Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter treats only the congenital anomalies of the mitral valve to the exclusion of the mitral valve in atrioventricular (AV) discordance, the mitral valve in univentricular hearts, and the mitral valve of the hypoplastic left heart syndrome. It also excludes all acquired mitral valve disease, including the mitral valve insufficiency secondary to myocardial infarction or stunning as seen in anomalous origin of the left coronary artery from the pulmonary artery. Because repair of secondary or recurrent left AV valve regurgitation is often found in studies of the congenital mitral valve, it is included here. Although the division between congenital valve stenosis and congenital mitral valve insufficiency is classical and seemingly practical, we shall try to avoid it. Congenital valve stenosis and congenital mitral valve insufficiency generate different pathophysiologies and mechanisms of adaptation from the cardiovascular system, but they do have similar pathologies and associated lesions, they are often combined, and they require similar surgical techniques for treatment. Mitral valve anomalies associated with congenital connective tissue disorders, in which the embryology of the mitral valve itself is normal, are also covered in this chapter.
The embryology of the mitral valve is complex. The understanding of the formation of the leaflets and suspension apparatus has evolved, and the current approach is based on immunohistochemistry, in vivo labeling of cushion tissue, and scanning electron micrograph of human and chick embryos. In humans, the mitral valve develops between the 5th and the 15th weeks of embryonic life. The leaflet and chordal tissue derive from the endocardial cushion tissue lying on the inner surface of the AV junction. The separation between atrial and ventricular myocardium is dependent on the sulcus tissue located on the epicardial side of the junction. As the cushion tissue elongates and grows toward the ventricular cavity, it becomes progressively delaminated from the underlying myocardium, and the leaflet transitions into a funnel-like structure completely attached to the myocardium. Then, perforations into the valve leaflet appear. The perforations grow and form the chordae tendineae. The atrial aspect of the cushion generates the spongy atrial layer, and the ventricular layer generates the fibrous part of the mitral valve and the chords. The development of the papillary muscle takes place at the same time and is originated from the myocardium. A horseshoe-shaped ridge lies in the left ventricle. Progressively, the anterior and posterior parts of the ridge lose contact with the ventricular wall. They will form the papillary muscles, and as they increase in size, they stay in contact with the cushion tissue at the tip of the papillary muscle. The midportion of the muscular ridge will be incorporated into the apical trabeculations of the left ventricle.
Several AV cushions participate to form the final mitral valve. The most important are the superior and inferior cushions. However, there is no symmetry in the role of these two cushions. The superior cushion tissue generates most of the anterior leaflet of the mitral valve, whereas the inferior cushion generates most of the septal leaflet of the tricuspid valve. Smaller cushions are involved in the formation of the mural leaflet of the mitral valve. The wedging of the aortic root into the superior bridging leaflet, which originated primarily from the superior cushion, separates the developing mitral valve from its septal attachments.
