Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital lung anomalies represent a heterogeneous group of pulmonary developmental disorders that affect the lung parenchyma, its arterial supply, venous drainage, or a combination thereof. The reported incidence of congenital lung anomalies ranges from 30 to 42 cases per 100,000 population. Although prenatal sonography, fetal magnetic resonance imaging (MRI) and advances in postnatal imaging have enhanced our understanding of congenital lung anomalies, substantial controversy continues regarding the nomenclature, classification, pathogenesis, description, and management of these lesions. In addition, considerable variability exists in their prenatal clinical presentation and outcome, ranging from in-utero involution to severe hydrops and fetal demise. Likewise, their postnatal clinical presentation also is variable, ranging from marked neonatal respiratory distress to a completely asymptomatic newborn to an older child or young adult with recurrent pneumonias. In this chapter, the underlying etiology, clinical presentation, imaging findings, and management of various congenital lung anomalies are discussed.
The classification of congenital lung anomalies is challenging and controversial from embryologic, radiologic, pathologic, and clinical viewpoints. Several classifications and terminologies with particular advantages and disadvantages have been suggested. Some investigators have used embryology as a basis and have classified congenital lung anomalies according to the stage of intrauterine development in which the insult resulting in the malformation developed. Others have categorized lesions based on their morphologic-radiologic features and divided them into two groups: whole lung malformations (e.g., lung hypoplasia) and focal malformations (e.g., bronchial atresia). Recently the Langston classification has become one of the most accepted classification systems for congenital lung anomalies, particularly from the pathologic standpoint, although it is by no means used by all clinical groups. Langston categorizes the wide spectrum of respiratory tract malformations primarily as bronchial atresia, congenital pulmonary airway malformation (CPAM), extralobar bronchopulmonary sequestration (BPS), congenital lobar overinflation (CLO), and bronchogenic cysts. These five congenital lung anomalies comprise approximately 90% of the anomalies seen in clinical practice. However, this classification system is limited because other congenital lung anomalies (e.g., pulmonary arteriovenous malformation [AVM]) are not included.
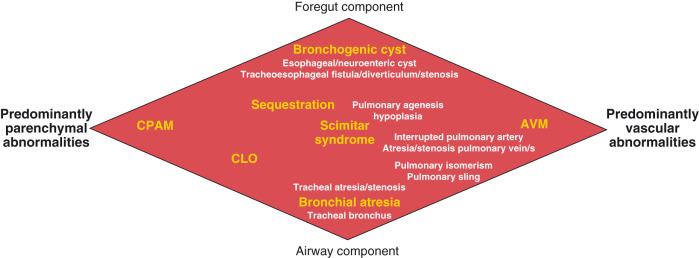
For the purpose of relatively clear classification, easy differentiation on imaging studies, and preoperative assessment of surgical lesions, congenital lung anomalies discussed in this chapter are categorized according to their morphologic-radiologic-pathologic features. Such a classification system views congenital lung anomalies as a continuum ranging from predominantly parenchymal abnormalities (i.e., abnormal lung parenchyma with variable airway/foregut components and relatively normal vasculature, e.g., CPAM), to combined parenchymal and vascular abnormalities (e.g., pulmonary sequestration and scimitar syndrome) in which foregut and airway components play an important role and the major abnormalities are intertwined ( Fig. 53.1 ), and lastly predominantly vascular abnormalities, such as pulmonary AVM or absent or interrupted pulmonary artery with secondary lung/airway abnormalities.
Given that specific terminology for these lesions may be controversial and occasionally confusing, we and other authors recommend that radiologists thoroughly describe all imaging findings of congenital lung anomalies rather than try to categorize the lesions by pathologic terminology. Specific imaging findings that need to be evaluated and described include (1) location of lesions; (2) vascular supply and drainage of the lesions; (3) internal architecture, presence of cystic components, and degree of aeration; (4) airway abnormality; (5) exclusion of communication with the gastrointestinal (GI) tract; (6) integrity of the diaphragm; and (7) an assessment of associated anomalies, such as vertebral anomalies.
