Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital Heart Disease;
Aortic valve Stenosis;
Atrial Septal Defect;
AtrioVentricular Septal Defect;
Coarctation of the Aorta;
Double Inlet Left Ventricle;
Double Outlet Left Ventricle;
Double Outlet Right Ventricle;
d extro-Transposition of the Great Arteries;
Fibroblast Growth Factor;
First Heart Field
Hypoplastic Left Heart Syndrome;
l evo-Transposition of the Great Arteries,
Patent Ductus Arteriosus;
Pulmonary valve Stenosis;
Patent Foramen Ovale;
Persistent Truncus Arteriosus;
Pulmonary Venous Obstruction;
Second Heart Field;
Tricuspid valve Atresia;
Total Anomalous Pulmonary Venous Connection;
Transient Ischemic Attack;
Transcriptional Factors;
Transposition of the Great Arteries;
Tetralogy of Fallot;
Ventricular Septal Defect.
Congenital heart disease (CHD) consists of a wide variety of anomalies and malformations involving the heart and great vessels that develop in utero during the development of the cardiovascular system and are present at birth, even if they might be diagnosed much later, and thus are popularly referred to as birth defects. These anomalies may be single or multiple. The CHDs frequently are the result of defective morphogenesis that occurs during the embryological development. The malformations may be limited to the cardiovascular system (nonsyndromic CHD) or occur in association with anomalies of other systems as part of defined syndromes (syndromic CHD). They may develop as a result of random alterations in morphogenesis and/or as a result of environmental factors. The cardiovascular anomalies also may be induced by genetic mutations, including chromosomal abnormalities or single gene defects. Improvement in the survival and quality of life of children with CHD due to accurate diagnosis and effective surgical treatment is one of the major achievements of contemporary medicine. This clinical accomplishment was necessarily preceded by and directly flows from the essential, creative, and accurate documentation of the pathologic anatomy and pathophysiology of CHD by a small cadre of dedicated and accomplished pathologists .
This chapter on CHD is aimed at emphasizing contemporary knowledge of etiology, pathogenesis, and pathophysiology of CHD, and is accompanied by conceptual illustrations. The reader is also referred to previous publications which have provided comprehensive photographic documentation of actual CHD specimens .
A commonly used general classification of CHD is as follows: left-to-right shunts, right-to-left shunts, lesions without shuts, including obstructive anomalies, and vascular anomalies .
According to the definition introduced in 1971 by Mitchell et al. , a CHD is a gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional significance. Abnormalities of systemic veins, such as persistent left superior vena cava azygos-continuity, and anomalies of systemic artery branches are excluded.
CHD consists of a wide variety of anomalies and malformations involving the heart and great vessels that develop in utero, are present at birth, and may come to clinical attention prior to birth, at birth, in infancy, adolescence, or adulthood. The cardiovascular anomalies generally result from defective morphogenesis during embryological development.
Anatomic anomalies of the cardiac conduction system already detectable at birth and prior to birth, such as a Mahaim fiber, and genetically altered electrical channel proteins (channelopathies) are important considerations for CHD inclusion, being sudden perinatal unexplained death and sudden infant death syndrome characterized by common histopathological substrates, congenital in nature . A channelopathy can also be detected in fetuses, and its diagnosis is considerably more challenging than the diagnosis of an anatomic anomaly. On the other hand, subtle anatomic abnormalities of the cardiac conduction system present at birth can be detected postmortem only with specialized analysis . It should be borne in mind that many cardiomyopathies also develop in utero and are present at birth, even if they are generally not strictly considered CHDs. Indeed, according to the classification of the cardiomyopathies of the American Heart Association , channelopathies are regarded as primary cardiomyopathies.
CHDs are the most common major congenital anomalies, representing a major global health issue. The increased survival of patients with CHD is associated with the need of long-term expert medical care with high healthcare-related costs, so that the global health burden as a result of CHD is high and is increasing .
It is challenging to identify the true prevalence of CHD, as major CHDs can be identified in the prenatal or neonatal period, but minor CHDs might not be detected until childhood or adulthood . The current prevalence of CHD does not include two conditions which may be the two most common congenital cardiac anomalies: the congenital, functionally normal bicuspid aortic valve, and the myxomatous mitral valve with prolapse . The true incidence of CHD could be significantly higher than the reported one, given that cardiac anomalies occur ten times more frequently in stillborn ad premature infants than in liveborn, full-term infants. In fact, the most severe forms of CHD are not compatible with intrauterine survival and they are common among stillborns. Congenital cardiomyopathies, subtle congenital anomalies of the cardiac conduction system, and channelopathies can also be detected in fetuses , but they are generally not counted as CHDs in the epidemiological studies, being their diagnosis more challenging than the diagnosis of anatomic anomaly.
Overall, the prevalence of CHDs is of 9 per 1000 (9‰) live births and of 4 per 1000 adults (4‰). Although a complete overview is missing, geographic variations in birth prevalence of CHD have been reported, being significantly lower in Africa (1.9‰), compared to North America (6.9‰), Europe (8.2‰), and Asia (9.3‰). Observed differences should be related to the limited access to health care and diagnostic facilities in many parts of the world . In general, the CHD has been considered to be similar in total incidence worldwide , but recently, it has been reported that the prevalence of CHD in Asia is the highest, suggesting higher genetic or environmental susceptibility to CHD among Asian people .
Over time, the reported total CHD birth prevalence has increased substantially, from 0.6 per 1000 live births in 1930–34 to 5.3 per 1000 live births in 1961–75, to eventually stabilize around 9.1 per 1000 live births in the last 25 years . The birth prevalence of CHD is likely to further increase over time, thanks to advancements in diagnostic and screening methods, such as fetal cardiac ultrasound, pulse oximetry, and echocardiography during infancy .
Twelve forms of CHD account for about 85% of all the CHD cases . The relative frequency of these twelve cardiac malformations is as follows: ventricular septal defect (VSD) 42%; atrial septal defect (ASD) 10%; pulmonary valve stenosis (PS) 8%; patent ductus arteriosus (PDA) 7%; tetralogy of Fallot (TOF) 5%; coarctation of the aorta (CoA) 5%; atrioventricular septal defect (AVSD) 4%; aortic valve stenosis (AS) 4%; transposition of the great arteries (TGA) 4%; persistent truncus arteriosus (PTA) 1%; total anomalous pulmonary venous connection (TAPVC) 1%; and tricuspid atresia (TA) 1% ( Table 6.1 ). In children, the most common types of CHDs are VSD, ASD, PS, and PDA. In adults, the most common types of CHDs are PDA and TOF .
| Malformation | Incidence per million live births | % | |
|---|---|---|---|
| 1 | Ventricular septal defect | 4482 | 42 |
| 2 | Atrial septal defect | 1043 | 10 |
| 3 | Pulmonary stenosis | 836 | 8 |
| 4 | Patent ductus arteriosus | 781 | 7 |
| 5 | Tetralogy of Fallot | 577 | 5 |
| 6 | Coarctation of the aorta | 492 | 5 |
| 7 | Atrioventricular septal defect | 396 | 4 |
| 8 | Aortic stenosis | 388 | 4 |
| 9 | Transposition of the great arteries | 388 | 4 |
| 10 | Truncus arteriosus | 136 | 1 |
| 11 | Total anomalous pulmonary venous connection | 120 | 1 |
| 12 | Tricuspid atresia | 118 | 1 |
| Total | 9757 |
a Determined from the upper quartile of 44 published studies.
