Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 2000, the Congenital Heart Surgery Nomenclature and Database Project classified congenital tracheal stenosis as congenital–complete tracheal rings, postintubation, traumatic, or congenital web. Localized stenosis was defined as less than 50% of the tracheal length, and long-segment stenosis as greater than 50% of the tracheal length. The initial classification of tracheal stenosis had been proposed by Drs. Cantrell and Guild from the University of Washington, Seattle, in 1964. They classified these patients into three morphologic types: (1) generalized hypoplasia, (2) funnel-like stenosis, and (3) segmental stenosis. They also noted the frequent association of a tracheal right upper lobe bronchus that is present in 20% of these patients ( Fig. 112-1 ). Dr. Hermes Grillo from Massachusetts General Hospital is considered to be the father of tracheal surgery. In a series of landmark publications in the 1960s, he defined the blood supply of the trachea, the surgical approach to the trachea, and tracheal release procedures for resection. The first successful operation for congenital stenosis of the trachea was reported by Dr. Ken Kimura from Kobe Children's Hospital in Kobe, Japan, in 1982. He used a costal cartilage graft to augment the tracheal lumen of a 12-month-old infant with tracheal stenosis secondary to complete tracheal rings (cartilage tracheoplasty). The use of pericardium to augment the tracheal lumen was first performed by Dr. Farouk Idriss from Children's Memorial Hospital, Chicago, in 1982. The operation was facilitated by the use of cardiopulmonary bypass for respiratory support during the procedure. Slide tracheoplasty was first reported by Drs. Victor Tsang and Peter Goldstraw from Brompton Hospital in London, England, in 1989. Its use in children was popularized by Dr. Grillo and his associates. Drs. Claus Herberhold and Martin Elliott from Great Ormond Street Hospital, London, performed the first successful tracheal homograft to augment the lumen of a patient with a failed prior intervention for tracheal stenosis from congenital tracheal rings in 1994. Drs. Carl Backer and Constantine Mavroudis reported the use of a free tracheal autograft for infants with congenital tracheal stenosis in 1998, 2 years after they performed the first operation. Pulmonary artery sling repair was first performed by Dr. Willis J. Potts at Children's Memorial Hospital in 1953. The left pulmonary artery was divided at its origin from the right pulmonary artery and reanastomosed anterior to the trachea.