Bronchial atresia refers to atresia of a lobar, segmental, or subsegmental bronchus at or near its origin resulting in a blind-ended atretic proximal bronchus. Bronchial atresia has been categorized as proximal or peripheral. While proximal atresia is less common, it is often lethal during pregnancy. Peripheral bronchial atresia most frequently affects a segmental bronchus and may be isolated or part of another congenital lung malformation. Peripheral bronchial atresia may be diagnosed as an incidental finding on chest radiographs later in life in asymptomatic older children or adults. However, it is increasingly being diagnosed in utero, given the widespread use of prenatal imaging.
The precise etiology of bronchial atresia remains unknown, but etiologies such as a vascular insult to the involved atretic or stenotic portion have been proposed. Several authors who used modern dissecting techniques found that bronchial atresia is more common than originally thought. Furthermore, bronchial atresia and BPSs coexist in nearly all cases, whereas it is found in nearly 70% of CPAM lesions. A malformation sequence resulting from airway obstruction during development has been proposed as the unifying element for such a wide spectrum of imaging appearances of the major bronchopulmonary malformations. Differences in degree, level, and timing of the bronchial obstruction are thought to be responsible for these variations.
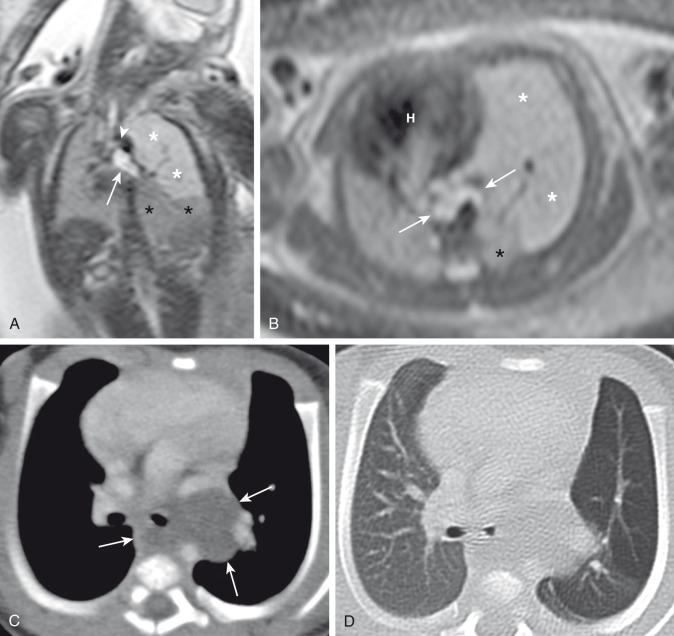
Prenatally, the involved portion of the lung appears hyperexpanded and shows increased homogenous echogenicity on US and high T2 signal on fetal MRI ( Fig. 53.2 ). The appearance may be difficult to distinguish from CLO, pulmonary sequestration, and microcystic CPAM. Identification of a centrally located, mucus-filled bronchocele/mucocele on prenatal US or MRI strongly suggests bronchial atresia ( e-Fig. 53.3 ).