Males have a higher incidence of AS, CoA, hypoplastic left heart syndrome (HLHS), pulmonary, and tricuspic atresia. Females have a higher incidence of PDA, Ebstein’s anomaly, and ASD .
According to a recent report from the American Heart Association , in 10 years, the mortality related to CHD has decreased by 18.1% and the mortality rate attributable to CHD is now 0.9 per 100,000. In live-born children with CHD, the all-cause mortality rate is 17.4% for children with severe CHDs and 3.0% for children with milder forms of CHDs, with declining mortality rates related to less frequent operative mortality and increasing pregnancy terminations.
According to the Centers for Disease Control and Prevention (CDC) , the infant deaths from CHD represent 48% of all deaths from birth defects and occur more frequently in neonates, that is, infants within the first 28 days of postnatal life. A total of 4.2% of all neonatal deaths are attributable to CHD.
The mortality rate attributable to CHD differs between ethnicities is and is 1.01 per 100,000 for white males, 1.28 for non-Hispanic black males, 0.91 for Hispanic males, 0.77 for non-Hispanic white females, and 1.03 for non-Hispanic black females, and 0.73 for Hispanic females . CHD mortality depends on the regional availability of medical and surgical care, being in underdeveloped countries with little access to cardiac surgery, a reflection of the natural history of the disease .
In developed countries, an increased risk of infant mortality for CHD is associated with maternal non-Hispanic-black and Hispanic ethnicity, female gender, low birth weight, preterm birth, presence of extracardiac defects, and earlier birth era. Prenatal diagnosis of CHD is not associated with lower mortality risk. To reduce mortality, universal pulse oximetry screening of newborn infants has been recommended to enhance early diagnosis and timely referral of neonates with critical CHD to a cardiac center .
Approximately 25% of infants affected by CHD require invasive treatment in the first year of life . Overall, 1-year survival for all CHDs is 87% , ranging from 75.2% for patients with critical CHDs to 97.1% for patients with noncritical CHDs . The overall 1-year survival is 87% and varies by CHD subtype, being the lowest for HLHS (17.4%) and greatest for VSD (95.5%). The 5-year survival is 85.4%, depending on CHD subtype, with survival for HLHS at 12.5% and survival for VSD at 97.7%. The 10-year survival is 81.4%, and after that the survival rate decreases very gradually with a 25-year survival of 72% .
Survival rates of CHD into adulthood have been significantly increasing, thanks to modern advances of health care, especially in the fields of cardiothoracic surgery and anesthesia. Accordingly, the numbers of adult CHD survivors have increased rapidly, in addition to the number of adults newly diagnosed with a minor form of CHD that has not be detected until adulthood. In the United States, 1 in 150 adults have some form of CHD . Adult patients with CHD are more numerous than child CHD patients, with adults representing two-third of all the CHD population .
A definitive cause of CHD is determined in only about 10% of cases. Nevertheless, multifactorial genetic and environmental etiologic factors have been implicated including chromosomal defects, gene mutations, single nucleotide polymorphisms, viruses, chemicals, and radiation . Many risk factors, intrinsic genetic and extrinsic nongenetic, contribute to the occurrence of CHD .
CHD can be hereditable, but the majority of CHDs occur in families without other history of CHD, supporting the possibility of a de novo genetic event. Evidence for genetic influences is manifest by two- to tenfold increase in the occurrence of CHD in siblings of an affected child or children of an affected parent . A parent affected by ASD or VSD has, respectively, 2.6% and 3.7% change to have a child similarly affected, that is 21 times the estimated population frequency . Twins have a 73% greater risk of developing CHD than singletons. Monochorionic twins have an 82% greater risk of developing CHD than dichorionic twins .
There is an association between CHD and extra-cardiac congenital anomalies, stronger for syndromic malformations compared to nonsyndromic malformations. About 25% of infants suffering from CHD are also affected by extra-cardiac congenital anomalies which are often multiple and significantly affect their clinical course. About one-third of infants suffering from CHD and extra-cardiac congenital anomalies have an established syndrome .
Many inherited CHDs are autosomal dominant and involve promoter genes and genes that encode transcription factors (TFs) (discussed later in Section “Molecular Biology of Genes Controlling Development of the Cardiovascular System”) ( Fig. 6.1 ; Tables 6.2 and 6.3 ).
| Gene | Promoter, length (kb) | Embryonic expression |
|---|---|---|
| αMHC | 5.5 | Atria |
| βMHC | 2.5 | Atria+ventricle |
| α-Cardiac actin | 3 | Atria+ventricle |
| Troponin I | 4.2 | Inflow tract+ventricle |
| MLC2c | 0.28 | Outflow tract+right ventricle |
| MLC3f | 2 | Right atrium+left ventricle |
| Desmin | 1 | Outflow tract+right ventricle |
| SM22α | 2 | Outflow tract+right ventricle |
| α B-crystallin | 4 | Outflow tract+right ventricle |
| Dystrophin | 0.9 | Outflow tract+right ventricle |
| Nkx2-5 | 14 | Outflow tract+right ventricle+left ventricle |
| ANP | 0.695 | Atria+left ventricle |
| TF | Phenotype |
|---|---|
| GATA-4 | Cardia bifida |
| GATA-5 | Cardia bifida |
| Nkx2-5 | Septation and differentiation defects |
| MEF2C | Right ventricle hypoplasia, septal defects |
| Tbx5 | Atrial hypoplasia, septal defects |
| dHand | Right ventricle hypoplasia |
| TEF-1 | Myocardial thinning |
| N -myc | Myocardial thinning |
| COUP-TFII | Atrial hypoplasia |
| RXR/RAR | Septal defects |
| Fog2 | Outflow tract defects |
| Tbx1 | Septation defects |
| Pax-3 | Septation defects |
| NFATc | Defective valve development |
| Hrt2 | Aortic coarctation |
| Smad6 | Valve thickening |
| Irx4 | Postnatal cardiomyopathy |
Overall, CHDs are mostly caused by sporadic genetic anomalies: mutations of a single gene, small chromosomal losses, numerical chromosome abnormalities (trisomies or monosomies) ( Tables 6.4 and 6.5 ). Identifiable chromosomal abnormalities constitute about 15% of cases , specifically monosomy XO (Turner syndrome), trisomies 5, 13 (Patau syndrome), 18 (Edwards syndrome), partial 20q, 21 (Down syndrome), 22, partial trisomy or tetrasomy 22 (Schmid-Fraccaro syndrome); deletion syndromes 4p (Wolf-Hirschhorn syndrome) 7q11.23 (Williams syndrome), 15q11 (Prader-Willi syndrome), 17p (Miller Dieker syndrome), 22q11 (CATCH-22, DiGeorge, and Velocardiofacial syndromes); rearrangement 5p15.1-3 (Cri du Chat syndrome), and recombinant chromosome 8 (San Luis Valley syndrome) ( Table 6.5 ). Detection of chromosome 22q11.2 occurs in up to 50% of patients with DiGeorge syndrome. The most common genetic cause of CHD is Down syndrome in which 40% of patients have one or more CHD, usually affecting second heart field (SHF) derivatives (atrioventricular septae).
