Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() For video accompanying this chapter see ExpertConsult.com . See inside cover for access details.
For video accompanying this chapter see ExpertConsult.com . See inside cover for access details.
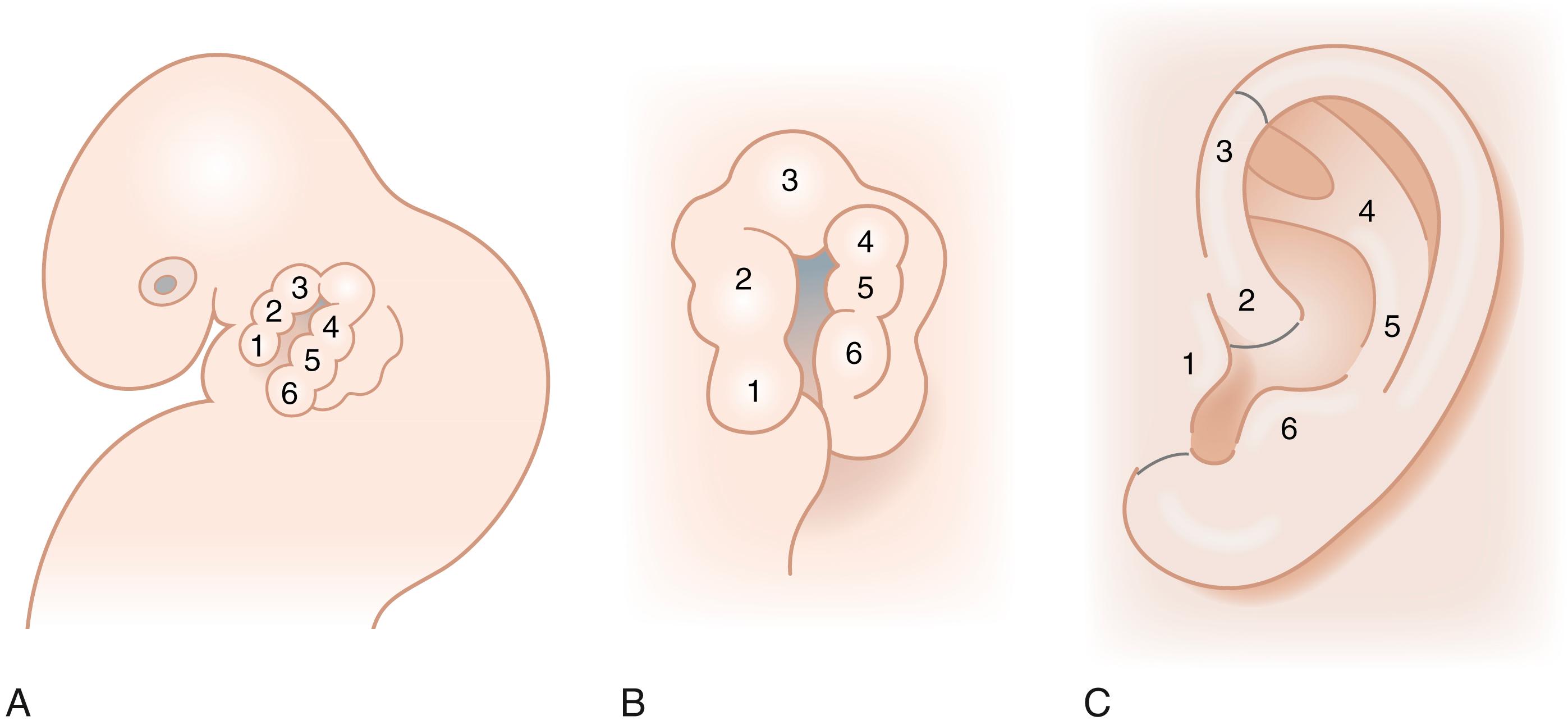
The intricate contours of the external ear are attributed to delicate elastic cartilage covered with a thin layer of subcutaneous tissue and skin. The ear develops around the first branchial cleft, which is the groove between the first (mandibular) and second (hyoid) pharyngeal arches. Auricular development begins during the fifth week of gestation. At the sixth week of embryogenesis six tubercles, the auricular hillocks of His, appear on the first and second branchial arches. The three anterior hillocks of the mandibular arch give rise to the tragus, root of the helix, and superior helix, respectively, while the three posterior hillocks of the hyoid arch form the remaining components of the auricle ( Fig. 18.1 ). The development of the ear is usually complete by the fourth month of gestation. The auricles are initially positioned on the embryonic neck, and progressively migrate from this anterocaudal location posterocranially as the mandible enlarges until it reaches its final anatomic location by week 20. As a result, remnants of the developing ear may be found at any point along this route, often localized to the preauricular region, so-called preauricular skin tags . Rarely polyotia may be encountered, considered an extreme form of the rudimentary skin tag, distinguished by both size and morphology. Polyotia bears resemblance to the normal external ear and is often termed mirror ear , with a conjoined conchal bowl in place of the tragus ( Fig. 18.2 ). The middle ear structures are also derived from the first and second branchial arches, and external auditory meatus and canal from the first branchial groove. Thus, patients with microtia typically have atresia of the external auditory canal and tympanic membrane, as well as variable deformities of the middle ear ossicles. Before subtleties of congenital anomalies can be recognized, the topography of normal ear must be appreciated and is illustrated in Fig. 18.3 .