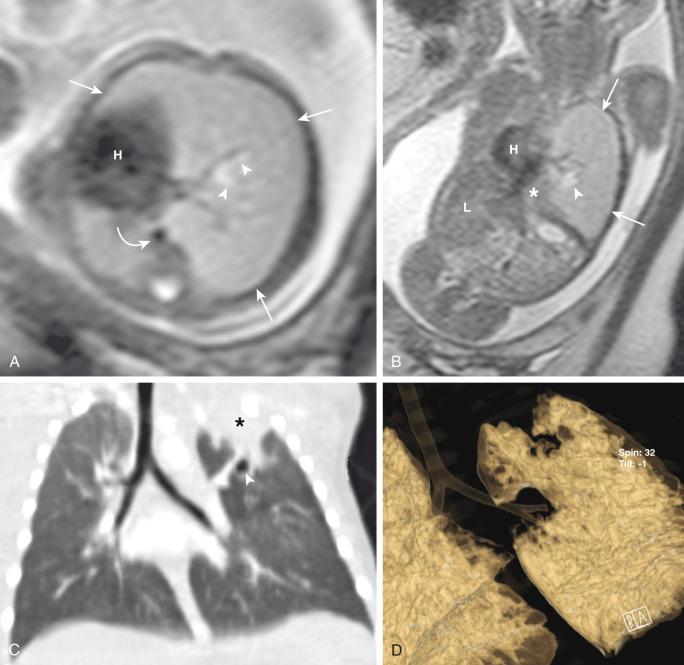
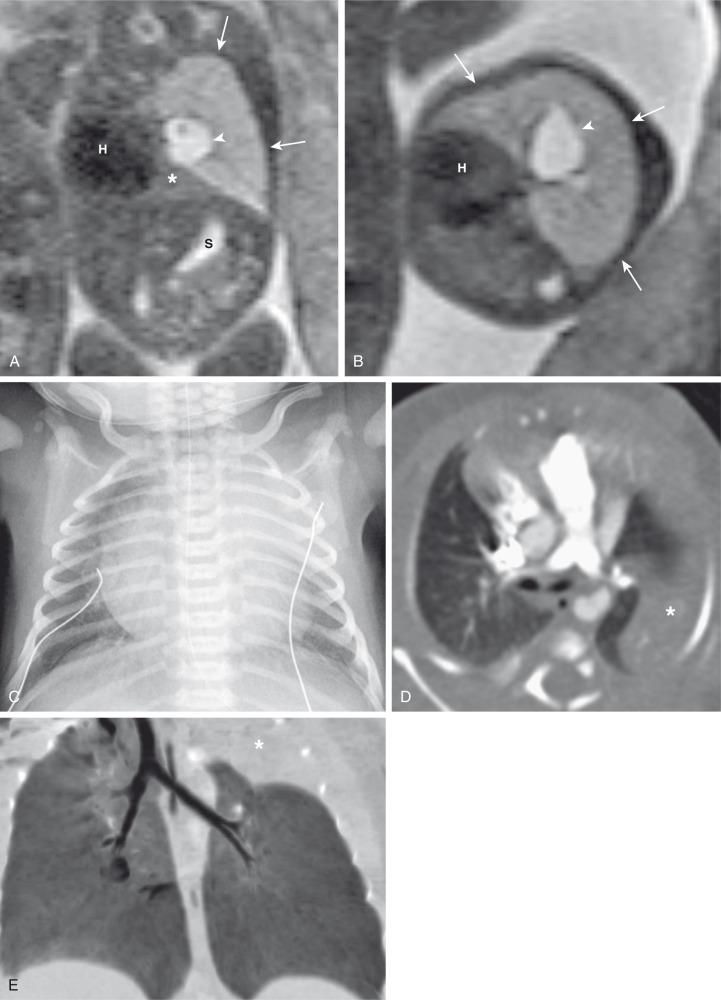
Characteristically, the apicoposterior segmental bronchus of the left upper lobe is most commonly affected, followed by the segmental bronchi of the right upper, right middle, and lower lobes. In children, radiographic and computed tomography (CT) imaging features of bronchial atresia are characterized by a tubular or glove-shaped opacity representing mucus plugging in the region distal to the atretic bronchus, surrounding segmental hyperlucency due to air trapping (associated with collateral aeration), and decreased underlying vascularity ( e-Fig. 53.4 ). However, in neonates, a portion of the lung distal to the atretic segment may remain atelectatic as a result of the in-utero mucostasis. Therefore some authors recommend avoiding immediate postnatal imaging evaluation because the underaerated lung related to bronchial atresia may be difficult to differentiate from the normal lung with expected fetal fluid retention in the newborn period. Identification of the hallmark atretic bronchus and the “bronchocele/mucocele” by means of two-dimensional (2D) multiplanar or three-dimensional (3D) reconstructions either on prenatal or postnatal imaging may be helpful.