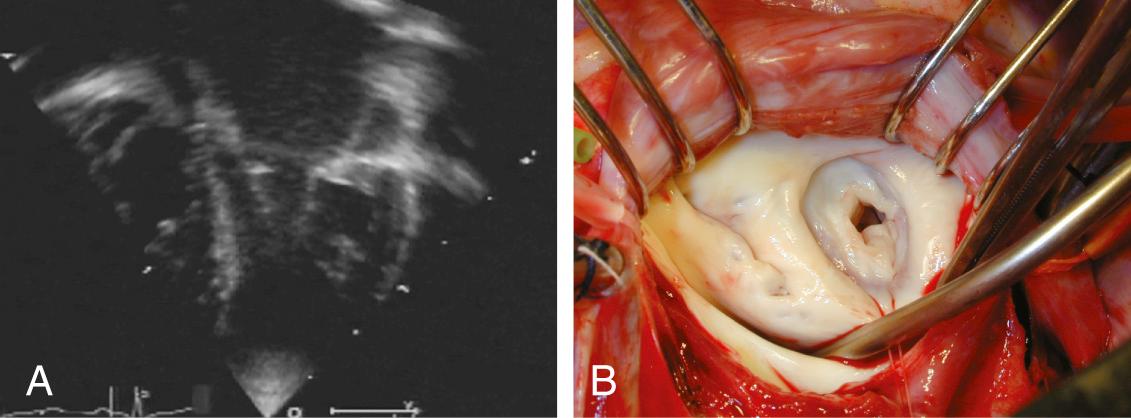
Often considered a congenital anomaly of the mitral valve, the supravalvar mitral ring is a fibrous construction attached to the posterior annulus of the mitral valve; it runs from both commissures to the mid height of the anterior leaflet. The lesion is stenotic, often to a greater extent than might be suggested by the extension of the ring. This is more a result of the limitation of the opening of the anterior leaflet than of the actual diaphragm effect of the ring ( Fig. 127-1 ). Strictly attached to the mitral valve annulus, it is to be differentiated from the cor triatriatum. Like the subaortic membrane in the left ventricular outflow tract, the supravalvar mitral ring is an acquired lesion resulting from turbulent flow through the mitral orifice. The primary lesion of the mitral valve responsible for the turbulent flow can be obvious, stenotic, and regurgitant, or it can be discrete or mild and difficult to identify. It can be related to a prominent coronary sinus, as found in the left superior vena cava draining into the coronary sinus. It is perhaps for these reasons that the supravalvar mitral ring is prone to reoccur after surgical resection, unless the underlying anatomic anomaly has been identified and corrected.
The cleft mitral valve is often isolated and can be easily differentiated from a left AV valve in a partial AV septal defect. It is an actual cleft with no suspension apparatus on the edges of the defect. The cleft is centered on the aortic commissure between the noncoronary and left coronary cusps. Each half of the anterior leaflet at the midportion bears the attachment of the strut chordae. With time, the cleft mitral valve regurgitation will generate secondary lesions at the edges of the cleft, such as thickening, rolling up, and retraction. The defect is never stenotic and may generate only little regurgitation for a long time.
