Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Several congenital abnormalities of the thorax have been described, but most are rare. Classification of these anomalies is difficult because the embryologic basis often is not clearly understood. A classification based on anatomic structures uses the categories of trachea, bronchi, lung, and pulmonary vasculature. Although often described as separate entities, bronchopulmonary malformations are frequently interrelated, and features of different imaging and pathologic entities may coexist in the same lesion or separately in the same individual.
In children and adults, most congenital bronchopulmonary malformations (CBMs) can be diagnosed noninvasively with radiography, ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). In this chapter, the more common congenital abnormalities encountered in adults are emphasized. Those more commonly identified in infancy are discussed in Pediatric Radiology: The Requisites . Congenital lesions of the heart, pericardium, and aorta are discussed in Cardiac Radiology: The Requisites .
The most common congenital anomalies of the trachea include tracheomalacia, tracheal stenosis, tracheobronchomegaly, tracheoesophageal fistula (TEF), and tracheal bronchus. Tracheal agenesis and atresia are not discussed because they are associated with dismal prognosis, resulting in near-immediate postnatal death.
Tracheomalacia refers to excessive pliability of tracheal cartilage, which leads to abnormal collapsibility of the trachea. It may be primary, associated with a localized absence or deficiency of the tracheal cartilage, or secondary, resulting from external compression by an extrinsic mass. Tracheomalacia may also occur in conjunction with other anomalies of the foregut, particularly TEF. Tracheomalacia must be differentiated from excessive dynamic airway collapse related to abnormal expiratory pressures. For example, collapse of a long segment of the intrathoracic trachea may occur in late expiration in patients with asthma, chronic bronchitis, and bronchiolitis caused by excessive anterior bowing of the posterior tracheal membrane rather than softening of tracheal cartilage.
Paired inspiratory and dynamic expiratory CT is excellent for evaluating tracheomalacia in adult patients. Anterior bowing of the posterior membrane and a greater than 70% cross-sectional area reduction of the trachea between inspiration and expiration are considered diagnostic. Other CT features of the tracheal lumen that are highly suggestive of tracheomalacia include a crescentic “frown” shape in expiration ( Fig. 8.1 ) and a “lunate” morphology (coronal:sagittal ratio >1) in inspiration. The accuracy of these techniques is comparable with that of bronchoscopy, the gold standard for diagnosing tracheomalacia.
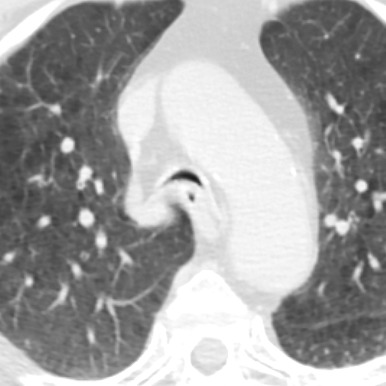
Congenital tracheal stenosis is a rare condition characterized by fixed narrowing of the trachea. The three patterns of congenital tracheal stenosis (CTS) are (1) a diffuse or generalized hypoplasia, (2) a funnel-like stenosis ( Fig. 8.2 ) often associated with an anomalous origin of the left pulmonary artery, and (3) segmental stenosis. The diffuse or funnel-like patterns may be associated with complete tracheal cartilage rings that replace the posterior membranous wall of the trachea. Patients with complete tracheal rings usually present with respiratory distress in the first few weeks of life, and this condition is associated with a high mortality rate. Rarely, the diagnosis is delayed until adulthood. Complete tracheal rings may be isolated events or may be associated with pulmonary vascular slings. CTS can be associated with abnormal bronchial branching patterns such as tracheal bronchus, bridging bronchus, and bronchial and lung agenesis. CTS has an association with other anomalies such as TEF, congenital heart disease, and skeletal abnormalities.