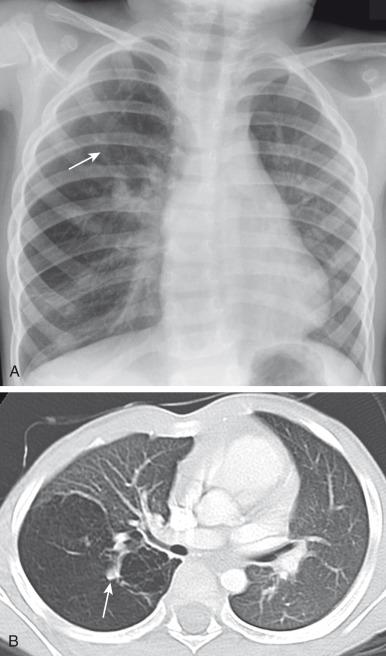
In general, surgical resection is the treatment of choice in symptomatic pediatric patients, with conservative management possible in asymptomatic children with small lesions. Although opinions vary, some centers advocate elective surgical resection of bronchial atresia even in asymptomatic pediatric patients because of potential future lung infection and especially when associated with CPAM.
Bronchogenic cysts result from abnormal tracheobronchial branching and probably originate from an aberrant bud of the developing foregut, similar to other foregut duplication cysts. Bronchogenic cysts typically are unilocular, fluid-filled or mucus-filled cysts lined by respiratory epithelium and are attached to, but usually do not communicate with, the tracheobronchial tree. Although most bronchogenic cysts are located within the mediastinum (predominantly near the carina), they may be encountered anywhere from the suprasternal area to the retroperitoneum. Bronchogenic cysts also may be found within the lung parenchyma, usually in the lower lobes located close to an adjacent airway. Such intrapulmonary bronchogenic cysts usually do not communicate with the airway unless superimposed infection occurs, which may further predispose them to recurrent infections. Symptoms depend on the mass effect the cyst exerts on neighboring structures including the airway, GI tract, and cardiovascular structures. Airway compression is usually mild, but it may be life-threatening in some instances when large bronchogenic cysts are located near the carina.
A bronchogenic cyst typically presents as a round or oval-shaped cystic lesion located near the right paratracheal or subcarinal area within the middle mediastinum. Bronchogenic cysts are anechoic on prenatal ultrasound and show high signal intensity on T2-weighted prenatal MRI ( e-Fig. 53.5 ). On chest radiographs, a bronchogenic cyst manifests as a well-delineated round or oval-shaped middle mediastinal mass. On CT, approximately 50% of bronchogenic cysts demonstrate fluid attenuation value (~0 Hounsfield unit) ( Fig. 53.6 ). The remaining bronchogenic cysts may have CT attenuation higher than water because of thick mucoid, milk-of-calcium, proteinaceous, or hemorrhagic contents. MRI, which can confirm the cystic nature of the bronchogenic cysts on T2-weighted images, is helpful for differentiating bronchogenic cysts with high attenuation value from a solid mass on CT. Typically, no internal contrast enhancement is seen within uncomplicated bronchogenic cysts. The presence of an air-fluid level, thick wall enhancement, or surrounding inflammatory changes often is associated with superimposed infection.
