Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We thank Drs. S. Morel, F. Molica, and O. Rusiecka for help with the preparation of figures and critical proofreading. The work of B.R.K. is supported by the Swiss National Science Foundation.
Connexins (Cxs) and pannexins (Panxs) have been implicated in a variety of cardiovascular diseases. Although their causal involvement in disease development has been clear in the case of certain mutations, protein function, location, and expression are often altered downstream of other pathologic events, where causality is often less clear. In these cases, connexin and pannexin dysfunction may still aggravate outcome by accelerating disease progression or adding to morbidities such as arrhythmias.
In specific conditions, genetics have confirmed the importance of connexins and pannexins in cardiovascular disease initiation and progression. In 2006, Gollob and colleagues showed that somatic mutations in Cx40 occurred in some patients with idiopathic atrial fibrillation (AF), and since then Cx43 has also been linked to the development of AF. The association between connexins and AF confirms our understanding of the pivotal role of these proteins in the propagation of action potentials but, in stark contrast, dominant negative mutations in Cx43, which cause oculodentodigital dysplasia, are not linked to cardiac electrophysiologic disturbances. This conundrum uncomfortably illustrates that despite extensive research, our understanding of connexins in cardiac conduction remains incomplete.
Genetics also points to the importance of connexins and pannexins in noncardiac cells of the cardiovascular system, in which single nucleotide polymorphisms (SNPs) in Cx37 and Panx1 increase the risk of atherosclerosis and thrombosis, respectively.
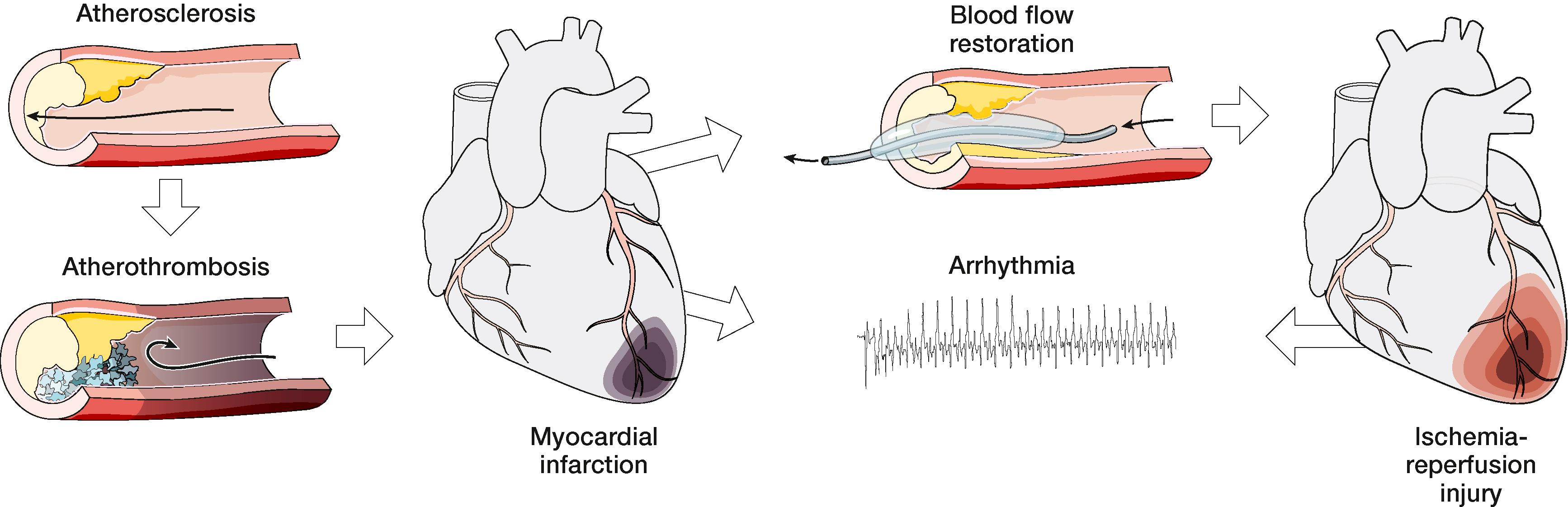
The majority of our knowledge, however, stems from investigations in acquired diseases; the involvement of connexins has been tested in a host of cardiovascular diseases, whereas our knowledge about pannexins is scarcer. An exhaustive review is beyond the scope of this chapter, but one striking example that involves and affects both connexins and pannexins in many cardiovascular cell types is acute cardiac ischemia. We briefly summarize the role of these proteins during acute myocardial infarction (MI) in the processes leading to this event and in the early complications associated with resolution of ischemia ( Fig. 15.1 ).