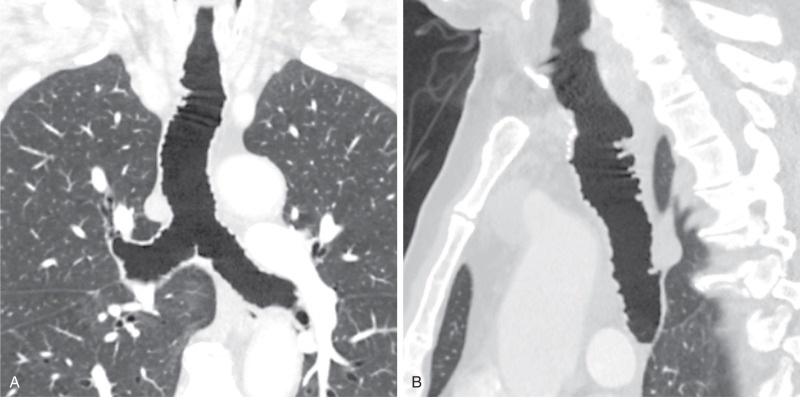
Tracheobronchomegaly (i.e., Mounier-Kuhn syndrome) is characterized by abnormal dilation of the trachea and bronchi thought secondary to atrophy of the elastic tissues and smooth muscle of the trachea and central bronchi. The disease is often diagnosed in adults, although symptoms of the condition may begin in childhood. In addition to tracheobronchomegaly, frequent outpouchings and possible diverticula of the intercartilaginous membranes are seen on radiography and CT, giving the trachea a characteristic corrugated appearance. The diagnosis can be established by chest radiography when the transverse diameter of the trachea is greater than 25 mm in men and 21 mm in women. Bronchomegaly is established when the diameters of the right and left main bronchi are greater than 21 and 18 mm in men and 20 and 17 mm in women. CT can confirm the increased diameter of the trachea in which a tracheal diameter greater than 3 cm and mainstem bronchi diameters greater than 2.4 cm are highly suggestive of Mounier-Kuhn syndrome ( Fig. 8.3 ). Frequently, there is concomitant cystic bronchiectasis. Collapse of the trachea and central airways can be observed on expiratory imaging.
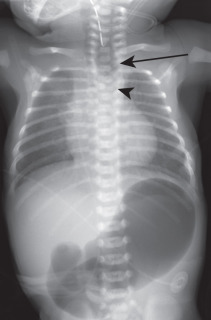
Congenital tracheoesophageal fistula (TEF) is invariably a pediatric disease. Most (70%–80%) occur in the presence of esophageal atresia with a distal TEF. However, about 4% of all TEFs occur with an otherwise normal esophagus (the so-called H-type fistula), and in these instances, patients may present in adult life with recurrent pneumonia. The esophagus communicates most often with the trachea (75%) and less frequently with the major bronchi. Congenital TEF carries a strong association with other congenital anomalies and is a component of the VACTERL association (vertebral anomalies, anal atresia, cardiac defects, TEF, renal anomalies, and limb defects). The chest radiograph may show evidence of bronchiectasis or an air-distended esophagus ( Fig. 8.4 ). Esophagography can identify the fistulous communication between the tracheobronchial tree and pooling of oral contrast within the alveoli. See Pediatrics: The Requisites for more information about TEF.
A tracheal bronchus is an abnormal bronchial branching pattern in which all or part of the upper lobe bronchial supply originates from the trachea. This usually occurs within 2 cm of the carina and most commonly on the right ( Fig. 8.5 ). Although classically a tracheal bronchus is considered to arise from the trachea, the literature also refers to an anomalous bronchus originating from the proximal main bronchus above the carina as a tracheal bronchus. In a bronchus suis, or “pig bronchus,” the entire upper lobe bronchial supply arises from the trachea. If all the segmental bronchi arise normally from the upper lobe bronchus (three segmental in the right upper lobe, four segmental in the left upper lobe), the bronchus that arises from the trachea is referred to as supernumerary. If one or all of the normal upper lobe segmental bronchi are absent, the tracheal bronchus is referred to as displaced. Tracheal bronchi are usually of no clinical consequence. In a minority of patients, impaired drainage may result in air trapping, recurrent infections, or bronchiectasis . During endotracheal intubation, an endotracheal tube balloon cuff may inadvertently obstruct the aberrant bronchus, causing lobar or segmental atelectasis.
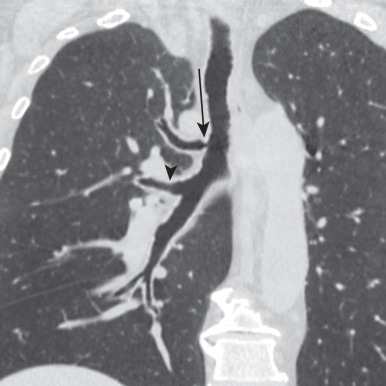
An accessory cardiac bronchus (ACB) is a supernumerary bronchus arising from the medial wall of the bronchus intermedius and extending toward the heart. ACB is uncommon, with a reported incidence of 0.07% to 0.5% but may be an incidental discovery on chest CT or bronchoscopy. Most are stumplike and blind ending ( Fig. 8.6 ) and contain cartilage in their walls, distinguishing them from a diverticulum. Others may be more elongate, supplying a small lobule of underdeveloped pulmonary tissue referred to as a “cardiac lobe.” ACB is often an incidental imaging or bronchoscopic finding in asymptomatic adults. Occasionally, an ACB may cause symptoms (e.g., hemoptysis) when associated with chronic inflammation or infection, retained secretions, foreign bodies, or rarely malignancy. Traumatic injury of ACB during bronchoscopy may lead to complications such as pneumothorax.