Three major anatomic types of lesions are almost always associated with a lack of valvar tissue to various degrees and are worth characterizing: parachute mitral valve, papillary muscle to commissure fusion, and hammock or arcade valve. There is, however, a continuum between these three types and with the normal anatomy. The functional lesion can be either predominantly regurgitant or predominantly stenotic, or it can be both stenotic and regurgitant. In rare cases, the valve functions normally.
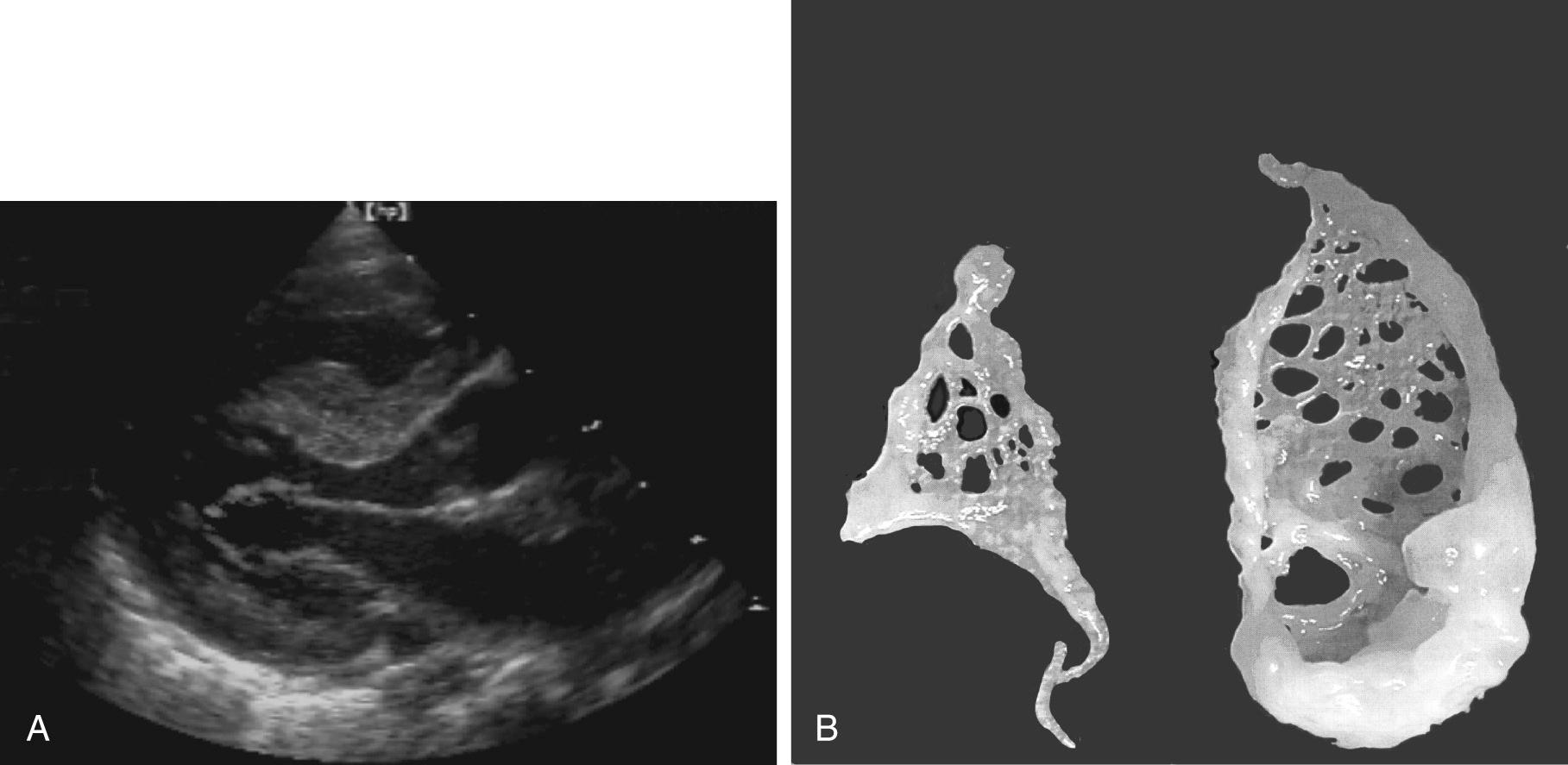
The parachute mitral valve can be found in isolation. It is, however, almost always associated with another cardiac anomaly, such as atrial septal defect (ASD), ventricular septal defect (VSD), or coarctation, and it is often integrated in Shone syndrome. It can also be seen in hypoplasia of the left ventricle, resulting in the need for univentricular palliation. The gross pathology shows a predominant single papillary muscle, with the orifice of the mitral valve overriding the tip of the papillary muscle. The spectrum of lesions for the suspension apparatus starts with complete fusion of the tip of the papillary muscle to the free edge of the valve. At the other end of the spectrum are chords that are relatively normal appearing, with good mobility of the leaflet ( Fig. 127-2 ). The accessory papillary muscle is usually small and devoted to a short segment of the free edge, or even to the undersurface of the leaflet tissue, as would be seen if it were a larger-than-normal secondary chord. The leaflet tissue can be intact or perforated.
