Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Apnea of prematurity is universal in preterm infants and a manifestation of greater inhibitory (rather than excitatory) influences on the central respiratory network.
In premature infants, excitation of peripheral arterial chemoreceptors by hypoxia predisposes to periodic breathing and the hypoxic ventilatory response is not sustained.
The protective upper airway reflex (laryngeal chemoreflex) prevents aspiration, but in premature infants, profound bradycardia, apnea, and oxygen desaturation can occur in addition to laryngeal constriction.
The excessively compliant chest wall and low lung volume in premature infants predispose them to intermittent hypoxia as a consequence of apnea.
Caffeine is the mainstay of therapy for apnea of prematurity, although the optimal duration and dosing of therapy remain uncertain.
By identifying the genetic mutations that are associated with marked abnormalities in respiratory control, we can obtain a better understanding of the key role of several neuromodulator systems.
How the respiratory network matures is of interest not only to physiologists, but also to clinicians who care for infants, newborns, and children with disorders of respiratory control. Neonatologists, in particular, are aware of the challenges of treating infants who are born prematurely. While breathing movements can be detected in the human fetus as early as 10 to 12 weeks’ gestation, the purpose of fetal breathing is not gas exchange, but instead, the lung stretch that occurs during breathing is essential for lung development. The transition from fetal to neonatal life requires a rapid conversion from intermittent fetal respiratory activity not associated with gas exchange to continuous breathing on which gas exchange is dependent. Breathing in the most premature infants is akin to fetal breathing, which is episodic, punctuated by periods of disturbingly long apneic pauses interspersed with frequent periods of hyperventilation and sighs (augmented breaths). For the infant who is born prematurely, frequent apneas and periods of hypoventilation associated with oxygen desaturations and bradycardia are of significant concern. With maturation, breathing becomes more stable. Thus, the premature infant provides a unique opportunity to observe how the respiratory system matures in humans.
Even though breathing is more stable in term infants than in premature infants, the respiratory system at term gestation is still undergoing significant maturation and can become unstable in response to stressors such as infection or hyperthermia. In some term infants who appear well, subtle developmental abnormalities in the anatomy and neurochemistry of the respiratory system can lead to profound disorders of breathing. Careful epidemiologic, genetic, neurochemical, and neuroanatomic studies in human infants with disorders of respiratoryss control have allowed for a better understanding of genes that regulate the development of the respiratory system and how environmental factors in fetal and early neonatal life may adversely affect the normal development of systems that control breathing.
The purpose of this chapter is to better understand how infants breathe, why premature infants have apnea of prematurity, why term infants have apnea of infancy, and how environmental exposures modify mechanisms that control breathing during fetal and early neonatal life.
Much of our understanding of the basic mechanisms that lead to stable breathing comes from studies performed in newborn and adult animals. Earlier studies in newborn pigs, dogs, and cats, as well as the fetal and newborn sheep, characterized the developmental physiology, and much has been gained from these models. However, a more detailed understanding of the neuroanatomy, neurocircuitry, and neurochemistry has been obtained with in vitro models from fetal and newborn rats and mice. Of particular relevance, the stage of respiratory development of the rat born at term is similar to that of the human born at 25 to 29 weeks’ gestation. Two different reduced in vitro preparations from rodents have been used to better delineate components of the respiratory network in the brainstem: (1) brainstem slices that include the area that contains the “pacemaker cells mediating rhythmogenesis,” and (2) isolated brainstem spinal cord preparations from fetal and newborn rodents. Because the stability and viability of these in vitro preparations are best when tissues are used from the late embryonic stage or within the first week of postnatal life, data from these in vitro preparations are relevant only to respiratory control during very early development. Using these reduced models, specific regions of the brain involved in respiration could be identified and characterized. We now know that the groups of neurons that control the different phases of respiration have a genetic signature, allowing the use of genetic tools to manipulate the activity of the different regions and observe the physiologic responses in intact animals. Specifically, optogenetics and pharmacologic techniques are often combined to explore the functionality of the specific brain regions related to breathing in unanesthetized adult animals. Thus, what we know about how the respiratory network is integrated and functions is based on a significant amount of information obtained from well-designed in vitro and in vivo animal experiments using immunohistochemistry, electrophysiology, fluorescent imaging, genetic manipulation, pharmacology, computational modeling and more recently optogenetics in rodents.