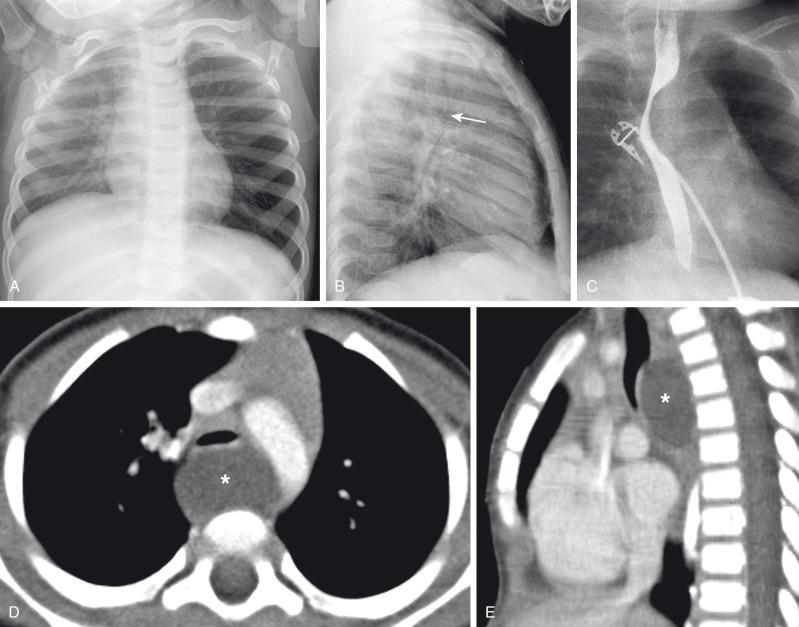
Complete surgical resection is the current management of choice for bronchogenic cysts, particularly in symptomatic pediatric patients. Temporizing or palliative procedures such as transparietal, transbronchial, or mediastinal aspiration may be considered in symptomatic patients who are not surgical candidates.
CLO, also referred to as infantile/congenital lobar emphysema or congenital lobar hyperinflation, is thought to be caused by an intrinsic or extrinsic bronchial narrowing, resulting in marked alveolar overdistension or overinflation of at least one pulmonary segment or lobe. The alveolar walls are maintained, therefore true emphysematous change is not present. Intrinsic bronchial narrowing can be caused by weakness or absence of underlying bronchial cartilage, whereas extrinsic bronchial narrowing may be due to the compression from adjacent mediastinal masses or enlarged vessels. Most cases of segmental overinflation, and some with lobar abnormality, are due to underlying bronchial atresia. CLO presents clinically with respiratory distress in the newborn period in nearly half of the symptomatic cases, and by the age of 6 months in 80% of cases. There is a slight male predominance, and the upper lobes are affected more frequently than the lower lobes, with the left lung affected more often than the right (see also Chapter 50 ).
On prenatal imaging, CLO manifests as a homogeneously hyperechogenic lesion on US or as a T2 hyperintense lesion on MRI without visible cysts. CLO often is indistinguishable from other congenital lung anomalies, particularly in the presence of bronchial atresia. In the immediate postnatal period, CLO initially may appear as an area of increased opacity related to retained fetal lung fluid, which will clear on subsequent studies. CLO usually is diagnosed postnatally by its typical clinical presentation and characteristic radiographic features of progressive lobar hyperexpansion and hyperlucency, producing displacement or compression of adjacent structures. Similar imaging findings are noted on CT, and the attenuated pulmonary vasculature is a helpful clue to distinguish CLO from a pneumothorax or other entities in cases of inconclusive chest radiographic findings ( Fig. 53.7 ). Similar to bronchial atresia, early CT imaging should be avoided because of a confusing appearance when the affected lung is still fluid-filled.
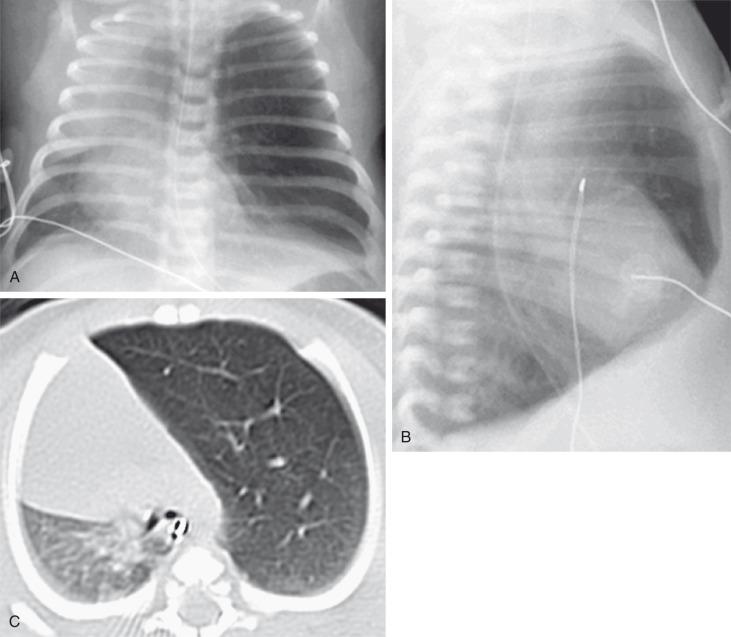
Surgical lobectomy is the current management for symptomatic pediatric patients with CLO. Some medical centers advocate expectant management for cases with minimal or no symptoms.