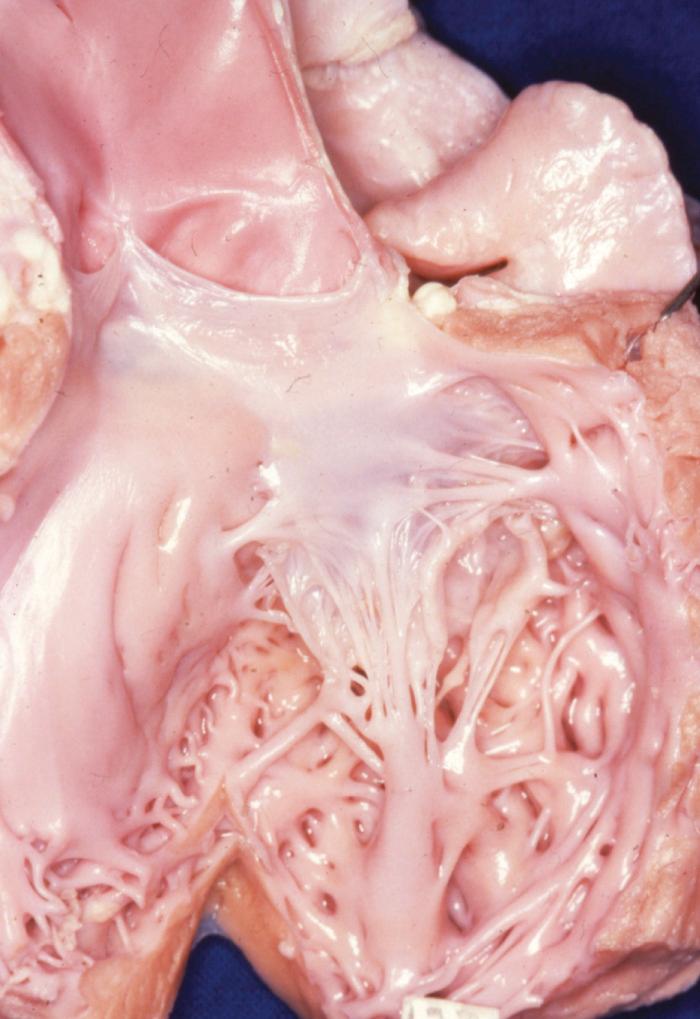
The functional class depends on the interaction between the amount of tissue and the mobility of the leaflet; presence and size of the fenestrations; and presence, length, and quality of the chords. The parachute mitral valve almost always has a stenotic component, because the gradient is fixed but the valve grows. These patients may not require a valve operation.
Double-orifice mitral valve, in which the lesser papillary muscle supports a complete orifice, is an exceedingly rare variation of the parachute mitral valve. It should be differentiated from the left AV valve, where an accessory orifice is often found when there is a diminutive or absent left lateral leaflet (mural leaflet).
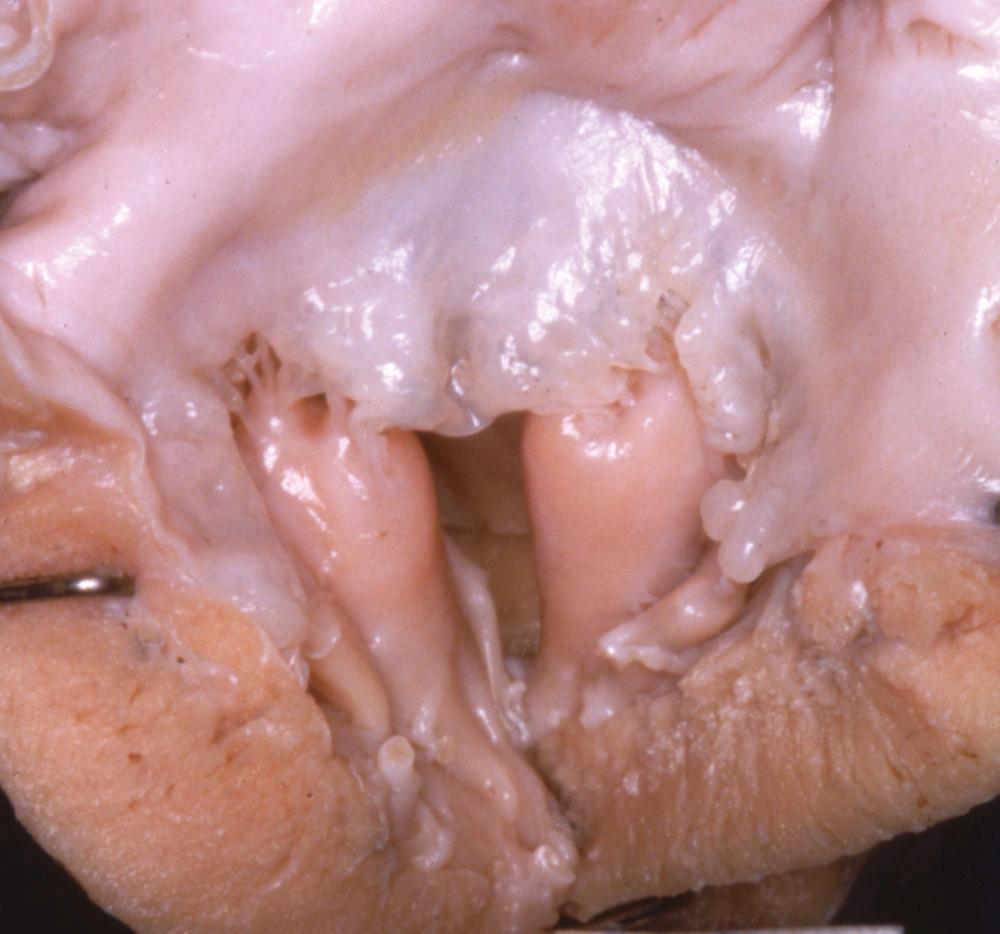
Fusion of papillary muscle to the commissure, also called short chordae syndrome, is defined by the presence of short chords and a papillary muscle tip that is attached or fused to the commissural area of the free edge ( Fig. 127-3 ). In the most extreme form, the chords are completely absent. The papillary muscles can be of normal size and volume. The valve is then generally more regurgitant than restrictive, which is because of the lack of valvar tissue and the restriction of the leaflet motion. When the papillary muscles are hypertrophied, the bulk of their mass is generally responsible for a predominantly restrictive valve.
In patients with hammock or arcade valve, the suspension apparatus may have lost all resemblance to the normal anatomy. The papillary muscles are not identifiable, or there are multiple small papillary muscles behind the posterior leaflet. The leaflets are suspended directly by a network of chords directly attached to the posterior wall of the ventricle. This attachment is generally displaced toward the base of the heart, with excess tension on the anterior leaflet and extreme limitation of posterior leaflet motion. The valve is most often predominantly regurgitant.
This rare condition is almost restricted to the middle scallop of the posterior leaflet.