The diaphragm is the major muscle of respiration, but other muscles of respiration are also essential for unobstructed breathing at rest and augmented breathing during exercise and stress. Thus, the muscles of respiration include the pump muscles (diaphragm, intercostal muscles, and abdominal muscles) and muscles of the upper airway (alae nares, pharyngeal muscles, and laryngeal muscles). The diaphragm is innervated by the phrenic nerve, which originates in the spinal column (C3 to C5). The upper airway muscles are innervated by motoneurons originating in the brainstem, specifically, the nucleus ambiguus, the dorsal motor nucleus of the vagus, and the hypoglossal nucleus. Different muscles are activated during different phases of the respiratory cycle: inspiration followed by post-inspiration and expiration. Upper airway muscles are particularly important in modulating the rate of inspiratory and expiratory airflow. During inspiration, the diaphragm, external intercostal muscles (in infants), and posterior cricoarytenoid (laryngeal dilator) contract.
During post-inspiration the diaphragm and the thyroarytenoid (laryngeal constrictor) contract. Diaphragmatic and thyroarytenoid post-inspiratory activity are common in newborns, both human and animal. Because the chest wall of newborns, particularly premature newborns, is highly compliant, diaphragmatic and laryngeal post-inspiratory activity of upper airway muscles is often audible, known as grunting , and is heard in infants with low lung volume disease states, such as surfactant deficiency and atelectasis to preserve lung volume. It is essential that the pump muscles, particularly the diaphragm and the upper airway muscles, contract in such a way that unobstructed breathing occurs. Because of lower airway tone or active closure of the glottis, premature infants may have an obstructed component during apnea. The resultant obstructed inspiratory efforts may prolong central apnea, resulting in so-called mixed apnea.
As shown in the anatomic illustration in Fig. 41.1 , the respiratory-related neurons are located in three main areas in the brainstem: (1) the dorsal respiratory group within the nucleus tractus solitarii (nTS); (2) the ventral respiratory column (VRC), which extends from the facial nucleus to the ventrolateral medulla at the spinal-medullary junction; and (3) the pontine respiratory group within the dorsolateral pons. The VRC should not be confused with the ventral respiratory group (VRG). The VRC can be subdivided into a rostral part—involved in rhythmogenesis—and a caudal part, involved in pattern formation. Bulbospinal neurons are neurons that originate in the medulla (bulbo) and synapse with motoneurons in the spinal column