CPAMs, formerly known as cystic adenomatoid malformations of the lung, were first described by Ch'In and Tang in 1949 as rare lung lesions occurring in premature or stillborn infants with significant hydrops. CPAMs are currently one of the most common congenital lung anomalies accounting for about 30% to 40% of the cases. CPAMs are characterized by a heterogeneous group of congenital cystic lung masses with an abnormal bronchial communication or bronchial atresia.
In 1977, Stocker et al. classified these lesions based on their clinical and pathologic features, with subdivisions based on the size of the cysts (types 1, 2, and 3) and according to the location of suspected development of the malformation along the airway. Type 1 CPAMs consist of cysts larger than 2 cm, with presumed bronchial/bronchiolar origin. Type 2 CPAMs consist of cysts smaller than 2 cm, with presumed bronchiolar origin. Type 3 CPAMs appear solid/microcystic, with a presumed bronchiolar/alveolar origin. However, Stocker later expanded his classification into five types that included type 0 CPAMs, with presumed tracheobronchial origin (hypoplastic lungs, usually lethal, not recognizable on imaging), and type 4 CPAMs, with presumed distal acinar origin. The term CPAM was implemented instead of cystic adenomatoid malformations, because cystic changes were observed in only three of the aforementioned types (types, 1, 2, and 4), and adenomatoid change was observed only in type 3. This expanded classification has proven problematic with increasing evidence indicating that the type 4 CPAM lesions is the same entity as type 1 cystic pleuropulmonary blastoma. Langston classifies CPAM lesions into two types, and terms the Stocker type 1 CPAM as “large cyst-type lesions” and the Stocker type 2 CPAMs as “small cyst-type lesions” based on cyst size and pathologic criteria. Langston proposed that the type 3 CPAM actually represents a form of pulmonary hyperplasia/dysplasia and should be excluded from the CPAM classification. On radiographs and CT imaging, these lesions are typically divided into large cyst (type 1, most common), small cyst (type 2), and solid (microcystic, type 3, very rare) lesions.
Prenatally, CPAMs have been classified on the basis of cyst size as microcysts (<5 mm) and macrocysts (≥5 mm) on fetal US and MRI. Type 1 CPAMs may contain one, several, or multiple macrocysts, some of which are ≥5 mm in diameter ( Fig. 53.8 ). Type 2 CPAMs have variable appearances, ranging from homogeneously hyperechoic or hyperintense lesions to microcystic lesions exhibiting multiple, uniform cysts that measure <5 mm ( e-Figs. 53.9 and 53.10 ).