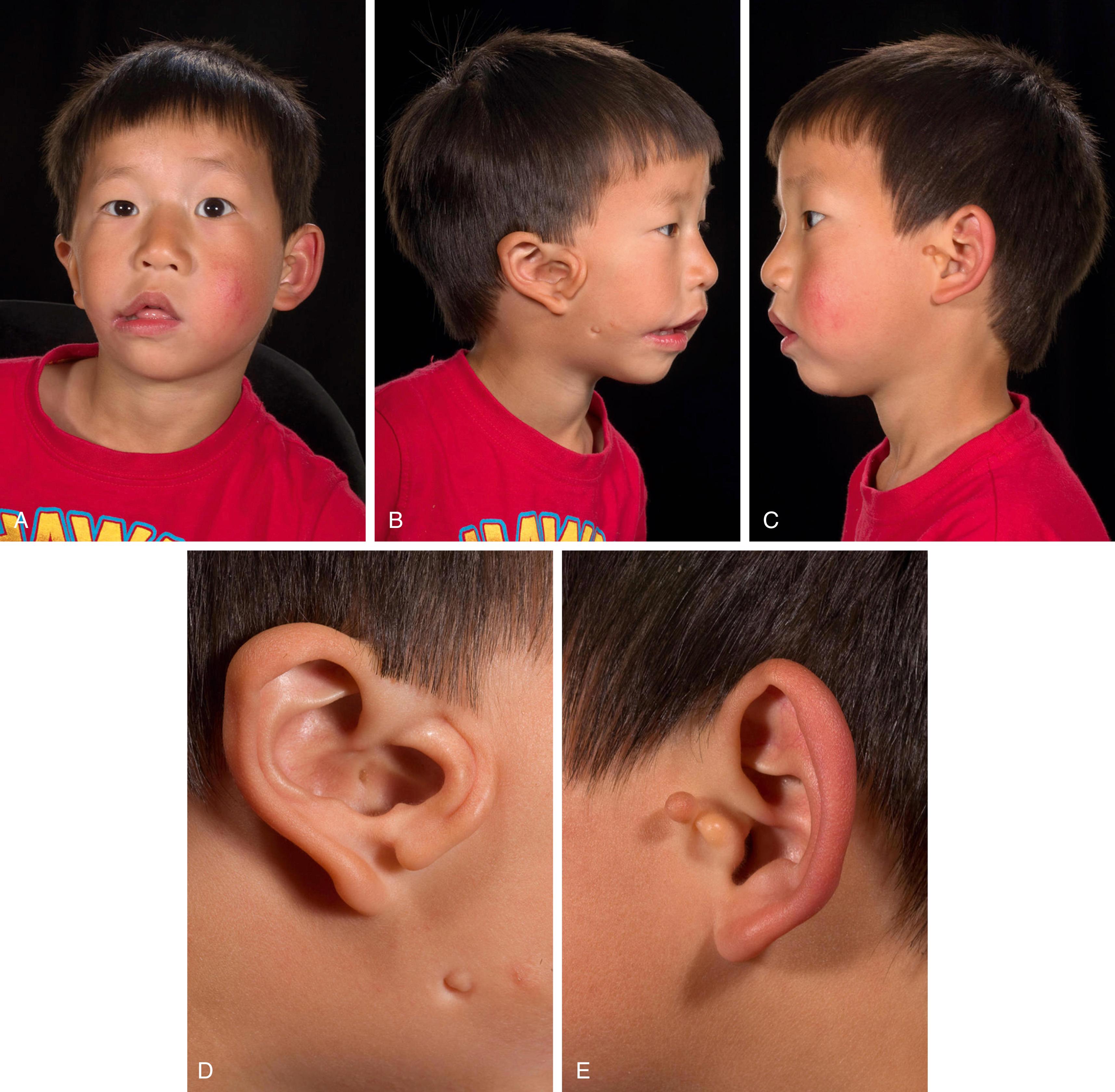
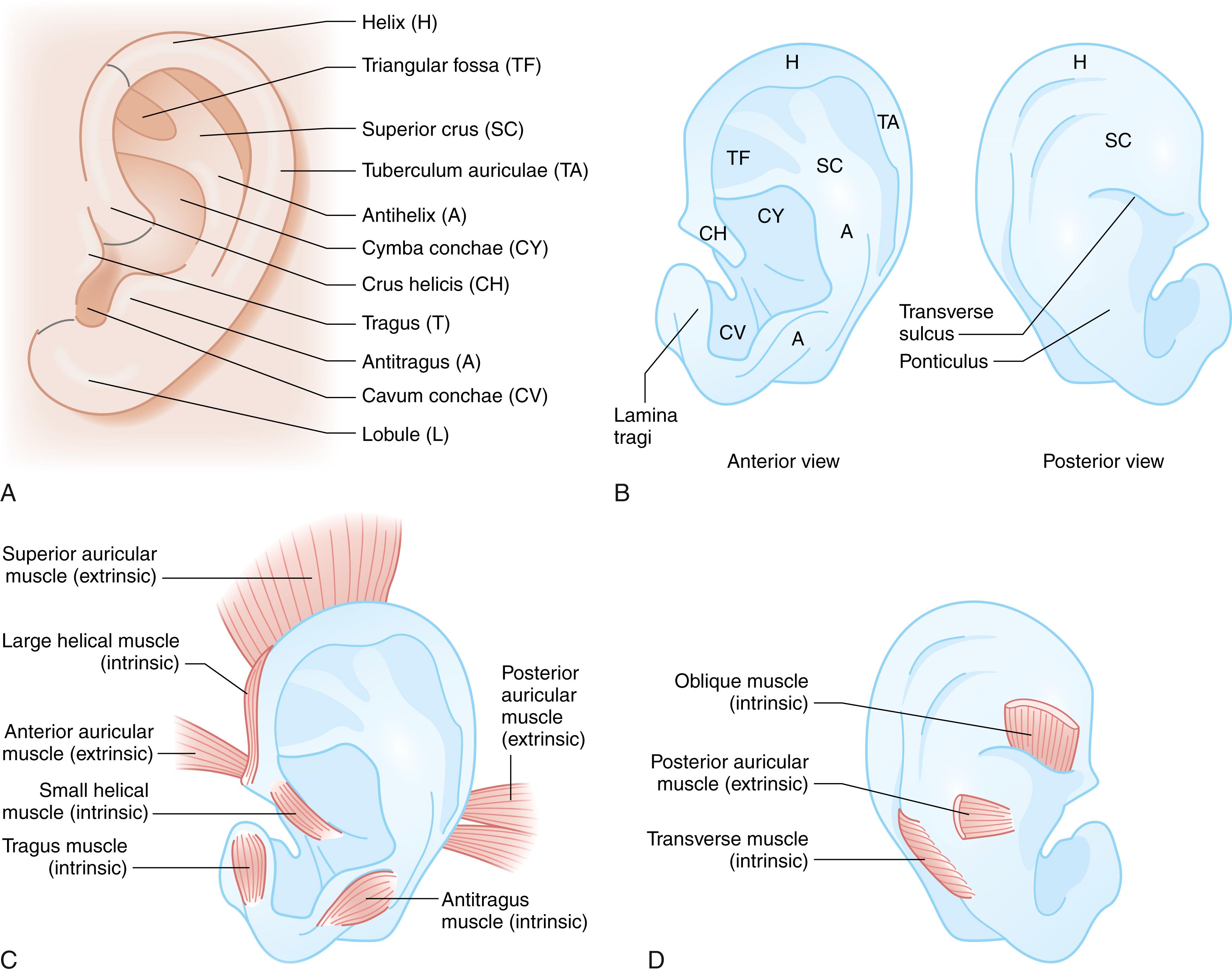
While the ear has its definitive form at birth, the auricular cartilage of the neonatal ear retains distinct malleability due to enduring high levels of circulating maternal estrogen. Estrogen upregulates the production of hyaluronic acid, which increases the pliability of cartilage. Estrogen levels are highest in the first 3 days after birth and decrease to a level comparable to older children at 6 weeks of age. During this privileged period, deformational anomalies of the auricle can be corrected by nonoperative means with external moulding devices. Authors have reported excellent results when correction is initiated within 3 days of birth. Thereafter, alteration of deformational irregularities, like auricular malformations, must be effected by surgical means. There is no consensus as to the upper age limit that splinting therapy can be considered, nor reliable evidence on the length of time needed for auricular moulding.