such as the phrenic motoneurons. The rostral VRC contains both the rostral VRG, consisting of a large proportion of bulbospinal inspiratory neurons that project directly to the phrenic and external intercostal motoneurons and the caudal VRG, containing bulbospinal expiratory neurons that project to abdominal and internal intercostal motoneurons. Propriobulbar neurons are neurons originating in the brainstem that send projections to other neurons in the brainstem.
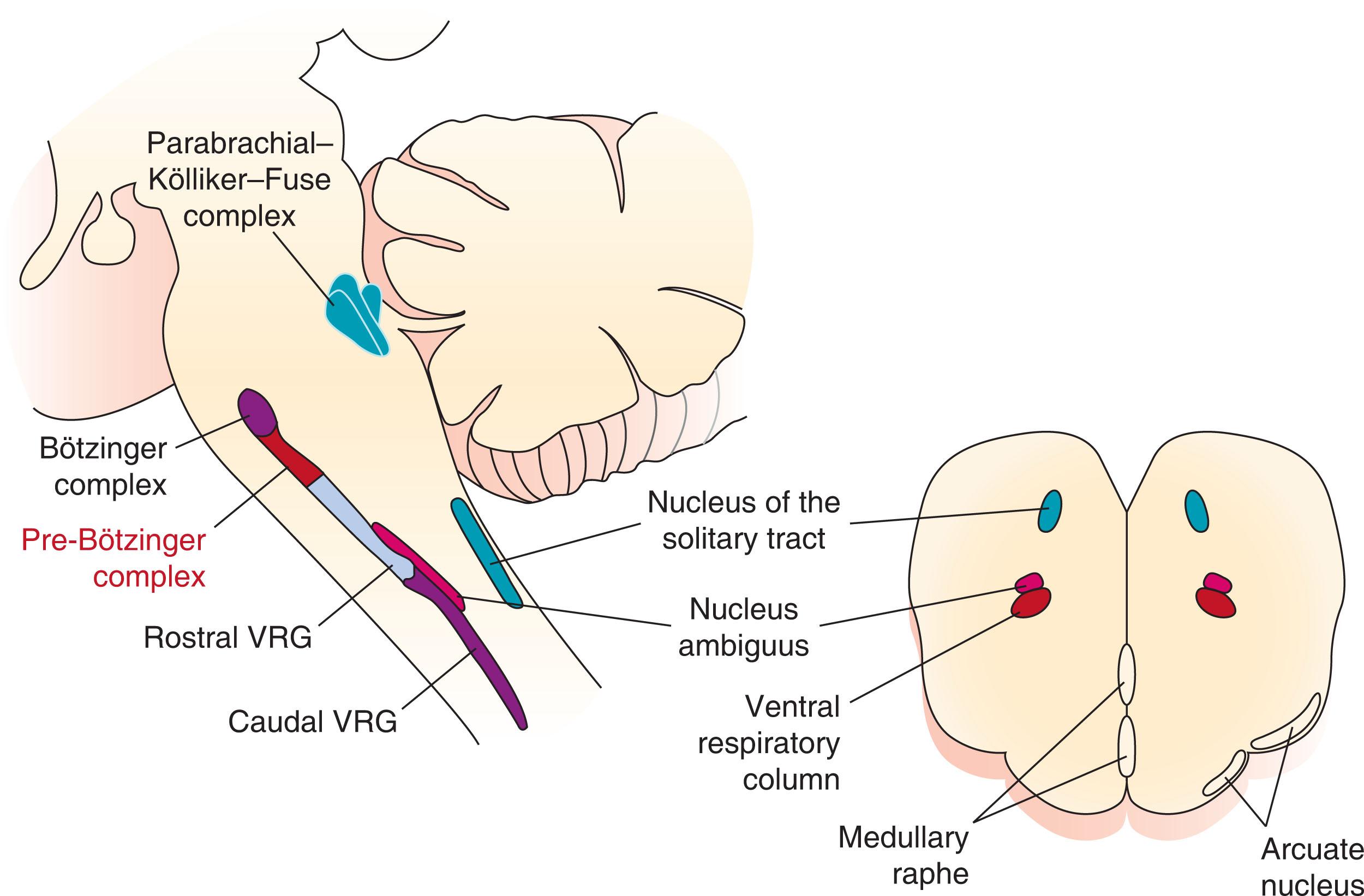
Within the rostral VRC are two areas that are essential to the formation of respiratory rhythm: the pre-Bötzinger (PBC) and the Bötzinger complex. As outlined already, the PBC contains a core group of synaptically coupled excitatory neurons that have pacemaker properties, similar to the pacemaker cells in the atrioventricular node of the heart. These pacemaker cells are rostral to the nucleus ambiguus, have both intrinsic inspiratory and expiratory bursting properties, and are essential to maintaining respiratory rhythm. Progressive destruction of the PBC disrupts rhythmogenesis, leading to death in animals. Using immunocytochemistry to identify NK1 and somatostatin positive neurons and anatomical approaches, a similar area has been identified in the human brain. In individuals who had died of neurogenerative disease with central respiratory deficits, reduced numbers of neurons were identified in the area of the putative PBC as compared with the brains of individuals with neurodegenerative disease without central respiratory deficits. Moreover, Bright et al. found that premature infants who died of sudden infant death syndrome (SIDS) had significantly lower levels of NK1 receptor binding (NK1R) in the nTS, and paragigantocellularis lateralis (PGCL) nucleus, the homologue of the PBC, than in premature infants who did not die of SIDS, and male sex was associated with less NK1R binding in the inferior olivary-cerebellar complex in preterm and term infants who died of SIDS vs control infants. Both male sex and premature birth increase the risk of SIDS. In newborn rats, destruction of NK1R expressing neurons within the preBötC causes abnormal respiratory pattern, and, the more immature the circuit is at the time of destruction, the more permanent the change in the pattern of respiration.
The post-inspiratory complex (PiCo) is another area, recently discovered, with autonomous rhythm-generating properties that controls post-inspiratory activity. The PiCo is medial to the nucleus ambiguus and caudal to the nucleus of cranial nerve VII. The Bötzinger complex contains propriobulbar expiratory neurons that provide strong inhibitory inputs to inspiratory and expiratory bulbospinal neurons in the VRC.