| Affected genes(s) | Normal function | Congenital cardiac disease | |
|---|---|---|---|
| Nonsyndromic | |||
| NKX2-5 | Transcription factor | ASD, VSD, conduction defects | |
| GATA-4 a | Transcription factor | ASD, VSD | |
| TBX20 a | Transcription factor | ASD, VSD, valve abnormalities | |
| NOTCH1 | Pass-membrane receptor | Bicuspid aortic valve | |
| ZFPM2 / FOG2 NKX2-5 | Transcription factors | TOF | |
| Syndromic | Syndrome | ||
| TBX1 | Transcription factor | DiGeorge | Cardiac outflow tract defects |
| TBX5 , NKX2-5 | Transcription factor | Holt–Oram | ASD, VSD, conduction defects |
| JAG1 , NOTCH2 | Notch signaling | Alagille | Pulmonary artery stenosis, TOF |
| Fibrillin | ECM structural protein TGF-β signaling | Marfan Loeys-Dietz | Aortic aneurysm Valve abnormalities |
| ELN | Elastin | Williams | Supravalvular aortic stenosis |
| PTP11 , KRAS , SOS1 | Signaling proteins | Noonan | Pulmonary valve stenosis, VSD, hypertrophic cardiomyopathy |
| TFAP2B | Transcription factor | Char | PDA |
| CHD7 | Helicase-binding protein | CHARGE | ASD, VSD, PDA, hypoplastic right side of the heart |
| Chromosome defects | Syndromes | Cardiac phenotype |
|---|---|---|
| Monosomy 45X0 | Turner syndrome | Coarctation of the aorta, ASD, aortic stenosis |
| Trisomy 5 | Interrupted aortic arch | |
| Trisomy 13 | Patau syndrome | CHD, VSD |
| Trisomy 18 | Edwards syndrome | CHD, VSD |
| Partial trisomy 20q | Dextrocardia | |
| Trisomy 21 | Down syndrome | CHD, AVSD, ASD, VSD, PDA |
| Trisomy 22 | VSD | |
| Partial trisomy or tetrasomy 22 | Schmid-Fraccaro syndrome | CHD, anomalous pulmonary venous return |
| Deletion 4p | Wolf-Hirschhorn syndrome | CHD |
| Deletion 7q11.23 | Williams syndrome | CHD, supravalvular aortic stenosis, hypertension, MVP |
| Deletion paternal 15q11 | Prader-Willi syndrome | CHD |
| Deletion 17p | Miller Dieker syndrome | CHD, ASD |
| Deletion 22q11 | CATCH-22, DiGeorge, and Velocardiofacial syndromes | CHD |
| Rearrangement 5p15.1-3 | Cri du Chat syndrome | CHD |
| Recombinant chromosome 8 | San Luis Valley syndrome | Tetralogy of Fallot |
Overall, the absolute risk of CHD given a maternal CHD is 5%, given a paternal CHD is 2.5%, given a sibling with CHD is 3%, second degree relative is <2%, third degree relative is about 1% .
There is an important role for genetic counseling for families with a child with CHD. In most situations, the risk for development of CHD in subsequent children is small, on the order of 1%–3%, so that it is seldom wise to discourage pregnancy. The risk rises if the original proband has a chromosomal defect associated with a well-defined multisystem syndrome or there is a likelihood of continued exposure to a noxious environment in the first trimester of the subsequent pregnancy. Also, if the family already has more than one member with CHD, the risk of a subsequent birth with CHD increases greatly. The risk of having another child with CHD when the mother is the carrier of a sporadic congenital lesion ranges from 2.5% to 18%, depending on the lesion. Obstructive lesions of the left ventricular outflow tract have the highest recurrence rates in offspring. When the father is a carrier of the same mutation, 1.5%–3% of the offspring is affected. Genetic screening for 22q11.2 microdeletion should be considered if patients with TOF intend to have children. Without the 22q11 deletion, the risk of CHD in the fetus is 4%–6% .
Many environmental, nongenetic risk factors contribute to CHD, playing a role in CHD alone or in combination with the above-described genetic factors .
A host of cardiac teratogens have been identified in experimental animals and humans including hypoxia, ionizing radiation, drugs such as thalidomide, lithium, dilantin, and other anticonvulsants, ACE-inhibitors; selective serotonin reuptake inhibitors; alcohol consumption; cigarette smoking; maternal diseases including diabetes, phenylketonuria, systemic lupus erythematosus, and Siögren’s syndrome if SSA/SSB autoantibody positive .
A well-defined environmental cause is maternal rubella infection in the first trimester of pregnancy. The rubella syndrome in the past was a serious condition, but nowadays it has been mostly eradicated thanks to vaccination. The spectrum of lesions consists of cataracts, retinopathy, deafness, microcephaly, and CHD which may take the form of PDA, pulmonary valvular or arterial stenosis or both, AS, TOF, and VSD, either alone or in combination . Other maternal infections, such as chlamydia, are associated with CHD . Maternal smoking is a known risk factor that accounts for 1.4% of all CHDs. Maternal smoking before and during the first trimester of pregnancy is associated with a ≥30% increased fetal risk of ASD, PS, PTA, and TGA and septal defects, particularly for heavy smokers of ≥25 cigarettes daily. Maternal exposure to secondhand smoke is also associated with an increased risk of CHD .
Maternal binge drinking can cause fetal alcohol syndrome, consisting of prenatal growth retardation, developmental delay, microcephaly, micrognathia, microphthalmia, and cardiac defects, often VSD, in about 45% of cases . The combination of smoking and binge drinking is the riskiest behavior to develop CHD .
Exposure to air pollutants, in particular, benzene, carbon monoxide, nitrogen dioxide, and sulfur dioxide, and particulate matters <2.5 microns (PM 2.5 ), particulate matter <10 microns (PM 10 ), during the first trimester pregnancy is a risk factor for CHD .
Maternal obesity and diabetes mellitus, pregestational or identified in the first trimester and gestational, are significantly associated with CHD, both isolated (CHD as the only major congenital anomaly) and multiple (CHD plus ≥1 noncardiac major congenital anomaly). In particular, they are associated with an increased risk of TOF .
Ingestion of lithium during pregnancy is associated with tricuspid valve anomalies, while ingestion of thalidomide is associated, in addition to with major limb deformities, to CHD without a predilection for a specific lesion .
A deficiency of folic acid is associated with congenital anomalies, including CHD, and folic acid supplementation is routinely recommended during pregnancy .
Preeclampsia is considered a risk factor for CHDs of noncritical type .
High altitude has been reported as a risk factor for CHD. Tibetan children living at higher altitude has an increased prevalence of CHD compared to children living at lower altitudes, in particular of PDA and ASD .
Paternal occupational exposure, especially to phthalates, is a documented risk factor for CHD. Paternal anesthesia is associated to TOF, sympathomimetic medication to CoA, pesticides to VSD, and solvents to HLHS . More investigations are needed to define the role of endocrine-disrupting chemicals (EDCs) on the development of CHD.