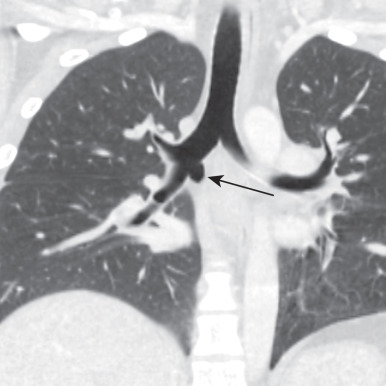
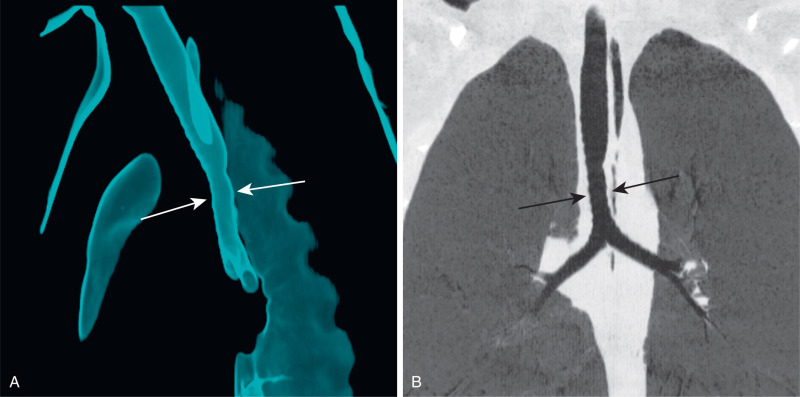
Bronchopulmonary isomerism refers to an identical pattern of bronchial branching and an equal number of lobes in each lung. Isomerism has a frequent association with abnormal left-to-right arrangement of the thoracic and abdominal organs, collectively known as heterotaxy syndrome. The most reliable imaging finding that defines right- or left-sided isomerism is the position of the mainstem bronchi in relation to the central pulmonary arteries. Bilateral eparterial bronchi (bronchus above the pulmonary artery in the hila) characterize the right bronchial isomerism type. Right-sided isomerism (bilateral right-sidedness) confers a poorer prognosis and frequent association with asplenia and severe congenital heart lesions. Bilateral hyparterial bronchi (bronchus below adjacent pulmonary arteries) define left isomerism (see Fig. 8.2 ). Left isomerism (bilateral left-sidedness) is associated with polysplenia.
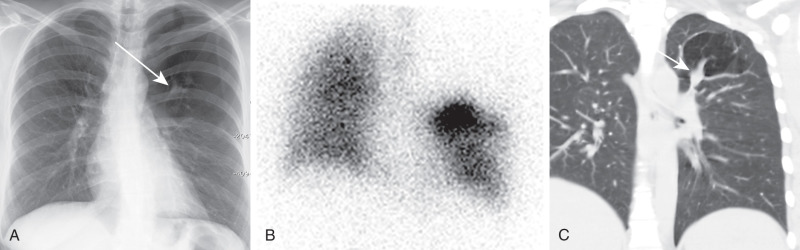
In bronchial atresia, there is focal atresia of a lobar, segmental, or subsegmental bronchus with focal obliteration of the lumen. Distal to the point of atresia, the airways remain patent and accumulate a mucous plug or mucocele, one of the characteristic features on imaging. The most common site is the left upper lobe, particularly the apicoposterior segment. The right middle and right upper lobes are less common sites. The lobe or segment of the lung distal to the point of atresia is aerated by collateral air drift and becomes hyperinflated because of air trapping distal to the point of atresia. The chest radiograph may show an ovoid or branching nodule or mass that corresponds to the mucocele. Chest radiography may also show an area of hyperlucency in the affected segment(s) of the lung ( Fig. 8.7 ). CT demonstrates a low-attenuation, nonenhancing, ovoid, or branching mass corresponding to the mucocele as well as characteristic hyperinflation in the lung distal to the mucocele (see Fig. 8.7 ). There may be an accompanying shift of the mediastinum and compression of the surrounding lung. It is important to consider and exclude an endobronchial mass (e.g., a carcinoid tumor) or a foreign body, which could have a similar appearance with a distal mucous plug and air trapping.