Cxs are members of a family of proteins encoded by 20 to 21 different mammalian genes that are expressed in virtually every tissue of the body. , Connexin genes are classified according to sequence homology and are divided into five subfamilies: α ( GJA ), β ( GJB ), γ ( GJC ), δ ( GJD ), and ε ( GJE ). However, connexin proteins are traditionally named after their specific molecular weight in kilodaltons. The general structure of a connexin consists of four α-helical transmembrane domains (TM1–TM4) and two extracellular loops (EL1 and EL2) that are highly conserved among the family members ( Fig. 15.2 ). Furthermore, the amino- and carboxy-termini (NT and CT) and the cytoplasmic loop (CL) of the proteins are located in the cytoplasm; the CL and CT differ considerably in length and composition, are nearly unique to each connexin type, and are importantly involved in the regulation of channel assembly, trafficking, and gating. Connexins are synthesized in the endoplasmic reticulum (ER) and oligomerize in the ER/Golgi or trans-Golgi network to form hexameric connexons. , Then, connexons traffic to the plasma membrane along microtubules. When two cells are closely apposed, connexons from one cell can dock with their counterparts in the neighboring cell to form an intercellular gap junctional channel that enables the direct intercellular exchange of ions and small messenger molecules. Gap junctional channels form densely packed plaques that appear as a pentalaminar structure in electron microscopy (see Fig. 15.2 ). Under specific conditions, connexons may function as a hemi-channel allowing the permeation of ions and small metabolites, such as Ca 2+ , adenosine triphosphate (ATP), and glutamate. A variety of factors regulate connexin channel gating including voltage, pH, and Ca 