There is marked variability in the phenotypic expression of congenital deformities of the ear, challenging classification of these anomalies. The classification system proposed by Tanzer in 1978 is still commonly used ( Box 18.1 ). Congenital auricular anomalies can be categorized as either malformations or deformations. The malformed ear presents hypoplasia of cartilaginous and cutaneous constituents and gross morphological aberrations, whereas the deformed auricle has a normal chondrocutaneous component but irregular architecture. Further, while malformational anomalies arise from a combination of genetic and environmental factors, deformational abnormalities are deemed to result from external forces applied to the developing or neonatal ear and/or abnormal morphology or function of the three extrinsic and/or six intrinsic auricular muscles. The intrinsic musculature is mainly vestigial at birth but during auricular morphogenesis has an integral role in folding of the ear.
Type 1: Anotia
Type 2: Completely hypoplastic ear (microtia)
With atresia of the external auditory canal
Without atresia of the external auditory canal
Type 3: Hypoplasia of the middle third of the auricle
Type 4: Hypoplasia of the superior third of the auricle
Constricted (cup or lop) ear
Cryptotia
Hypoplasia of entire superior third
Type 5: Prominent ear
At birth, the normal auricle is approximately 66% of its adult size. The superior aspect of the helix is at the level of the tail of the eyebrow and the inferior aspect of the lobule corresponds to the base of the columella, with the long axis of the auricle inclined posteriorly at approximately 20 degrees from vertical. At 5 years of age, the ear has grown to within 8 mm of its full adult vertical height (approx. 5.5–7 cm), and is therefore approximately 87% complete. Ear length reaches its mature size at age 13 in males and age 12 in females. In contrast, ear width at age 5 years has attained approximately 97% of its adult size. The width of the ear continues to grow until age 7 and 6 in males and in females, respectively (width is approximately 55% of the length, 3–4.5 cm). This has important implications regarding timing of operative correction.
Ideal projection of the normal external ear is often cited as 1.5–2 cm from the scalp, and the projection of the helix from the mastoid as 10–12 mm in the superior portion, 16–18 mm and 20–22 mm in the middle and inferior portions, respectively. The auricle is considered as prominent if on frontal view it projects beyond these limits.
Prominent ears or prominauris is the most common congenital abnormality of the ear. It is associated with an autosomal dominant inheritance pattern with variable penetrance. This deformational anomaly is qualified by one or a combination of the following features:
Effacement or reduced definition of the antihelical fold
Prominence of the concha (overdevelopment of the posterior wall of the concha and/or increased angle between mastoid and concha)
Prominence of the lobule.
Other commonly associated features include excessive angulation of the helical root from the temple, absence of the helical roll ( shell ear deformity), and macrotia. There is no consensus regarding the optimal age at which to perform correction (otoplasty), and it may be considered as early as age 4 years. Ideally, the authors’ preference is to delay correction until at least age 6 years. At this age the ear has nearly attained its final adult size and, although anecdotal, the increased rigidity of the auricle at age 6 appears to decrease the occurrence of inadvertent damage to the cartilage, which can result in irregularities in auricular form.