Another important group of neurons are those within the retrotrapezoid nucleus (RTN) located along the ventral medullary surface beneath the facial nucleus. These neurons have chemosensitive properties and depolarize in response to increasing carbon dioxide (CO 2 ) concentration and decreasing pH and synapse with rhythm- and pattern-generating neurons in the VRC. All these neuronal groups and networks that contribute to rhythmogenesis are present in newborn animals born at term (e.g., sheep, cats, and pigs) or born prematurely (e.g., rodents) in which rhythmogenesis is well established before birth. Episodic spontaneous fetal breathing movements occur in human fetuses as early as 10 weeks’ gestation. In rodents, respiratory rhythmogenesis is first detected at embryonic day 15 in rats and embryonic day 17 in mice. The emergence of this respiratory-related activity in rats is coincident with the characteristic expression of NK1 receptors of the PBC.
In summary, respiratory rhythm and inspiratory-expiratory patterns emerge from dynamic interactions between (1) excitatory neuron populations in the PBC and rostral VRG, which are active during inspiration and form the inspiratory motor output; (2) excitatory neurons in the PiCo, which are active during post-inspiration; (3) inhibitory neurons in the PBC that provide inspiratory inhibition within the network; and (4) inhibitory neurons in the Bötzinger complex, which are active during expiration and provide inhibitory inputs to inspiratory and expiratory neurons within the network and to phrenic motor neurons. Because of the limitations in performing mechanistic experiments in humans, much of what we know about how we breathe is extrapolated from animal models, but many similarities exist between animals and humans regarding the respiratory network. Harper et al. have described the relationship between damage to specific brain regions observed on brain imaging and specific disorders of respiratory control in humans.
Glutamate is the major neurotransmitter mediating excitatory synaptic input to brainstem respiratory neurons and respiratory premotor and motor neurons. Gamma aminobutyric acid (GABA) and glycine are the two major inhibitory neurotransmitters mediating inhibitory synaptic input in the respiratory network; they have a key role in pattern generation and termination of inspiratory activity. GABA (via GABA A receptors) and glycine (via glycine receptors) mediate fast synaptic inhibition via activation of chloride channels. Throughout development, glutamate always functions as an excitatory neurotransmitter; however, it is not the case that GABA and glycine are always inhibitory neurotransmitters. In early development, GABA and glycine mediate excitatory neurotransmission in many neuronal networks, including the respiratory network. GABA and glycine signaling modify the level of chloride in the cell. Activation of the sodium (Na + )-potassium (K + )-chloride (Cl) cotransporter (NKCC1) and the potassium-chloride transporter (KCC2) on the cell modulates intracellular ion concentrations. Specifically, NKCC1 brings Na + , K + , and 2Cl − into the cell, while activation of KCC2 moves K + and Cl − outside the cell. Low expression of KCC2 during early development, resulting in a high NKCC1/KCC2 ratio, causes high intracellular chloride concentrations in immature neurons. When GABA then binds to GABA A receptors, a net outward movement of Cl − ions occurs, leading to membrane depolarization. With maturation, KCC2 expression increases, reversing the NKCC1/KCC2 ratio and lowering intracellular Cl − ions. Now when GABA binds to GABA A receptors, more Cl − ions come into the cells, leading to hyperpolarization. With brain injury, NKCC1 expression increases, making the GABAergic system less inhibitory. Moreover, myoclonic jerks are associated with midazolam exposure (GABA A receptor agonist) in premature infants. GABA B receptors, which are metabotropic G protein-coupled receptors, also have a greater role in inhibiting respiratory rhythm in adult animals as compared with newborn animals. Finally, the baseline excitatory and inhibitory influences mediated by glutamate and GABA-glycine, respectively, on major neuronal networks are further altered by many endogenously released neuromodulators that shape and fine-tune respiratory pattern and rhythm throughout development, as outlined in Table 41.1 .