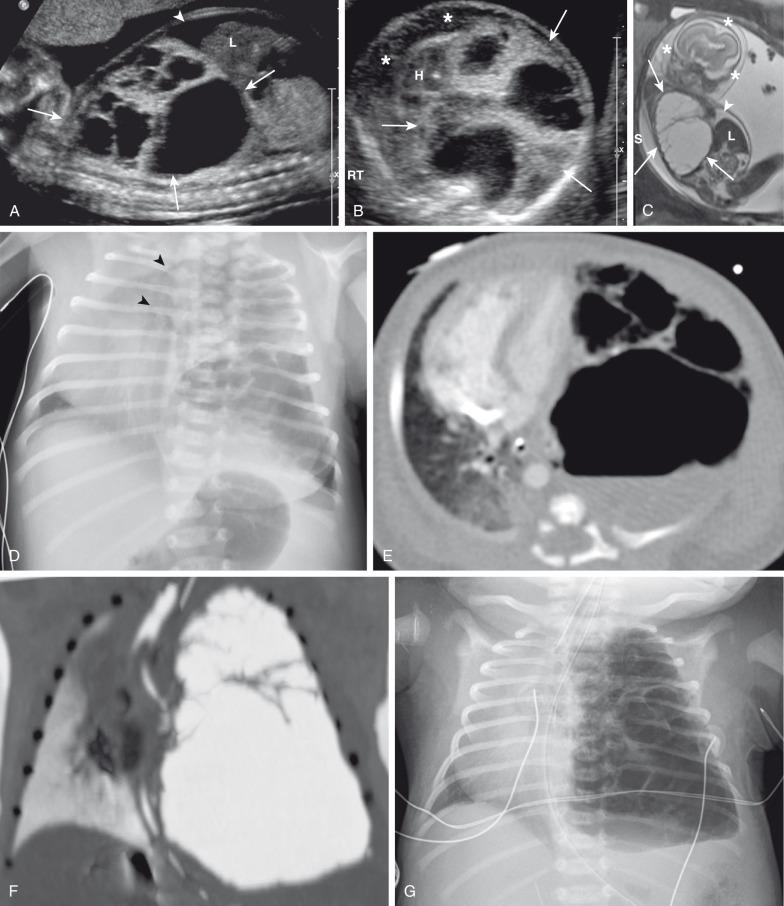
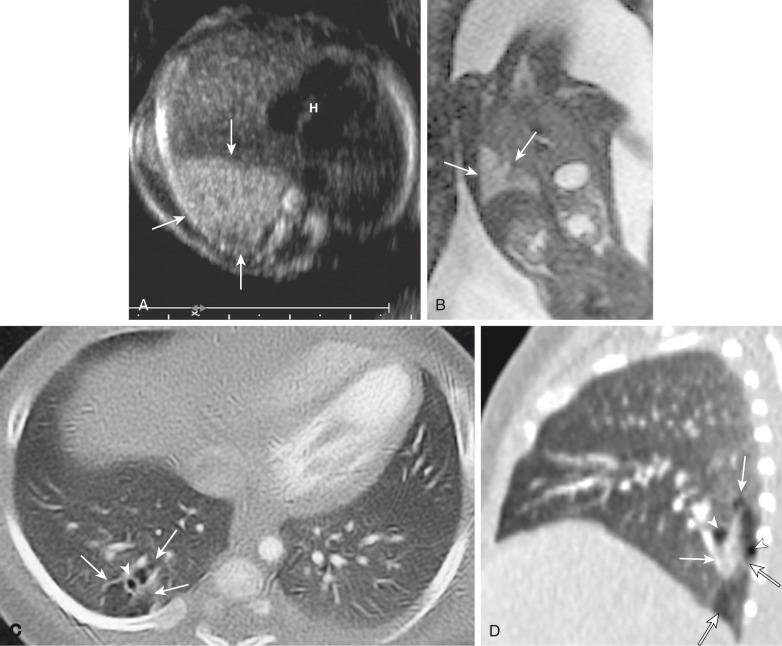
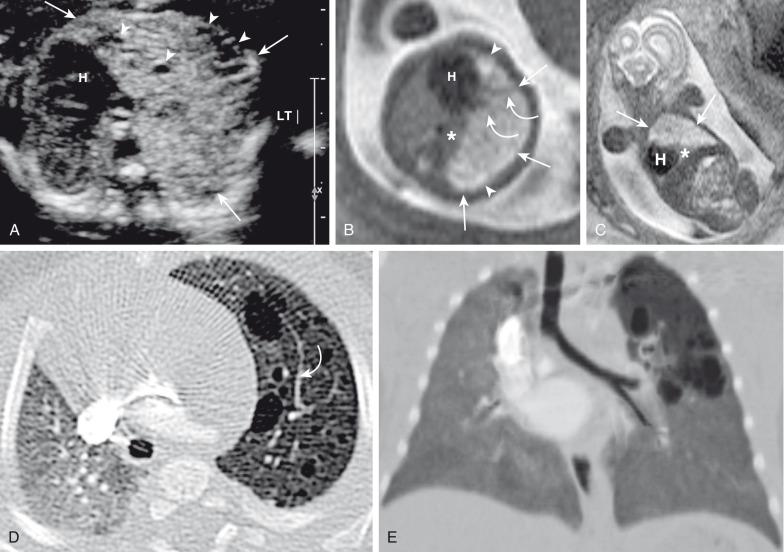
Postnatal imaging findings of CPAMs usually correlate with underlying histopathologic features. Large cyst-type CPAMs typically present with one or several larger air-filled cystic structures with intervening solid, unaerated lung parenchyma. The cysts of type 1 CPAMs are larger than 2 cm and may be accompanied by several smaller cysts, whereas small cyst type or type 2 CPAMs usually manifest as partially air-filled multicystic masses, with individual cysts smaller than 2 cm and with variable degrees of solid-appearing, unaerated lung tissue. Type 3 CPAMs typically appear as solid lesions with mild contrast enhancement because of microscopic cysts that can be identified only at histologic evaluation. Pleuropulmonary blastoma in infancy usually present as large cysts arising from the peripheral portion of the lung, this lesion may be difficult to distinguish radiographically from other cystic lung lesions (see Chapter 55 ) ( e-Fig. 53.11 ).
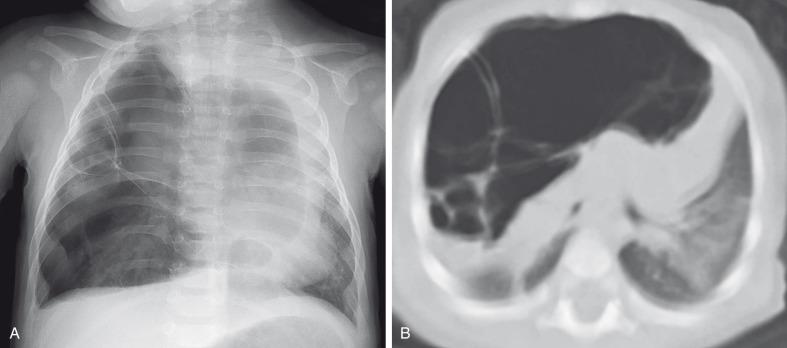