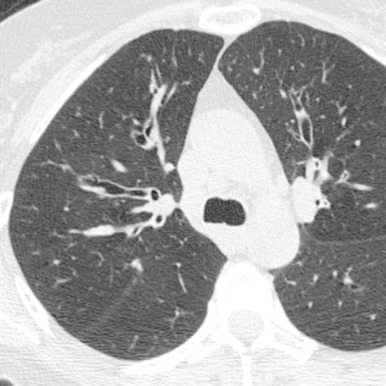
Congenital bronchiectasis (i.e., Williams-Campbell syndrome) is rare. Williams-Campbell syndrome results from a congenital deficiency of bronchial cartilage affecting fourth- to sixth- order subsegmental bronchi. The cartilage deficiency leads to airway collapse, bronchiectasis, and pulmonary hyperinflation ( Fig. 8.8 ). Acquired bronchiectasis (from chronic or recurrent infection) may also occur early in life as a result of other congenital, developmental, or genetic disorders. These conditions are listed in Box 8.1 .
Congenital cystic bronchiectasis
Primary hypogammaglobulinemia
Yellow nail syndrome
Cystic fibrosis
Immotile-cilia syndrome (Kartagener syndrome)
Pulmonary underdevelopment, also known as lung agenesis–hypoplasia complex, results from disordered in utero formation of the lungs and airways varying in severity from agenesis, to aplasia, to hypoplasia. In pulmonary agenesis, there is total absence of lung tissue, bronchi, and vasculature distal to the carina. This is distinguished from pulmonary aplasia in which a rudimentary bronchus ends in a blind pouch without lung tissue or pulmonary vasculature. In pulmonary hypoplasia, lung tissue and bronchi are present but are decreased in number or size. Pulmonary underdevelopment may be primary or secondary to an in utero pathologic process that prevents full development of the thoracic cavity. These conditions include decreased pulmonary vascular perfusion, oligohydramnios, or compression of the lung by a space-occupying mass (e.g., congenital diaphragmatic hernia). More than half of affected fetuses with pulmonary underdevelopment exhibit additional cardiovascular, gastrointestinal, or skeletal abnormalities.
The imaging appearance of agenesis–hypoplasia complex varies depending on the degree of pulmonary underdevelopment. In agenesis, chest radiography shows complete absence of an aerated lung in one hemithorax, pronounced decrease in volume of the affected hemithorax, and shift of the mediastinum to the affected side. There is usually compensatory overinflation of the contralateral normal lung that extends across the midline ( Fig. 8.9 ). In pulmonary hypoplasia, the lung is decreased in size, and there may be similar signs of volume loss, but the appearance varies depending on the degree of pulmonary hypoplasia. In some cases, it may be difficult on radiography to distinguish severe pulmonary hypoplasia from aplasia or agenesis. In such instances, chest CT differentiates these entities by the presence or absence of a bronchus, pulmonary artery, and lung tissue. In pulmonary hypoplasia, a small pulmonary artery associated with transpleural collateral arteries may be seen in the affected lung.
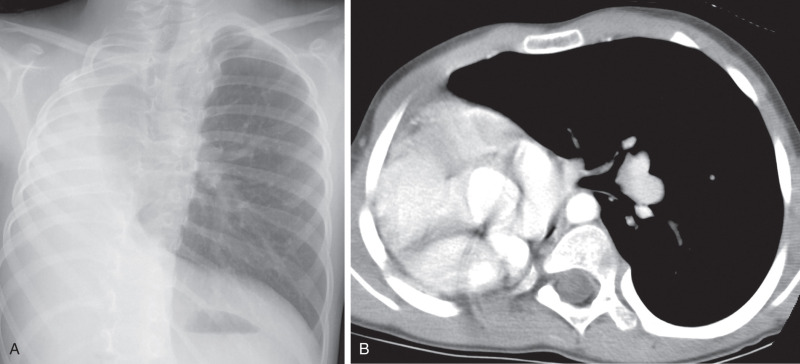
Pulmonary hypoplasia must be differentiated from other conditions that can produce volume loss, including total atelectasis of the lung, severe bronchiectasis with collapse, and advanced fibrothorax caused by chronic pleural disease. CT is likely to be diagnostic in these cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here