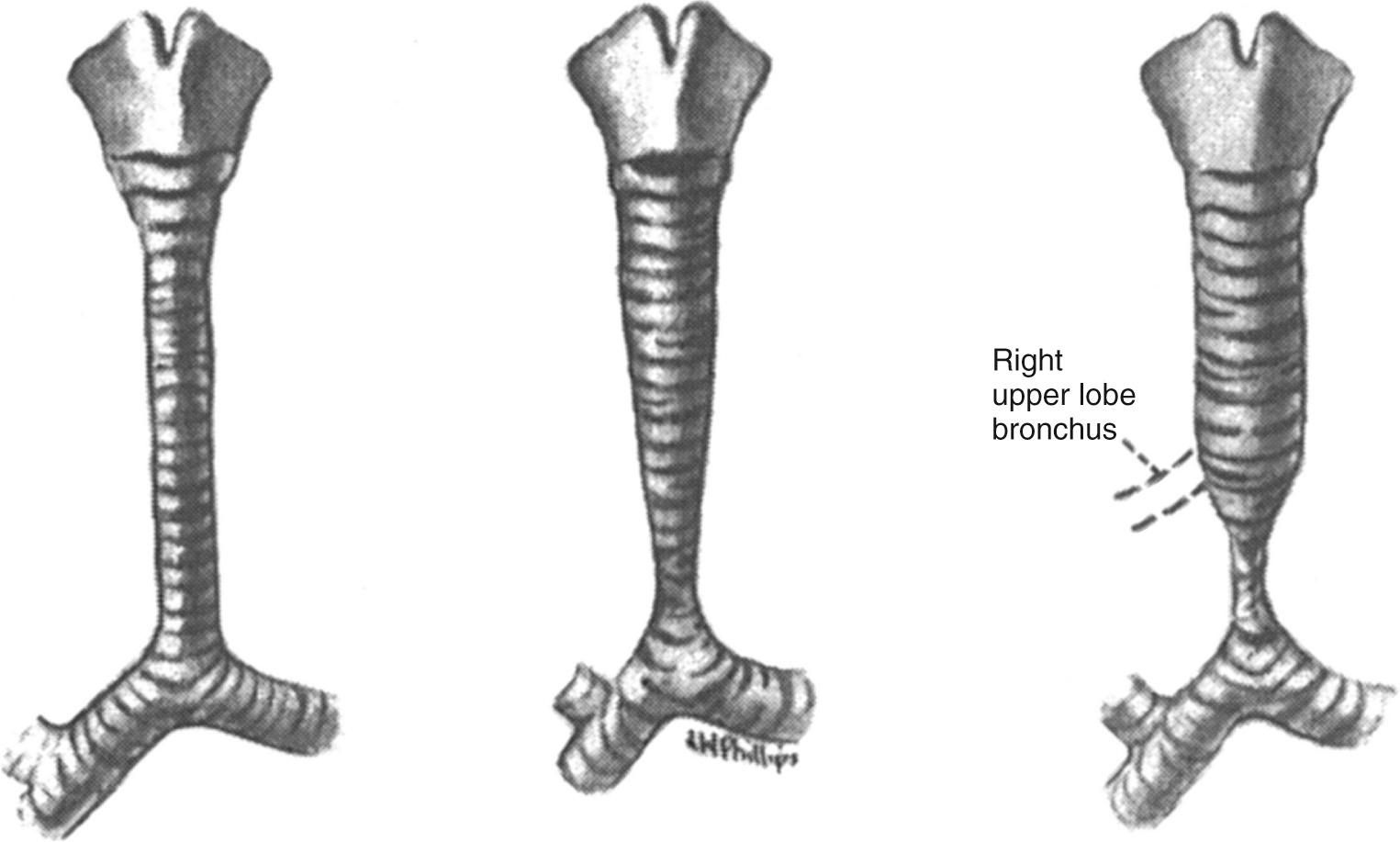
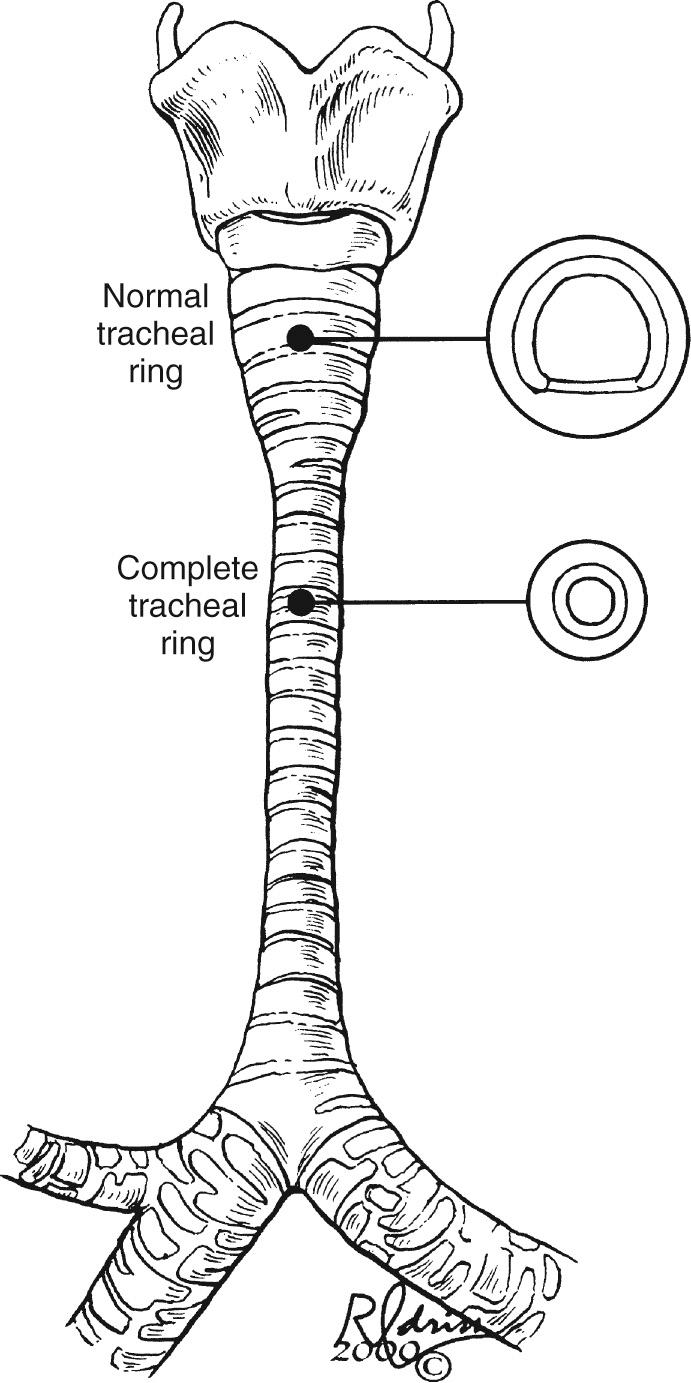
The two most common congenital tracheal anomalies in infants and children are tracheomalacia and tracheal stenosis. Congenital tracheal stenosis is most commonly caused by complete cartilage tracheal rings, and it is the most common indication for surgical intervention. The normal trachea has an anterior arch-shaped cartilage and a posterior membranous trachea. In children with complete tracheal rings, the cartilage ring is circumferential and the membranous trachea is absent ( Fig. 112-2 ). In contrast, tracheomalacia is associated with a normal or sometimes broad membranous trachea. Failure of the C-shaped cartilaginous portion to maintain a minimum of rigidity to the trachea results in “floppiness,” which results in functional tracheal narrowing, particularly during expiration. The circumference of the trachea is typically normal during inspiration, or when a patient is intubated (e.g., for a computed tomography [CT] study). Tracheomalacia is a chronic condition that infrequently requires surgical intervention. It often improves with time and growth of the child. Patients with tracheal stenosis often have associated anomalies, most commonly pulmonary artery sling and congenital cardiac anomalies. Pulmonary artery sling is present in a third of patients with congenital tracheal stenosis. Major intracardiac anomalies are present in 25% of the patients with congenital tracheal stenosis.
The diagnosis of congenital tracheal stenosis should be suspected in any infant who has stridor (noisy breathing), respiratory distress, apnea, cyanosis, wheezing, retractions, or respiratory failure requiring intubation. Tracheal stenosis can result in a difficult intubation (e.g., the endotracheal tube selected for the patient's size fails to pass into a normal location in the trachea), and this traumatization of the trachea can cause future tracheal stenosis.