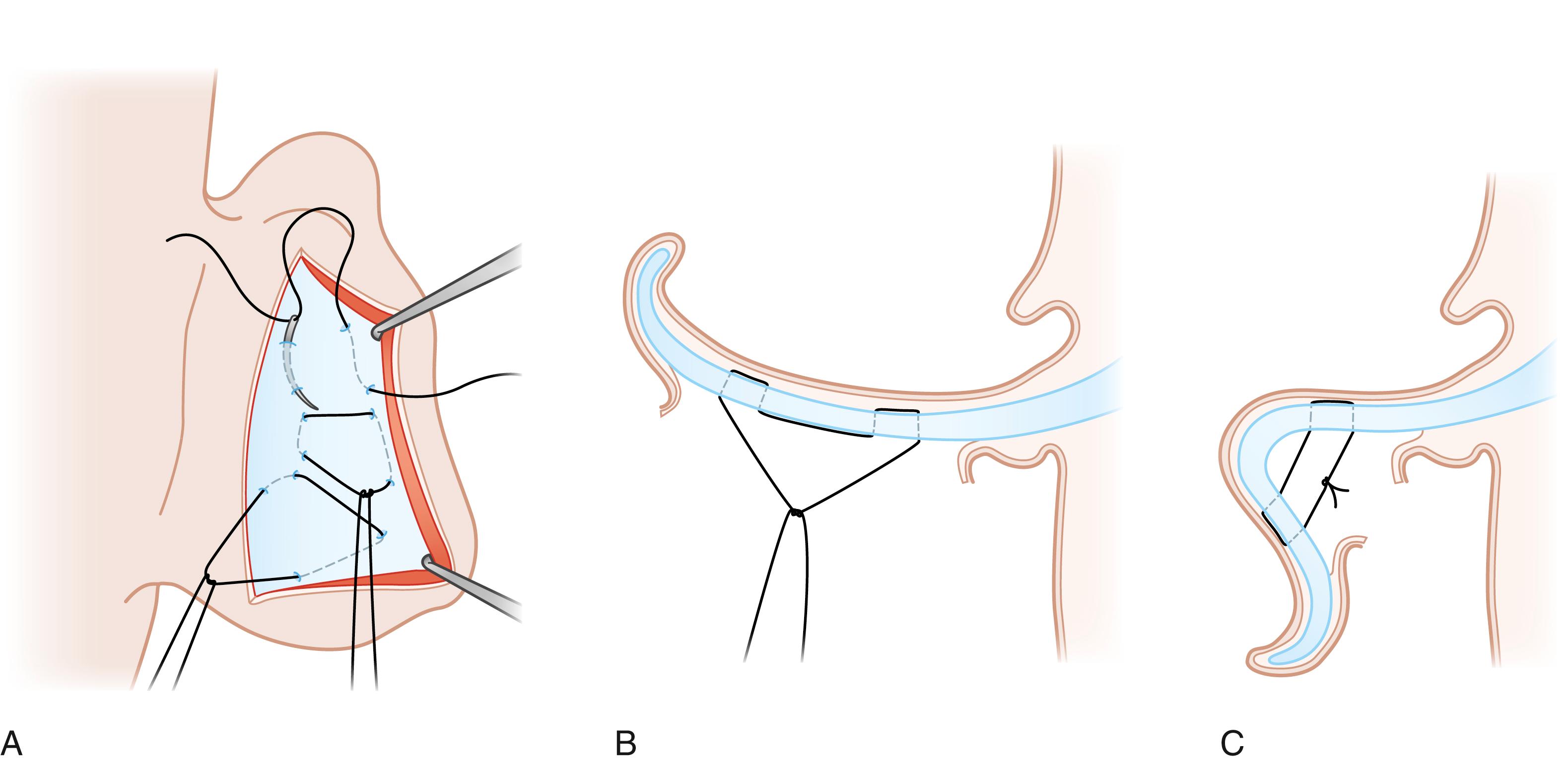
Numerous techniques have been described for correction of each of the three above-mentioned features of the prominent ear. Methods to recreate the antihelical fold are classified as cartilage suturing, cartilage scoring, and cartilage breaking. Cartilage breaking, initially proposed by Luckett in 1910, involves placing a full-thickness cartilage incision or excision along the curve of the antihelix and superior crus, which permits the cartilage on either side of the incision to fold into position. As an alternative to this technique, some surgeons made a pair of incisions parallel to the desired antihelical fold and placed sutures between the two. The latter technique was modified by Converse to obviate the sharp fold of the antihelix that often results. Mustardé is credited for creation of the antihelical fold with suture fixation, wherein at least three full-thickness mattress sutures are placed on the posterior surface of the cartilage to span the antihelix, with each suture purchase including cartilage and both perichondrial layers ( Fig. 18.4 ). Interestingly, Luckett in his original paper suggested that the antihelical fold could be created by appropriate suture placement, although he did not explore the concept. Cartilage scoring techniques, as popularized by Stenström and Chongchet, are based on the principle of Gibson and Davis that cartilage tends to warp away from the abraded perichondrial surface. Some authorities contend that so-called conchal excess is in fact secondary to a lack of antihelical fold, and once the fold is restored any apparent “excess” will be corrected. In fact, both Mustardé and Stenström addressed conchal protuberance by rolling back the posterior conchal wall, such that it is integrated into antihelix formation. Alternatively, conchal hypertrophy can be corrected with excision of a crescent-shaped cartilage segment (with or without overlying skin), through either an anterior or posterior approach, with care taken to avoid violation of the antihelix. Conchal mastoid sutures, elaborated by Furnas, may be added to effect desired conchal setback, where the concha is sutured to the mastoid fascia and/or periosteum ( Fig. 18.5 ). This maneuver effectively rotates the concha forward and outward, thereby increasing the protrusion of the angular ledge that marks the junction of the concha with the external auditory canal. Thus, when this procedure is