2+ ; phosphorylation; and other posttranslational modifications. , Increasing evidence points to an important regulatory function for the “connexin interactome,” a protein interacting platform with the connexin as the central mediator. The cardiac intercalated disc (ID), for example, appears to host such an interacting complex of gap junctions, desmosomes, and sodium channels that together control electrical coupling, intercellular adhesion, and excitability. ,
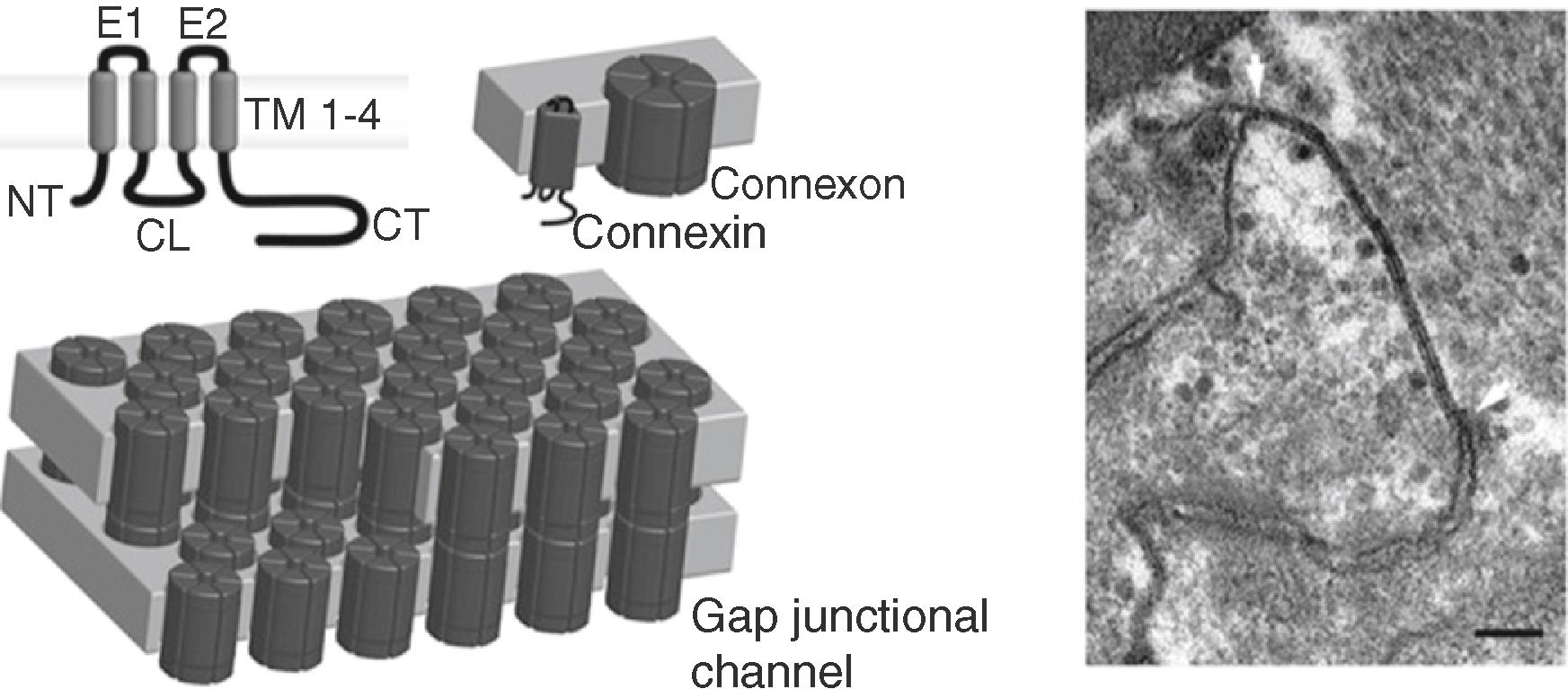
Pannexins represent yet another small family of three membrane proteins (Panx1–3) that exhibit a similar tetraspan topology as the large family of connexins, although they share no sequence homology. , Although it was initially thought that pannexins form hexameric single-membrane channels, recent structural data showed that Panx1 channels are heptamers, whereas no detailed structural data are yet available for Panx2 and Panx3. Important glycosylation sites are found in the ELs of Panx1 and Panx3. , The occupation of these sites likely prevents docking of pannexons. Similar to connexin hemi-channels, pannexin channels allow for intercellular communication by the release of small molecules, for instance, purines or prostaglandins, that can signal via targeting surface receptors in a paracrine fashion. Panx1 channels can, however, be opened at physiologic membrane resting potential and regular extracellular [Ca 2+ ], by mechanical stretch and after purinergic P2 receptor stimulation ; whereas connexon opening is generally induced under more pathologic conditions such as low extracellular [Ca 2+ ] or very large depolarizations.
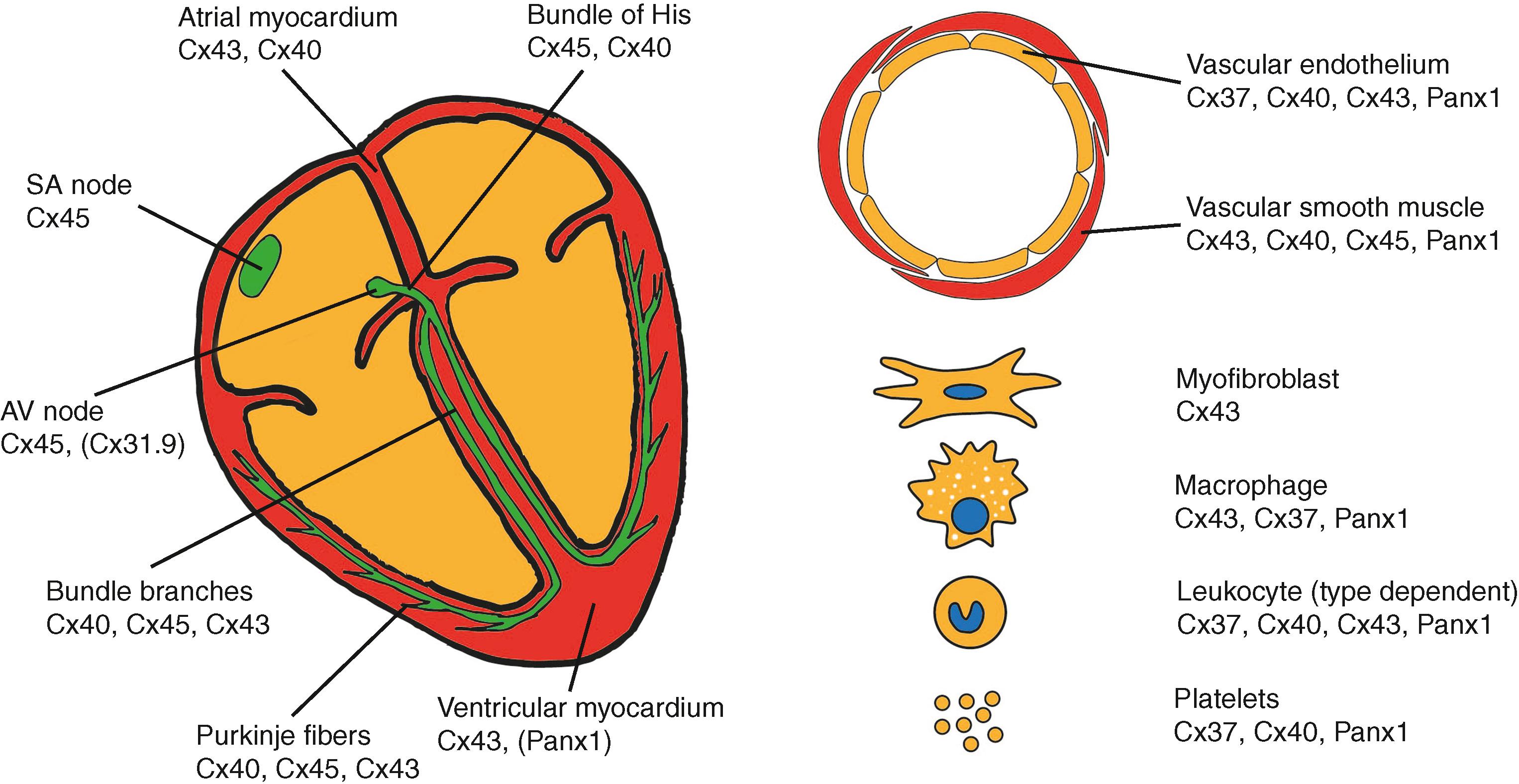