Patients with known or suspected tracheal problems should be evaluated with a chest radiograph, CT or magnetic resonance imaging (MRI) (or both), bronchoscopy, and echocardiography ( Box 112-1 ). The chest radiograph often demonstrates the diminutive nature of the tracheal lumen. It reveals lung agenesis or hypoplasia. A CT scan or MRI shows the tracheal lumen in cross section and provides a measurement of the tracheal stenosis. A tracheal right upper lobe can be identified if present. Three-dimensional reconstruction can generate a very good representation of the tracheal pathology ( Fig. 112-3 ).
Chest radiography
Computed tomography (CT) with contrast, including three-dimensional reconstruction or dynamic CT
Rigid bronchoscopy
Echocardiography (to rule out pulmonary artery sling, congenital cardiac anomalies)
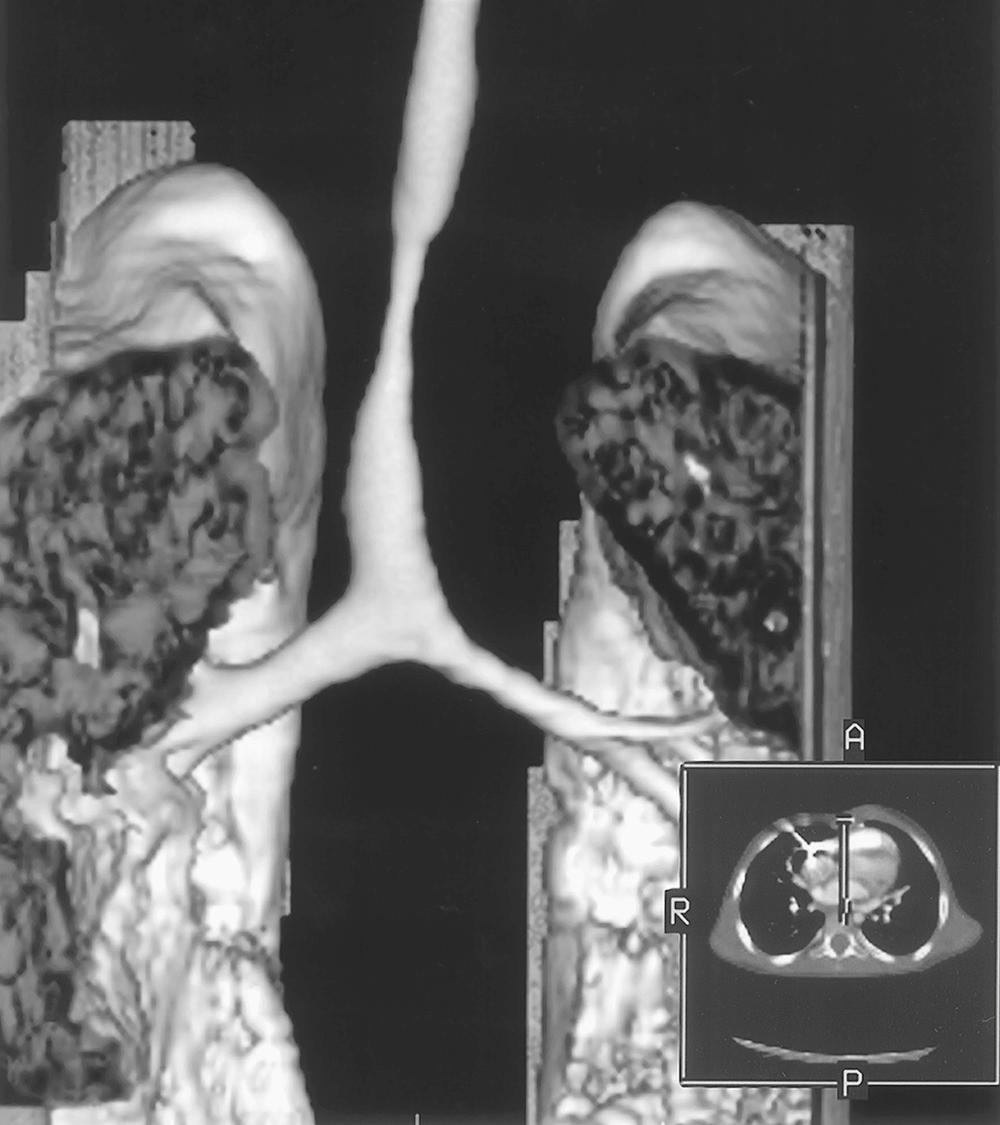
In our experience, CT has been more helpful than MRI because it is usually quicker (i.e., requires less sedation, or obviates the need for intubation), and there is less movement artifact. It can be performed as a dynamic expiratory CT scan—that is, in synchrony with the child's inspiration and expiration. End-inspiratory and dynamic expiratory volumetric imaging can be obtained. This type of dynamic study is helpful when evaluating children who might have tracheomalacia but do not fit a typical symptomatic pattern. However, CT results in a much higher radiation burden, which should be taken into consideration if the child is likely to require repeated imaging studies. Contrast tracheobronchography, which generates high-quality images, is still used sometimes. However, CT generates such excellent images that the risks of contrast tracheograms mostly outweigh the benefits, so this technique is usually used only in conjunction with a catheterization. A pulmonary artery sling can be demonstrated by CT if the CT is performed with contrast, or by MRI. For the sick neonate who is transferred with a critically positioned endotracheal tube and congenital tracheal stenosis, transthoracic echocardiography is the diagnostic procedure of choice to demonstrate a pulmonary artery sling. Echocardiography should be performed on all of these infants because of the 25% incidence of congenital heart disease found in patients with congenital tracheal stenosis.