carried out one must be cautious to avoid narrowing of the external auditory meatus. On the basis of cadaveric studies, Goulian and Conway described the cartilage of the helix and concha as two discrete components, separated by a distinct fissure, and advocated relocation of the cauda helicis (helical tail) onto the cavum conchae to reposition the lobule. The latter is based on the commonly advanced premise that lobular protrusion is due to a displaced cauda helicis. Many surgeons have subsequently adopted this approach and developed various technical modifications to retroposition the helical tail. Various other methods to address lobule prominence have been illustrated, such as fishtail excision of skin on the posterior surface of the lobule, wedge resection of the medial aspect of the lobule, and suture fixation of the lobule to the mastoid fascia.
There a number of complications associated with otoplasty, which differ depending on the technique used. With cartilage suturing, suture extrusion and granuloma formation may occur, necessitating suture removal and resulting in recurrence of ear prominence. Cartilage irregularities and/or deformity are more likely to occur with cartilage scoring and/or breaking techniques. Hematoma and infection are two early complications that can occur with any technique and merit more detailed mention. With regards to hematoma formation, rapid recognition and appropriate management is essential. In the early postoperative period, onset of severe often unilateral pain should prompt the immediate removal of the head dressing and if hematoma is confirmed, sutures released to drain the collection. Infection is another potentially devastating complication of prominauris correction, which mandates aggressive management with repeated washouts and intravenous antibiotics, as chondritis and cartilage deformity may ensue if not effectively controlled. Either of the latter two complications can result in significant cartilage destruction, for which correction may involve formal ear reconstruction.
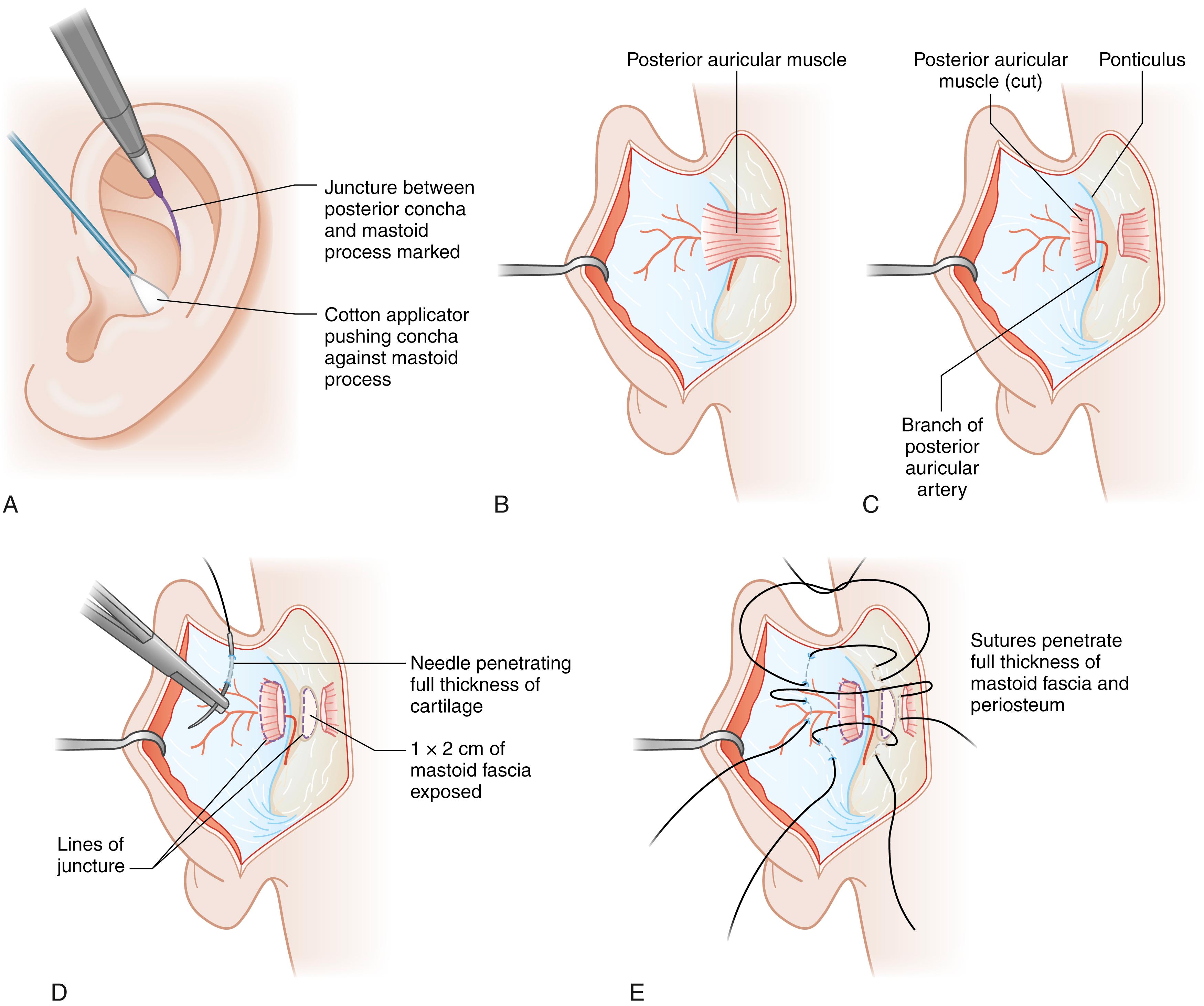