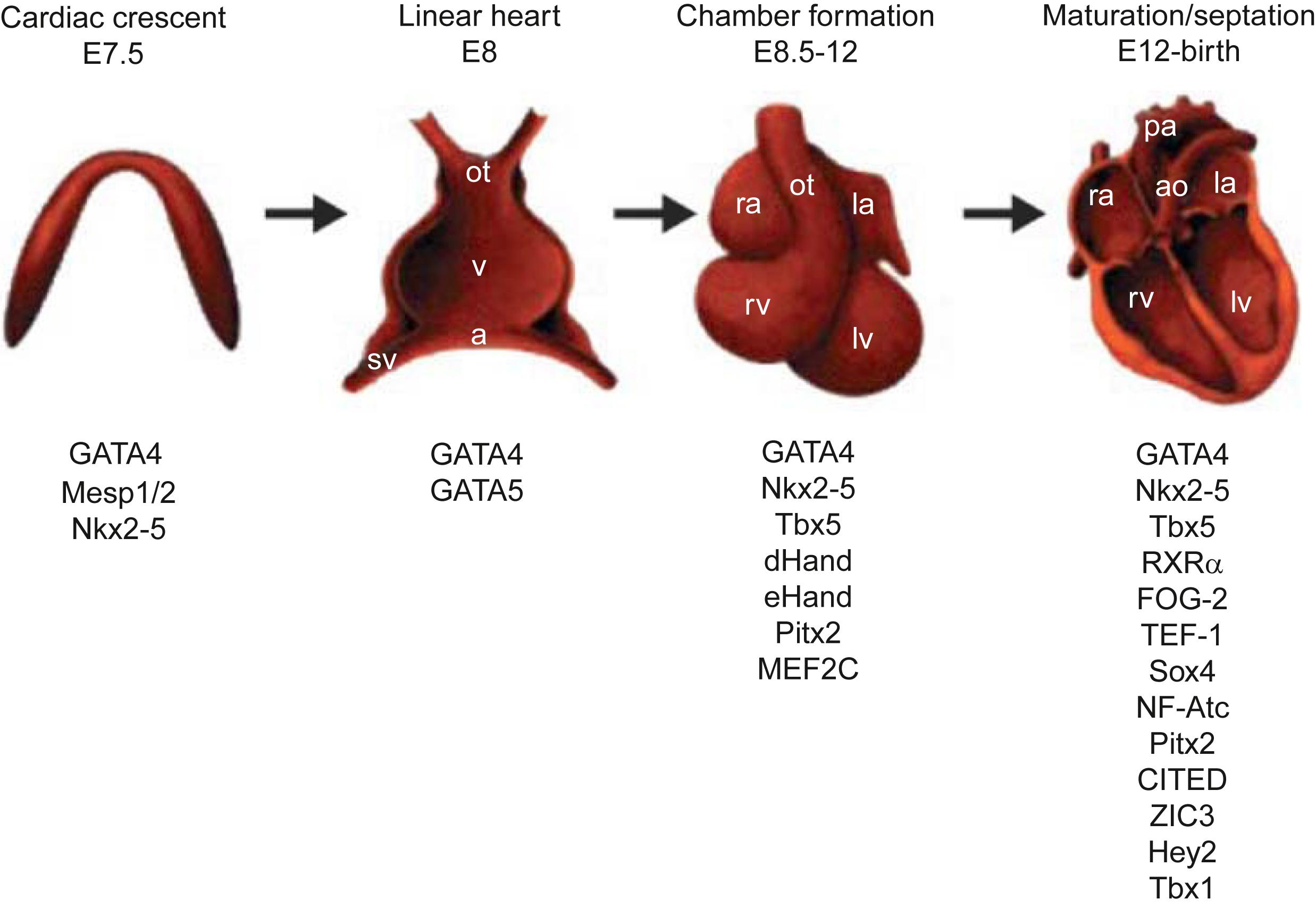
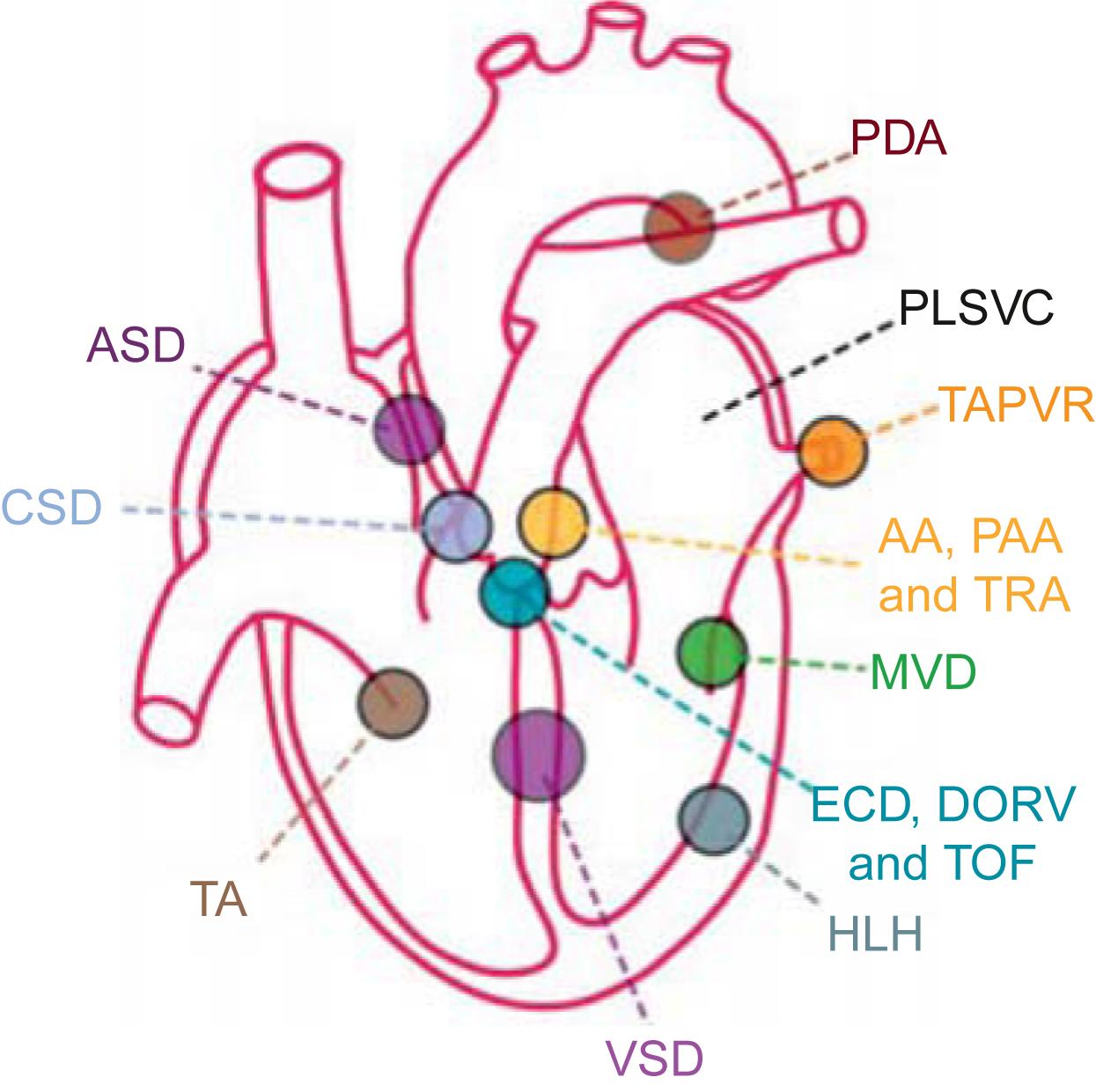
Molecular biological studies in mice utilizing inactivation of genes (knockouts) have provided identification of proteins involved in heart morphogenesis and chamber development . These studies have also linked alterations in these genes to the development of specific cardiac and vascular anomalies ( Figs. 6.1–6.3 ; Tables 6.2–6.4 ).