The most important diagnostic procedure in all patients is a rigid bronchoscopy. This can be performed as a separate procedure before the planned tracheal operation, or it can be done at the time of the tracheal procedure. In some severe cases, distal visualization of the trachea cannot be obtained by bronchoscopy because of the risk of occluding the tracheal lumen. The child can then be placed on cardiopulmonary bypass, and bronchoscopy can be performed safely.
In general, the incidence of airway complications, including anastomotic dehiscence, rises with the length or amount of trachea that is resected. Younger patients and reoperative tracheal procedures also have significantly higher complication rates. Tracheal anatomy, including blood supply, should be well understood. Grillo expressed concern about devascularization in adults, but this appears to be less important in children, who have an excellent mediastinal blood supply. Right and left recurrent laryngeal nerves should be protected at all cost. A good relationship with pediatric otorhinolaryngologists with special expertise in endoscopic and surgical airway management is essential.
Postoperative management differs with the age of the child. For older children, adult principles can be applied, and a “chin stitch” can be used to prevent neck extension. In all cases, a flexible bronchoscopy is performed at 5 to 7 postoperative days. Younger children can be fitted with a neck prosthesis to prevent important neck excursions, but this is rarely used. It may be best to leave neonates and young infants sedated and intubated for 5 to 7 days, and to perform flexible bronchoscopic examination before extubation.
Cantrell and Guild published an early report of a successful tracheal resection in a child in 1964, and they also reported the three morphologic varieties of congenital tracheal stenosis. The 7-year-old patient had a tracheal right upper lobe and a bridging bronchus to the carina. The bridging bronchus, which was stenotic, was excised, and the carina was brought up to the main trachea. An anastomosis was performed with interrupted sutures of braided stainless steel. The child survived the operation and was asymptomatic on follow-up.