| Neurotransmitter/Neuromodulator | Receptor Subtype | Source of the Endogenous Ligand | Excitatory or Inhibitory on Respiratory Rhythm | Comment |
|---|---|---|---|---|
| Glutamate | NMDA, AMPA, GluR | Ubiquitously expressed | Excitatory | Major excitatory neurotransmitter |
| Ach | M3 | PAG, LC, X | Excitatory | |
| NE | α1-Adrenergic | LC | Excitatory | |
| Serotonin | 5-HT2A2B, 5-HT3, 5-HT4 | Raphé | Excitatory | |
| Dopamine | Likely D 1 | PVN, hypothalamus | Excitatory | |
| ATP | P2X2 | Ventral medulla; CO 2 /H +- sensitive cells in the RTN | Excitatory | |
| Adenosine | P2Y1 | Ventral medulla | Excitatory | |
| Substance P | NK1 | nTS, NA | Excitatory | |
| CCK | CCK1 | nTS, raphé | Excitatory | |
| TRH | TRH-R (1 and 2) | Raphé | Excitatory | |
| GABA | GABA A , GABA B | Ubiquitously expressed | Inhibitory | Major inhibitory neurotransmitter (can be excitatory during fetal life) |
| Glycine | GlyR | Inhibitory | Can be excitatory during fetal life | |
| NE | α2-Adrenergic | Pons | Inhibitory | |
| Dopamine | D4 | PVN, hypothalamus | ||
| Adenosine | A 1 , A 2 | Ubiquitous from metabolism of ATP that increases during hypoxia | Inhibitory | Contributes to respiratory depression at the baseline (A 1 ), and mediates HVD |
| Opioid | μ, δ, κ | nTS, PBN, PVN, raphé | Inhibitory | Prominent inhibitory effect during early development |
| PDGF | PDGF-β | nTS | Inhibitory | Contributes to HVD |
Some neuromodulators may be more critical in supporting respiratory rhythmogenesis than others. By identifying the genetic mutations that are associated with marked abnormalities in respiratory control, we can obtain a better understanding of the key role of several neuromodulator systems. For example, serotonergic neurons in the caudal medullary raphé nuclei have extensive projections to phrenic and hypoglossal motoneurons, the nTS, the RTN, and the PBC. The serotonergic system has a significant influence on the modulation and integration of diverse homeostatic functions, including cardiorespiratory responses and thermogenesis. Individuals with Prader-Willi syndrome, who may exhibit breathing abnormalities at birth, have mutations in the necdin gene ( NDN ) on chromosome 15 leading to abnormalities in the brainstem serotonergic system. Mice lacking the necdin gene also have abnormal brainstem serotonergic neurochemistry. Medullary serotonergic neurons are also CO 2 sensitive. In genetically modified mice that do not develop medullary serotonergic neurons, CO 2 sensitivity is reduced by 50%.
In some infants who have died of SIDs/sudden unexplained infant death (SUID), neuropathologic studies have shown disruptions of the brainstem serotonergic system. While specific single-gene mutations that regulate serotonin production and function have not been identified in infants who have died of SIDS, some studies have shown a higher proportion of specific polymorphisms in the 5′ regulatory region of the SLC6A4 gene, which encodes serotonin transporter, which regulates the reuptake of serotonin from the extracellular space. Specifically, infants who have died of SIDS have an increased frequency of the long allele variant and the variable number 12-tandem repeat in intron 2 polymorphisms in the promoter. The long allele occurs more frequently in African-Americans; African-Americans also have a 2.0-fold greater incidence of SIDS/SUID than whites. The higher risk of SIDS/SUID in non-Hispanic Blacks when compared to non-Hispanic whites has remained consistent even though the overall incidence of SIDS/SUID has decreased in both groups. Effectively, the polymorphisms result in increased activity of serotonin transporter, thereby decreasing the time that serotonin stays in the synapse, leading to a relative serotonin deficiency causing dysregulation of the cardiorespiratory system.
Rett syndrome is an X-linked disorder with mutations in several genes, but the most common genetic defect (90%) is in the methyl CpG binding protein 2 gene ( MECP2 ). Affected individuals are normal at birth and then experience progressive deterioration leading to severe motor, cognitive, and autistic behaviors. They also have characteristic severe respiratory disturbances with prolonged apnea and hyperventilation that can be fatal. Genetically modified mice that lack the MECP2 gene have reduced levels of norepinephrine and serotonin in the medulla and have breathing patterns similar to those of humans with Rett syndrome. Pharmacologic treatment to increase brain norepinephrine and serotonin levels stabilizes breathing and prolongs the life of these mice. A case report demonstrating the efficacy of fluoxetine and buspirone in reducing breathing dysregulation in a patient with Rett syndrome has been published. Altered GABA neurotransmission might also be causative as suggested by experiments using stem cells from a patient with Rett syndrome that demonstrated that the functional switch of GABA neurotransmission from excitation to inhibition was impaired.