At about day 15 of the human development, a horseshoe-shaped area of the splanchnic mesoderma develops as the cardiogenic region moving to the midline in two migratory waves to create a crescent of cells consisting of the first heart field (FHF) and SHF. The FHF expresses the transcriptional factors (TFs) HAND1 and TBX5, and is destined to give rise to left ventricle and part of the atria. The SHF expresses the TFs HAND2 and fibroblast growth factor (FGF)-10, and will give rise to the outflow tract, right ventricle, and part of the atria. The two heart fields consist of multipotent progenitor cells that can differentiate to endocardium, myocardium (working and specialized), pericardium, or smooth muscle cells. TBX1 regulates neuronal crest migration and the expansion of cardiac progenitors in the SHF.
Around human embryonic day 20, the heart is a functional beating tube propelling blood unidirectionally, caudo-cranially from the venous to the arterial poles. It then elongates with addition of cells to both poles, loops to the right and, by embryonic day 28, begins to form the cardiac chambers, while extra-chamber myocardium specializes as conducting tissue. At about day 28, the extra-cellular matrix (ECM) known as cardiac jelly enlarges to produce the endocardial cushions and the future atrioventricular canal and outflow tract, while cells derived from the neuronal crest migrate into the outflow tract to the correct position to form the aortic arches. By day 50, septation of the atria, ventricles, and atrioventricular valve give rise to the four-chambered heart .
The multipotent cardiac progenitors express positive cell cycle activators, such as cyclins and proto-oncoproteins, which are downregulated in adult cardiomyocytes, insufficiently able to regenerate in response to injury. They are of great interest also for their therapeutic potential to regenerate or replace damaged cardiac cells, offering effective therapies for heart failure . Cardiogenesis is regulated by cross-talk among developing endodermal, mesodermal, and ectodermal cells, involving multiple steps to commit progenitor cells to the myocardial lineage, formation and looping of the heart tube, segmentation and growth of the cardiac chambers, development of the cardiac conduction system, cardiac valve formation, and connection of the great vessels to the heart. These remarkable transformations depend on networks of TFs, regulated by signaling pathways. Bone morphogenetic proteins (BMPs) produced by the endodermal cells induce the cardiomyocyte specification, while the Wnt proteins produced by the ectodermal cells suppress alternative pathways. Vascular endothelial growth factor produced by mesodermal cells stimulates the endothelial cells migration, proliferation, and formation of vascular lumen.
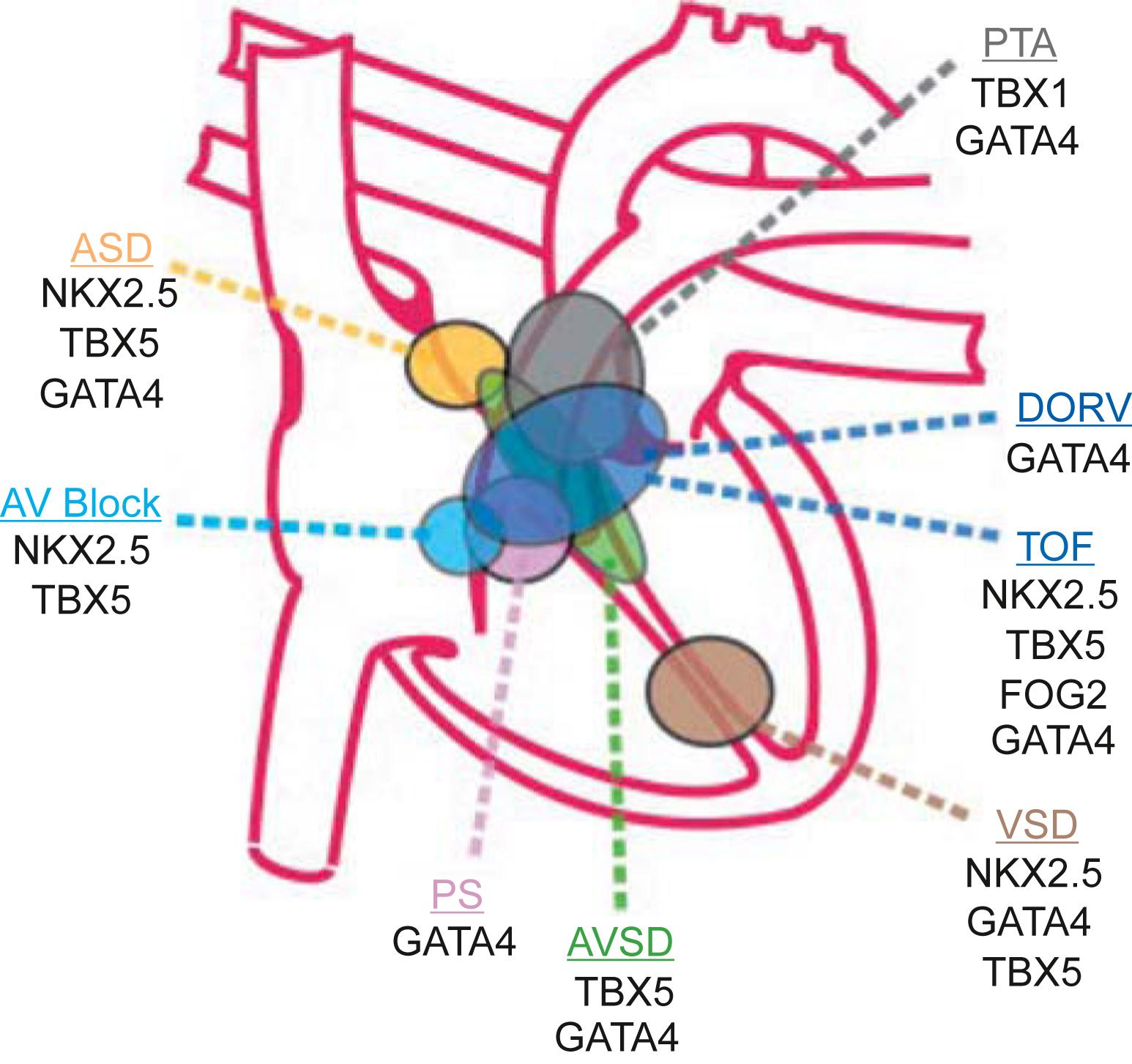
The cardiac conduction system originates from mesodermal multipotent progenitor cardiac cells that have retained primitive phenotype aspects rather than being specified from different precursors. The Notch pathway regulates the endothelial-to-mesenchymal transition during ventricular, atrioventricular canal, and outflow tract development, and promotes the expression of specific genes of the conduction system, programming cardiomyocytes into conduction system cells. Micro-RNAs also play important roles in heart development coordinating the TFs expression. The mechanical force of the flowing blood exerted by the developing heart also plays an important role in the development of heart and vessels . Alterations of TFs during heart development can lead to defects in cardiac looping, septation, and compartmentalization underlying CHD ( Table 6.3 ).
