson Cancer Research Center, USA |
|
a Horse ATG 15 mg/kg twice daily days −6 to −4, if no recent immunosuppressive chemotherapy or previous autologous transplant,
In children, the myeloablative regimen with cyclophosphamide (CY), FLU, and 1200 to 1320 Gy total body irradiation (TBI; TCF regimen) developed by the University of Minnesota has been widely used and has resulted in high rates of engraftment. In a recent multicenter study, this conditioning regimen improved outcomes among children and young adults with acute leukemia, as evidenced by a comparison with chemotherapy only and non-FLU-containing TBI regimens. Taken together, these data support the use of TCF as a preferred regimen for patients with leukemia who are candidates for MA.
Given that this is the main indication for allogeneic transplantation in this age group, it was extrapolated for other conditions. In adults, the TCF regimen has also been shown to result in high rates of engraftment and progression-free survival compared to transplantation using other donor types. At least in part, the long-term disease control seems to be driven by a potent graft versus leukemia effect. The use of this myeloablative regimen in centers with less experience in CBT has proved challenging. This led to increased interest in reduced toxicity FLU-based regimens with thiotepa. These regimens have been shown to produce very promising outcomes in single and double CBT and potentially better applicability (see Table 108.1 ). An advantage of these regimens is the ability to extend intensive conditioning regimens to older fit patients, potentially improving outcomes.
Reduced-intensity and non-myeloablative conditioning regimens are mostly used for the treatment of adult patients who are older or those who have comorbid conditions that would otherwise increase their risk of TRM. Similar to the myeloablative setting, FLU is a critical component of these conditioning regimens. Non-myeloablative conditioning consisting of CY 50 mg/kg, FLU 150 to 200 mg/m 2 , and TBI 200 cGy (Cy-Flu-TBI-200) described by the University of Minnesota is the most commonly used regimen. Other reduced-intensity non-myeloablative conditioning regimens have been described, but a retrospective registry-based comparison suggested that Cy-Flu-TBI-200 resulted in superior long-term outcomes. However, the reported outcomes from the centers that developed each conditioning regimen are very similar. Thus, center experience with a treatment platform that includes conditioning regimen, immune suppression, and supportive care impacts outcomes.
In contrast to myeloablative conditioning regimens, indications for CBT transplantation using a reduced-intensity or a non-myeloablative conditioning regimen include not only acute leukemia, but also chronic leukemia, myelodysplasia, and lymphomas. To facilitate engraftment in these older patients whose disease was not treated with potent immunosuppressive chemotherapy, ATG was added to the conditioning regimens to improve engraftment. However, ATG was associated with a higher risk of viral reactivations, post-transplant lymphoproliferative disorders, and slower immune reconstitution. Thus, investigators have developed ATG-free regimens by replacing it with thiotepa, treosulfan, busulfan, or TBI. A new strategy dosing the ATG based on the absolute lymphocyte count may allow for a better therapeutic window of this drug as part of the conditioning regimens while allowing adequate immune reconstitution after UCB transplantation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here