When the anatomy of the mitral valve is otherwise normal, it is difficult to ascertain the congenital origin of dilation of the mitral valve annulus and elongation of the suspension apparatus, but they are included in most studies of congenital anomalies of the mitral valve and account for 15% to 40% of the patients in published studies of congenital mitral valve regurgitation. However, there is no evidence of their congenital origin. Elongation of papillary muscles can be found at birth in the mitral or the tricuspid apparatus, but the muscles usually have an ischemic, beige aspect. Sometimes, the ischemic origin is demonstrated by acute rupture at or shortly after birth. Isolated dilation of the annulus is not found at birth.
Both dilation of the annulus and elongation of the suspension apparatus are usually associated with significant volume loading of the left ventricle (e.g., with a large VSD or large patent ductus arteriosus). The pathophysiology is of initial dilation of the posterior annulus under the effect of the volume loading, followed by elongation of the marginal chords and prolapse of the free edge of the anterior leaflet. In rare cases, minor anomalies of the valvar tissue or the papillary muscles indicate a true congenital origin. When a patient older than 4 years has an isolated mitral regurgitation combining anterior leaflet prolapse and various degrees of posterior leaflet retraction, especially if the latter is thickened, a rheumatic origin should be ruled out when the patient originates from a geographical area of high prevalence.
Functional mitral regurgitations secondary to cardiomyopathies are not discussed here.
The interchordal spaces are filled with a dense network of valve tissue. When there is continuity between the anterior and the posterior leaflet, the accessory valvar tissue may generate a gradient directly related to the size of the perforations in the accessory tissue ( Fig. 127-4 ). When the accessory valve tissue is entrapped in the left ventricular outflow tract, the mitral valve may become regurgitant because of the traction exerted by the accessory valvar tissue on the anterior leaflet, which results in the valve's opening at mid systole ; however, in that case, the left ventricular outflow tract obstruction is the predominant hemodynamic lesion and is usually responsible for the diagnosis. Often, the accessory mitral valve tissue does not generate significant gradient or insufficiency.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here