Nonsyndromic, sporadic CHDs are associated with mutations of the genes NKX2-5 , GATA-4 , or TBX20 that may cause atrial and VSDs, associated with conduction defects ( NKX2-5 ) or valve abnormalities ( TBX20 ). Considering that the affected patients are heterozygous for a single gene mutation, a 50% reduction or less of the involved TF can be enough to impair cardiac development. The genes GATA-4 and TBX20 also play a role in maintaining normal postnatal heart function and are mutated in some forms of adult cardiomyopathies . Mutations in gene NOTCH1 are associated with the bicuspid aortic valve. Different mutations can cause the same phenotype; for example, TOF can be caused by mutations of genes ZFPM2 / FOG2 , or NKX2-5 , or JAG1 , NOTCH2 . Mutations in the same gene can cause multiple phenotypes; for example, mutations of the gene NKX2-5 can cause ASD, VSD, conduction defects, and can also cause TOF ( Table 6.4 ).
Syndromic CHDs are associated with mutations of the genes TBX5 , NKX2-5 (Holt–Oram syndrome), TBX1 (DiGeorge syndrome), JAG1 , NOTCH2 (Alagille syndrome), fibrillin (Marfan syndrome, Loeys-Dietz syndrome), ELN (Williams syndrome), PTP11 , KRAS , SOS1 (Noonan syndrome), TFAP2B (Char syndrome), and CHD7 (CHARGE syndrome). Environmental stresses at a critical developmental time can also cause changes in TFs leading to the defects produced by heritable mutations ( Table 6.4 ).
Multimodality genetic studies have identified several genomic loci linked to congenital cardiac defects. Two important general principles have been elucidated. First, a given structural defect is often linked to more than one genomic locus ( Fig. 6.2 ). An exception is the rare cases of autosomal dominant diseases, such as Holt–Oram syndrome, which is caused by mutations of the genes in TBX5 and NKX2-5 ( Table 6.4 ). Second, even in families with CHD linked to a specific gene mutation, variable expressivity of the phenotype is often observed. These features point to the existence of modifier genes and the importance of gene–environment interactions .
The heart in higher vertebrates, in addition to being the organ most essential for life, is also the first functional organ in the developing embryo, able to beat spontaneously by week 4 of human development. Multipotent progenitor cardiac cells within the anterior lateral mesoderm are recognizable before the end of gastrulation, at approximately human embryonic day 15, with commitment to the cardiogenic lineage and their migration and organization into the cardiac crescent ( Fig. 6.1 ). Inductive signals from the underlying endoderm are responsible for the commitment to a cardiac fate of this mesodermic tissue. BMPs, FGFs, and Wnt proteins are critical for this first stage. The second step involves the formation of the beating linear heart tube as a result of migration and fusion of specified precursors from the cardiac crescent along the ventral midline. The complex process of cardiogenesis is under the regulation of multiple TFs and promoter genes ( Fig. 6.1 ; Table 6.2 ) .
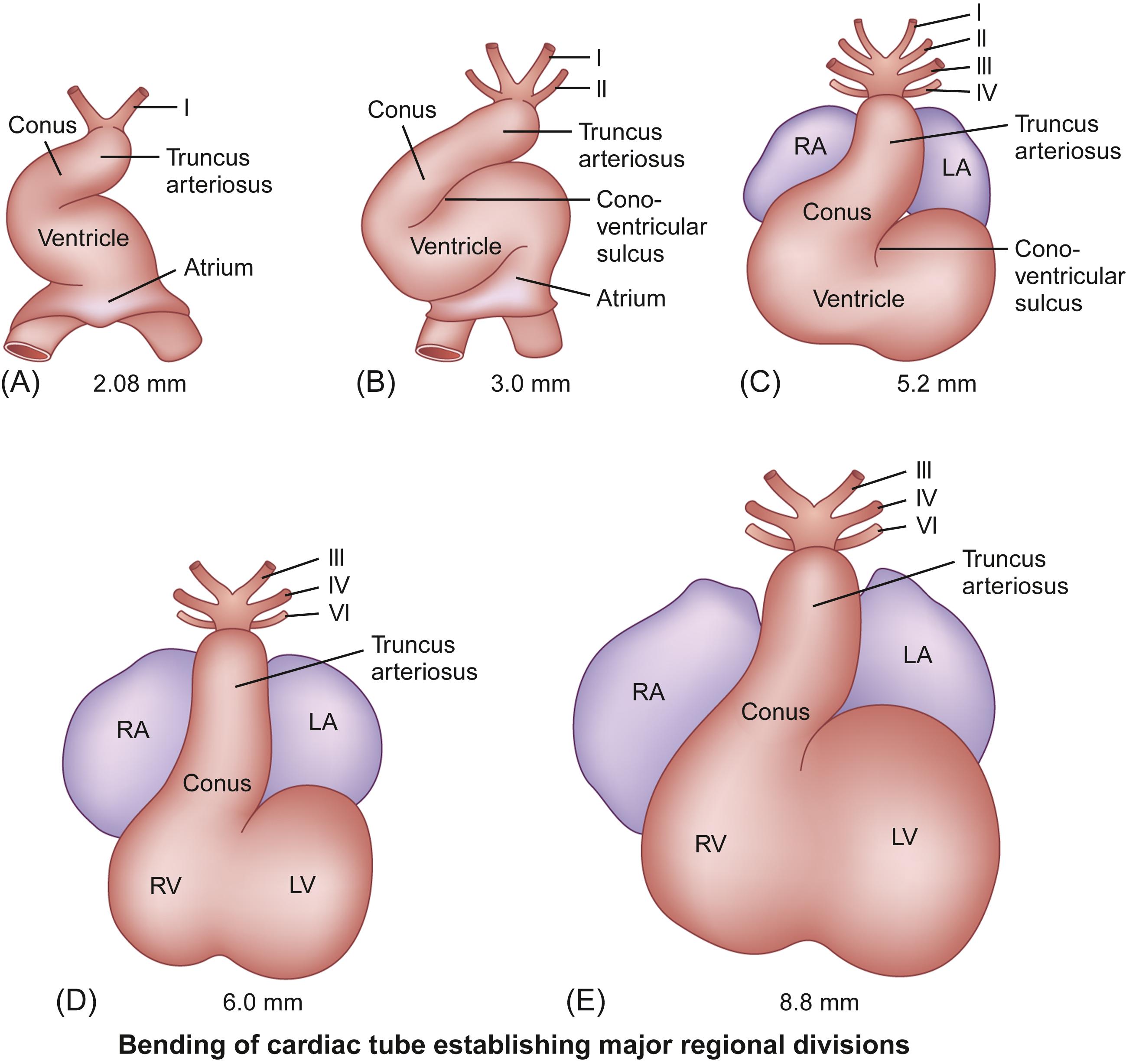
Beginning at about 3 weeks’ gestation, components of the splanchnic mesoderm differentiate into a primitive cardiac tube and pericardial cavity, and vascular channels develop and fuse to form blood vessels. The primitive cardiac tube forms from the joining together and fusion of two lateral endothelial heart tubes. Then, the epimyocardial mantle and cardiac jelly develop. These components differentiate into the endocardium, constituting the internal endothelial lining of the heart, the myocardium forming the muscular wall, and the epicardium or visceral pericardium forming the outer covering of the heart. Initially, the cardiac tube is a single-chambered structure and is composed of the following structures, extending from inferior (caudal) to superior (cephalad): the sinus venosus, which connects to the major veins; the atrium; the ventricle; the bulbus cordis or conus; and the truncus arteriosus, which connects via six pairs of aortic arches to two dorsal aortae .