Most connexin and pannexin research in the cardiovascular system involves Cx37, Cx40, Cx43, Cx45, and Panx1. , In cardiac cells, the dominating connexin is Cx43, which is almost the sole connexin expressed in the working ventricular myocardium, whereas that of the atria also express Cx40 (as schematically shown in Fig. 15.3 ). The connexin expression of the conduction system is more diverse, with Cx45 dominating the sinoatrial (SA) and atrioventricular (AV) nodes and varying degrees of Cx45, Cx40, and Cx43 when moving from the bundle of His, through the bundle branches, to the Purkinje fibers. In addition, mouse nodal tissue expresses Cx30.2, which paradoxically slows AV conduction, and although mRNA for the human ortholog, Cx31.9, has been detected, it has not been possible to demonstrate its presence by immunohistochemistry. Although Panx1 has been found in cell lysates of the (left) ventricle, it remains uncertain which cell type expresses this protein. , Other interesting newcomers to the cardiac conduction field are resident macrophages, which couple to cells of the AV node and regulate its conduction. In the vasculature, endothelial cells (ECs) lining large arteries typically express Panx1, Cx37, and Cx40 and, to a lesser extent Cx43, depending on the blood flow pattern experienced. Vascular smooth muscle cells (SMCs) in the media of arteries and arterioles express Cx43, Cx40, and Cx45. Panx1 is also expressed in vascular SMCs of resistance arteries. Finally, Cx43, Cx40, Cx37, and Panx1 have been detected in inflammatory cells, dependent on the type of leukocyte, , and Cx37, Cx40, and Panx1 have been found in platelets. Maintenance of Panx1 channels, connexin hemi-channel, and/or gap junction functionality and integrity is fundamental to conserve intercellular communication and thus homeostasis in a wide range of tissues.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here