CPAM blood supply is often from the pulmonary artery and venous drainage is into the pulmonary veins, although hybrid sequestration/CPAM lesions with systemic arterial supply are common (especially CPAM type 2). Although unilobar involvement of CPAM is far more common, multilobar and even bilateral lung involvement may occur. Although any lobe of the lung can be involved, predilection exists for the lower lobe. CPAMs that are complicated as a result of superimposed infection may have an imaging appearance similar to pneumonia or a lung abscess ( Fig. 53.12 ).
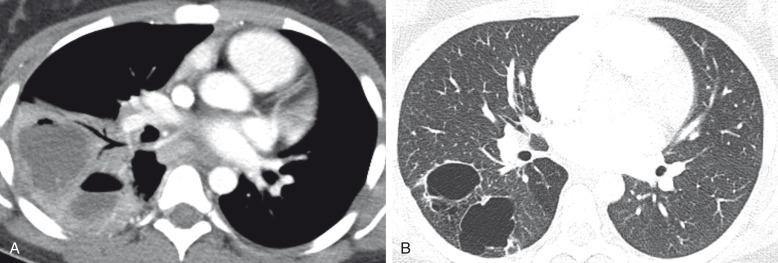
The generalized consensus is that symptomatic CPAMs should be resected, typically by lobectomy, regardless of the patient's age at presentation. However, considerable controversy exists with regard to the management of prenatally diagnosed, asymptomatic, small CPAM lesions, and no consensus exists on the timing of or need for resection. Although there is some support for a nonsurgical strategy with imaging follow-up, most medical centers advocate surgical resection before 1 year of age because of the potential risk of associated complications, such as infection, pneumothorax, and the small risk of malignant transformation, particularly in the case of CPAM type 1 lesions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here