Tracheal stenosis amenable to tracheal resection with end-to-end anastomosis was originally thought to be feasible for stenosis involving up to 50% of the total tracheal length. However, a report from Massachusetts General Hospital has indicated that resections of more than 30% of the trachea have a substantial failure rate. We believe that tracheal resection is the preferred technique if the stenosis is less than a third of the total tracheal length. This is usually six to eight complete tracheal rings.
We use the technique developed by Grillo for tracheal resection and reanastomosis (TRR). In adults, most TRRs are performed via a transverse cervical (“collar”) incision. The airway can be safely managed intraoperatively with careful endotracheal tube positioning. In children, especially small children and infants, control of the airway is much more precarious. As in most experienced pediatric centers, we believe that most if not all tracheal resections in younger children should be performed through a median sternotomy approach with cardiopulmonary bypass. If the tracheal stenosis extends up to the cricoid, a collar incision is made in the neck, forming a “T” with the median sternotomy incision. This incision tends to heal better than a midline extension of the median sternotomy onto the neck. The strap muscles are divided in the midline. The thymus is partially or entirely excised. The innominate artery and vein are dissected free and encircled with vessel loops. These may then be retracted superiorly or inferiorly to address the various components of the trachea. The aorta is mobilized from its pericardial attachments and retracted with a small pledgeted suture to the left. Care is taken to avoid injury to the right or left recurrent laryngeal nerves. The anterior surface of the trachea is freed from pericardial attachments so that it may be completely visualized from cricoid to carina if necessary.
The site of the stenosis is identified externally. At the site of the stenosis and up to one ring above and below the stenotic segment (at most, two rings above or below), dissection must be performed circumferentially and posteriorly to facilitate the removal of the stenotic portion of the trachea. Attention must be paid to the lateral blood supply of the tracheal wall and a decision made regarding which vessels can be preserved for an adequate blood supply to the eventual anastomosis. In most cases, the carinal attachments are freed, as are the pericardial attachments of the right and left mainstem bronchi. Cardiopulmonary bypass is initiated with a single right atrial and aortic cannula, and the aorta is retracted to the left. Cooling is not necessary, and we aim for a temperature of 32° to 34° C. The trachea is opened in the midline through the extent of the tracheal stenosis. If the stenotic portion can be identified from the outside, bronchoscopic guidance is not needed. Three-dimensional CT scan reconstruction has been used to identify the area of stenosis with enough accuracy to allow opening at the correct site. If the focal area of stenosis cannot be identified externally, intraoperative bronchoscopy, using the illuminated tip of the bronchoscope for guidance, can demonstrate the area of stenosis.
The technique of passing a 25-gauge needle through the lightest portion of the stenosis also is useful for some patients. The anterior tracheal incision is extended proximally and distally until the extent of the complete tracheal rings or stenosis has been encompassed. If this length is less than 30% of the trachea (six to eight complete tracheal rings), the segment is then simply excised. The two ends are brought together, and the anastomosis is performed with interrupted polydioxanone (PDS) sutures ( Fig. 112-4 ), with the knots tied outside. If there is little tension, it is acceptable to run the posterior wall. Before the anterior sutures are tied, the trachea is suctioned free of secretions and blood with a fine plastic suction catheter. At completion, flexible bronchoscopy is always performed before leaving the operating room. A tension-free anastomosis is the key, and we liberally use the Grillo-derived lateral stay sutures, placed one ring above and one below the anastomosis, to bring the two edges together and take some of the tension away from the suture line itself.

It is important to determine before the resection of tracheal tissue (including the stenotic portion) whether a TRR or a slide tracheoplasty will be performed. In the slide tracheoplasty, the extent of tracheal resection should be minimized to use the tracheal wall as a flap. If the length of the stenosis is too long for a successful end-to-end anastomosis, the operation can be converted to the tracheal autograft technique. Results of tracheal resection in infants and children are shown in Table 112-1 . Complications include anastomotic leak, restenosis, and tracheomalacia.
| Surgeon | Year | Patients (N) | Mortality |
|---|---|---|---|
| Ziemer | 1998 | 8 | 1 (12%) |
| Jones | 1999 | 6 | 1 (16%) |
| Grillo | 2002 | 46 | 2 (4%) |
| Backer | 2002 | 12 | 1 (8%) |
| Totals | 72 | 5 (7%) |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here