The single-chambered heart initially is a straight tube positioned in the pericardial cavity that generates unidirectional blood flow from the caudal/venous pole to the cranial/arterial pole. The bulboventricular region grows much more rapidly than the pericardial cavity. As a result, further extension in a longitudinal direction is blocked, and the heart tube is forced to bend. The cephalic ventricular component of the tube bends in a ventral and caudal direction and to the right, while the caudal atrial component moves in a dorsal and cranial direction and to the left. This process is known as dextro ( d ) -bulboventricular looping , and it results in the atrial region developing a position superior to the ventricular region and the cardiac apex pointing to the left ( Fig. 6.4 ).
The external shape changes are accompanied by a complex process of internal septation that results in the formation of a four-chambered heart, while subregions of nonchamber myocardium specialize into the cardiac conduction system. In the atrium, a septum primum forms and then develops two openings: ostium primum and ostium secundum . The septum primum is a crescent-shaped membranous posterior ingrowth partially separating the right and left atria. The ostium primum is an anterior opening that allows blood flow from the right-to-left atrium during fetal development. A septum secundum then forms to the right of the septum primum , as a subsequent membranous ingrowth. The foramen ovale forms in the midportion of the developing interatrial septum due to the growth and positioning of the septum secundum adjacent to the septum primum . The sinus venosus becomes incorporated into the superior portion of the developing atria. Ventricular septation involves upward growth of muscular tissue to form the muscular interventricular septum . Specialized mesenchymal tissue, the endocardial cushion tissue , develops and provides the essential tissue for formation of the atrioventricular valves, the closure of the ostium primum in the atrial septum, and the formation of the membranous interventricular septum structures in the forming heart ( Fig. 6.5 ).
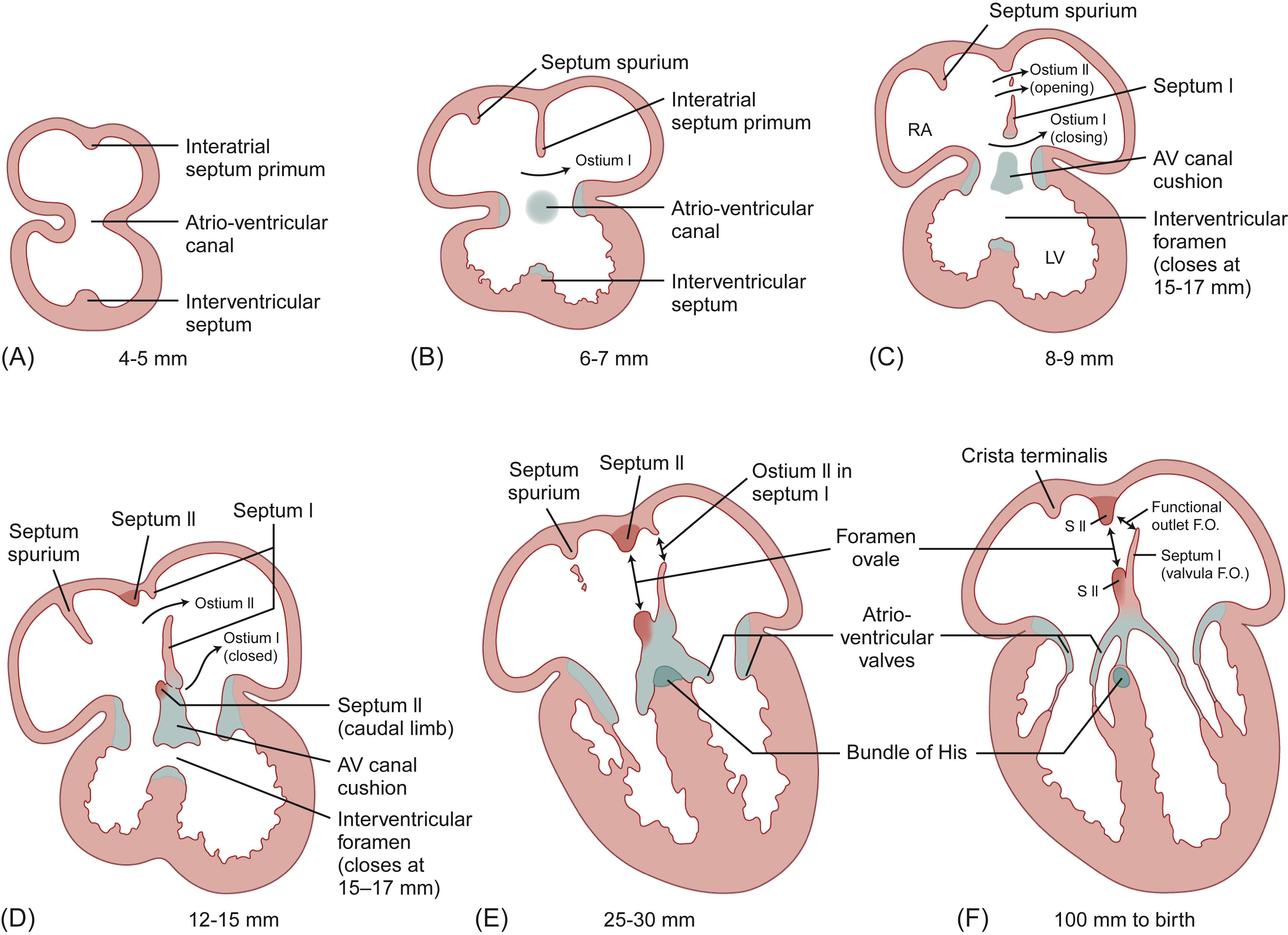
The primitive bulbus cordis contributes to the formation of several key of the developing heart. The proximal third of the bulbus cordis forms the trabeculated part of the right ventricle; the midportion, or conus cordis , forms the outflow tracts of both ventricles; and the distal third, the truncus arteriosus, forms the proximal aorta and pulmonary trunk. The junction between the primitive ventricle and the bulbus cordis is called the primary interventricular foramen. As the ventricular septation process proceeds, the primitive ventricle develops into the major part of the definitive left ventricle, and the proximal one-third of the bulbus cordis gives rise to the major part of the definitive right ventricle.
A spiral aorticopulmonary septum develops in the truncus arteriosus leading to the formation of separate aortic and pulmonary channels. As a result of extensive remodeling of the double aortic arch system, the mature vascular structures form, including a single aorta with left-sided aortic arch, a pulmonary trunk and main right and left main pulmonary arteries, and the ductus arteriosus , also known as ductus Botalli , which connects the aortic arch and the left pulmonary artery.
The aorta has a complex embryological origin . Extending cephalad from the developing heart, the truncus arteriosus and a connected ventral aortic sac are formed. Also formed are the dorsal aortae, which are paired structures that extend the entire length of the embryo and have segmental branches, the umbilical arteries, which establish connections to vessels in the chorion and later the placenta, and the vitelline arteries that communicate with the yolk sac. The dorsal aortae subsequently fuse. Six pairs of aortic arches develop as branches from the aortic sac and connect to the dorsal aortae, although not all branches are present at one time ( Fig. 6.6 ).