The authors’ preferred method for prominent ear correction is a modified Mustardé technique for antihelix formation with or without Furnas’ method for conchal setback. In the majority of cases, otoplasty is performed under general anesthesia. The procedure begins with design for a conservative dumb-bell-shaped outline for skin excision on the posterior (medial) side of the auricle. Following infiltration of local anesthesia to the posterior auricular surface in the subcutaneous plane, skin excision is performed and the posterior auricular skin is dissected off the perichondrium. If placement of Furnas sutures is anticipated, dissection is extended to the ponticulus (conchal ridge, site of muscle insertion) and an area of mastoid fascia for suture placement is exposed. Many often use a needle charged with methylene blue to mark the points for recreation of the antihelix. The needle is passed from the anterior (lateral) side and thus tattoos the exposed posterior cartilaginous surface at the desired location for suture placement. The authors do not utilize the latter technique, relying instead on familiarity with the architecture of the cartilage as viewed from the medial surface, to identify the conchal border, triangular fossa, and scapha, as underscored by Rubin et al. A nonabsorbable braided suture is used for cartilage fixation. Following antihelical fold formation, conchal prominence is assessed. If deemed excessive, Furnas sutures are placed with no conchal cartilage excision, in an effort to reduce the distance between concha and mastoid prominence. In his original technical description, Furnas divided the posterior auricularis muscle fibers and posterior auricular ligament, which extend from the ponticulus to mastoid fascia, prior to suture placement. Given that sutures are placed posterior to the muscle insertion on the concha and that great auricular nerve fibers span this area, the authors prefer to not divide these structures, ensuring rather that the posterior auricularis muscle is not strangulated with suture positioning. The latter is thought to avert the postoperative pain that may ensue following placement of conchomastoid sutures. If the lobule requires retropositioning, dissection is extended into the lobe such that a deep suture is placed from both the tail of the helix and the subcutaneous lobular tissue to the floor of the concha. The latter is an effective means to address lower third prominence and avoids tissue excision in and scar on the earlobe. Once hemostasis is assured, the incision is closed with a running horizontal mattress suture with absorbable suture and a head dressing is applied for 5 days. Once the dressing is removed, the patient is instructed to wear a headband at night for 3 months.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here