Congenital central hypoventilation syndrome (CCHS), also known as Ondine’s curse , is another rare autosomal dominant genetic disorder, occurring in 1 in 200,000 live births. Affected individuals characteristically have adequate ventilation during wakefulness but profound hypoventilation during sleep as well as impaired ventilatory responses to CO 2 and hypoxia during sleep and wakefulness. Although the disorder most commonly presents during infancy, milder forms may present later in childhood or even during adulthood. More than 90% of individuals with CCHS have mutations in the PHOX2B gene. PHOX2B is a homeobox gene located on chromosome 4 that is specifically expressed in limited types of neurons involved in autonomic processes. Its expression is required for the development of the carotid body, nTS, and catecholaminergic neurons. It is also expressed in chemosensitive glutamatergic neurons in the RTN that receive polysynaptic inputs from peripheral arterial chemoreceptors. Neurons in the nTS that express PHOX2B also appear to be chemosensitive to CO 2 in preclinical models . Thus, mutations in the PHOX2B gene alter the development of key structures that regulate the chemical control of breathing.
The PHOX2B gene has a stretch of 20 alanine repeats in exon 3. Most affected individuals have the classic mutation that adds additional alanine repeats to the 20-alanine repeat, resulting in a stretch of 25 to 35 alanine repeats instead of 20. This mutation is classified as polyalanine repeat mutations (PARMs). The severity of the disease is correlated with the number of extra alanines. Patients with PHOX2B 20/25 have 25 versus 20 alanines; they have the mildest disease, may never need 24-hour ventilatory support, and may present only after infection or exposure to agents that inhibit respiration. On the other hand, patients with 28 to 32 alanine repeats in the gene ( PHOX2B 20/28-32 ) often need continuous ventilatory support. Fewer affected individuals with CCHS have non-polyalanine repeat mutations (NPARMs) resulting in deletions in exon 3 that cause frameshift mutations. Depending on the mutation, some patients require tracheostomy and long-term ventilation and/or diaphragmatic pacing. While respiratory stimulants are not effective in increasing respiratory drive, drugs that cause respiratory depression may be harmful.
From genetically modified mouse models with mutations in the PHOX2B gene, we know that its expression is essential for the development of the RTN in the brainstem and catecholaminergic and cholinergic traits in the autonomic nervous system. The RTN contains putative central chemoreceptors that have intrinsic pH sensitivity and release the excitatory neurotransmitter glutamate, thereby stimulating breathing during hypercapnia. However, abnormalities throughout the autonomic nervous system are often seen in patients with CCHS. Specifically, patients can present with Hirschsprung disease (Haddad syndrome) and neuroblastomas (neuroblastoma-Hirschsprung disease-CCHS syndrome). As reviewed by Moreira et al. Hirschsprung disease occurs in 80% of patients with NPARM and 10% of patients with PARM. Similarly, neural crest tumors are found in 1% of PARM patients and in 41% of NPARM patients.
The nTS in the brainstem is where sensory information from vagally mediated reflexes and chemical signals from the blood (arterial chemoreceptors) and cerebrospinal fluid (central chemoreceptors) and information from higher brain regions are integrated; neurons from the nTS synapse onto respiratory-related neurons, thereby augmenting or attenuating minute ventilation.
Essentially all bronchopulmonary reflexes that modify the depth and duration of inspiration and expiration are mediated through the vagus nerve. The vagus nerve has both myelinated and unmyelinated fibers. Myelinated vagal afferent fibers are activated via (1) slowly adapting stretch receptors (SARs), which are activated by volume and stretch of the lung (mediating the Breuer-Hering reflex); or (2) rapidly adapting receptors (RARs) that are activated in response to inhaled irritants (e.g., ammonia, cigarette smoke) and large inflations or deflations of the lung. Activation of SARs changes the duration of inspiration and expiration, whereas activation of RARs causes sighs (i.e., augmented breaths) and cough. Unmyelinated vagal afferents, specifically C-fibers in the airway, are activated by a multitude of chemical stimuli, including CO 2 and capsaicin, in addition to lung edema and elevated temperature. Activation of C-fibers in the lung causes rapid shallow breathing and apnea. Table 41.2 lists bronchopulmonary and upper airway reflexes and their physiologic responses.
| Receptor | Characteristics | Stimulant | Responses | Comment |
|---|---|---|---|---|
| Slowly adapting stretch receptors |
|
Lung volume and transmural pressure |
|
Breuer-Hering reflex more active in infants than in adults |
| Rapidly adapting receptors |
|
|
|
Responsible for inducing sighs in premature infants—restoring functional residual capacity |
| Bronchial and pulmonary C fibers |
|
|
|
J-receptors located in alveoli activated by lung edema |
| Laryngeal chemoreflex |
|
|
|
May contribute to apnea and bradycardic events associated with oral feedings in premature infants. Immature responses are exacerbated during hypoxia |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here