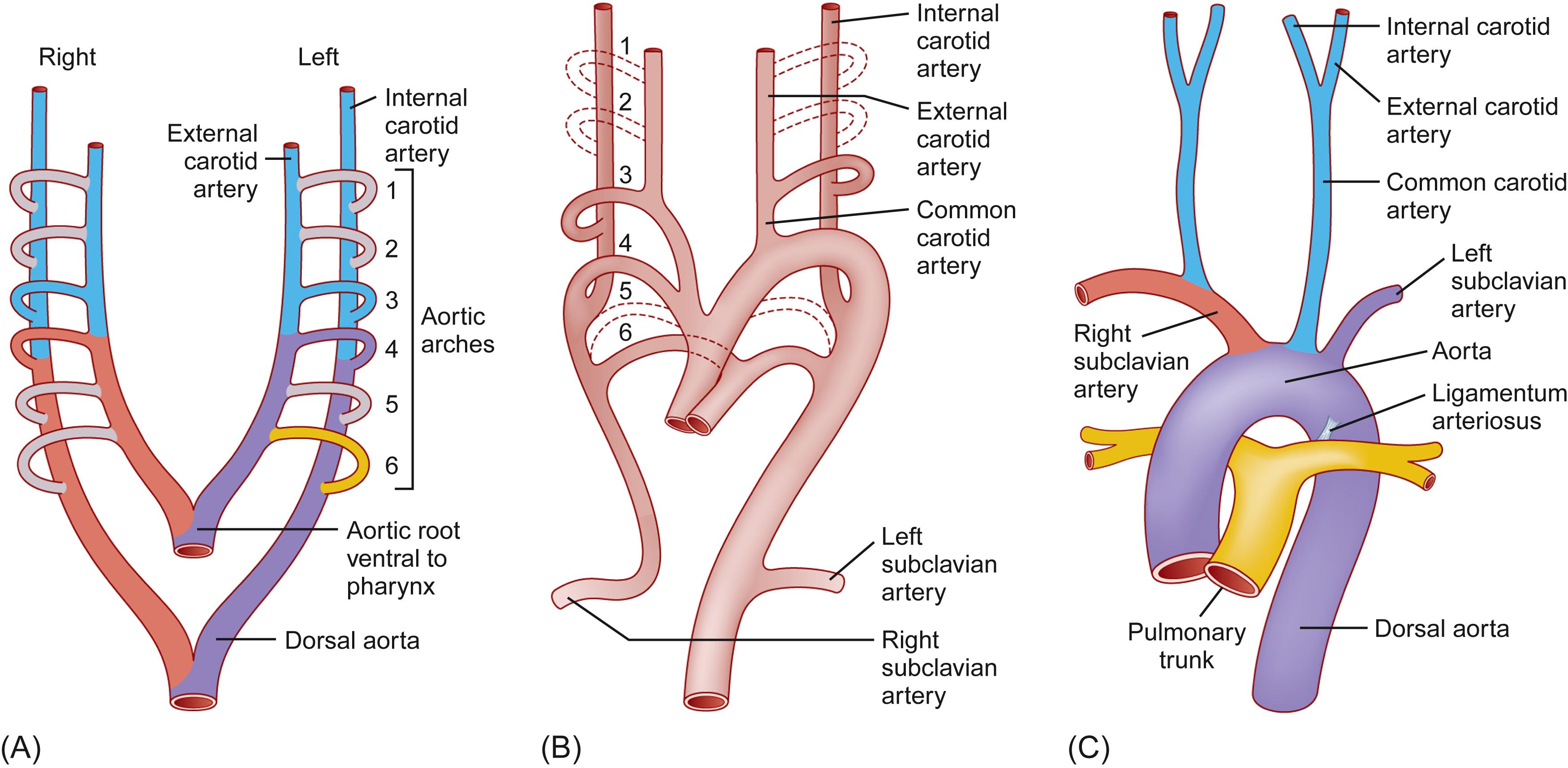
A complex process of selective resorption and migration of components of the aortic arches occurs, which results in formation of the major branches of the mature aortic arch. Some arches disappear while others develop further. Those that ultimately disappear are the first, second, fifth, and distal part of the right sixth arches. The remaining portions of the arches contribute as follows: first arches—portions of the maxillary and external carotid arteries; second arches—stapedial arteries; third arches—common carotid arteries and portions of the internal carotid arteries; fourth arches—aortic arch on the left, proximal subclavian artery on the right; fifth arches—absent or rudimentary in the adult; and sixth arches—portions of the right and left pulmonary arteries and ductus arteriosus on the left. Thus the important contributions are from the third, fourth, and sixth arches ( Fig. 6.6 ).
Anatomic variation and abnormalities in the complex events that change the six pairs of aortic arches to a mature aorta and its branches include persistence of parts that normally disappear, disappearance of parts that normally persist, and combinations of the two. Three patterns of normal variation of the aortic arch are as follows: type A—three aortic arch branches; that is, the innominate (brachiocephalic), left common carotid, and left subclavian arteries; type B—innominate and left common carotid sharing the same trunk and a separate left subclavian artery (bovine aortic arch); and type C—left vertebral artery arising from the aortic arch.
Each of the paired dorsal aortae has lateral, ventral, and dorsal segmental branches that supply each segment of the embryo. During the fourth week of gestation, the paired dorsal aortae fuse starting at the level of the seventh cervical vertebra to form the thoraco-abdominal aorta. The segmental branches persist and change to become the vertebral arteries in the neck, the intercostal arteries in the thorax, and the lumbar arteries in the abdomen. The lumbar segments of the fifth lumbar arteries give rise to the common celiac arteries and the ventral segments give rise to the vitelline arteries. The vitelline arteries become the celiac axis, the superior mesenteric artery, and the inferior mesenteric artery. At this time, the vascular supply to the upper and lower extremities is formed.
As the primitive heart develops in the fetus, myocardial contractile activity begins and a functional circulation is established. The foramen ovale in the interatrial septum and the ductus arteriosus remain open. In the fetus, pulmonary vascular resistance in the unexpanded lungs is high and systemic vascular resistance is low. These anatomic and physiologic features regulate the fetal circulation which involves delivery of oxygenated blood via the umbilical blood vessels and the inferior vena cava to the right atrium, right-to-left shunting of blood across the open foramen ovale , and the PDA to provide oxygenated blood from the placenta to the systemic circulation. The ductus venosus , also known as Arantius ductus venosus , shunts a portion of the left umbilical vein blood flow directly to the inferior vena cava, allowing oxygenated blood from the placenta to bypass the liver. Because the fetal lungs are not inflated and the fetus obtains oxygen and carbon dioxide gas exchange via the placenta, prenatal physiological shunts involve mixing and redirection of blood flow through (1) the foramen ovale , (2) the ductus arteriosus , (3) the ductus venosus , (4) the umbilical vessels, and (5) the placenta. Because of the fetal flow patterns, the workload of the right and left ventricles are different, as the right ventricle provides about 65% of the fetal cardiac output and the left ventricle ejection provides the remaining 35%. The uninflated lungs receive ≤10% of the cardiac output. After birth, the lungs expand, pulmonary vascular resistance falls, and systemic vascular resistance increases. These postnatal physiological changes lead to functional closure of the foramen ovale and ductus arteriosus with subsequent fibrous closure of these structures. The end result is complete separation of the right-sided and left-sided components of the mature circulatory system .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here