Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Confocal microscopy provides high-resolution images of all corneal cell layers in vivo.
Confocal microscopy through focusing can be used for three-dimensional assessment of temporal changes in epithelial, stromal, and corneal thickness, nerve regeneration, cell density, and haze estimation after surgery, injury, infection, or disease.
Ultrafast, laser-based nonlinear optical imaging of second harmonic generated (SHG) signals from collagen provide an important emerging technology for evaluating the structural organization of the human cornea with high spatial and lateral resolution.
Structural and mechanical studies suggest that the distribution of lamellar branching in the corneal stroma establishes a self-stabilizing structure that defines corneal shape.
By combining in vivo confocal imaging with ex vivo SHG analysis, important insights into cell/matrix patterning and corneal regeneration and remodeling during healing can be obtained.
It is well established that confocal microscopy provides higher-resolution images with better rejection of out-of-focus information than conventional light microscopy. The optical sectioning ability of confocal microscopy allows images to be obtained from different depths within a thick tissue specimen, thereby eliminating the need for processing and sectioning procedures. In this chapter, we provide background on the technological development of in vivo confocal microscopy for corneal imaging. In addition, we also discuss multiphoton-second harmonic generation confocal imaging, a more advanced technology that enables the noninvasive assessment of collagen matrix organization in intact corneal tissue.
The optical design of confocal microscopy is based on the principle of Lukosz, which states that resolution may be improved at the expense of field of view. In 1955, Marvin Minsky developed the first confocal microscope, which was used for studying neural networks in the living brain. In modern confocal microscopy, a point light source is focused onto a small volume within the specimen, and a confocal point detector is used to collect the resulting signal. This technique results in a reduction of the amount of out-of-focus signal from above and below the focal plane, which contributes to the detected image and produces a marked increase in both lateral (x, y) and axial (z) resolution.
The use of a point source/detector in the confocal optical design trades field of view for enhanced resolution; therefore a full field of view must be built up by scanning. Petran et al. , developed the first scanning confocal microscope, named the tandem scanning confocal microscope (TSCM), which used a spinning disk containing thousands of optically conjugate (source/detector) pinholes arranged in Archimedean spirals ( Fig. 14.1A ). In 1986, Lemp et al. were the first to apply confocal imaging techniques to the study of the cornea ex vivo. This work led to the design of a TSCM with a horizontally oriented objective (Tandem Scanning Corp, Reston, VA), which was the first system suitable for use in ophthalmology.
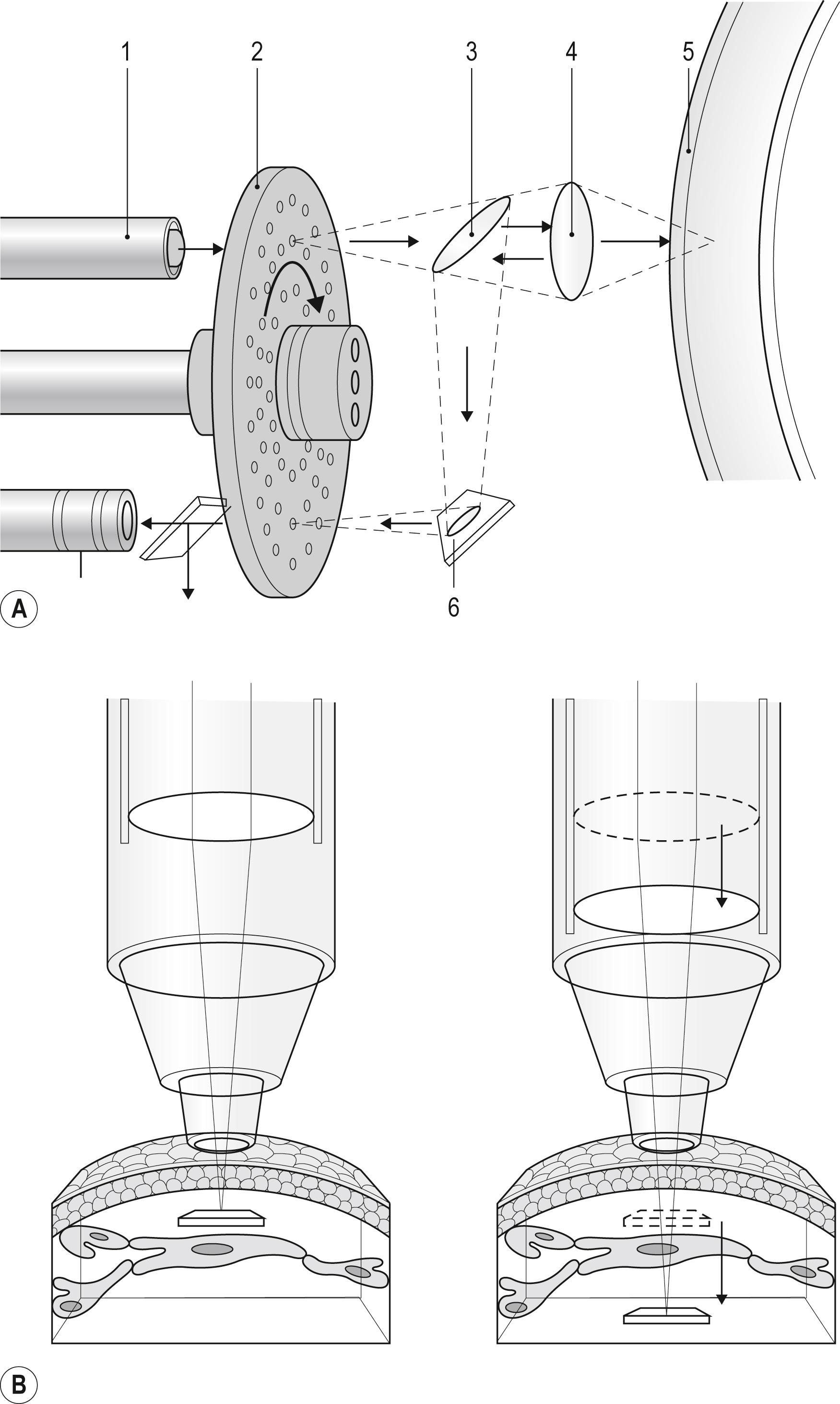
Three main confocal imaging systems have been used clinically: the TSCM, the Heidelberg Retinal Tomograph with Rostock Corneal Module (HRT-RCM) from Heidelberg Engineering, and the Confoscan 4 from Nidek, Inc. Most early in vivo confocal imaging was accomplished with the TSCM design. Most TSCM systems still in use employ a specially designed surface contact objective (24×, 0.6 NA, 1.5 mm working distance). The position of the focal plane relative to the objective tip is varied by moving the lenses within the objective casing (see Fig. 14.1B ). Thus the depth of the focal plane within the tissue can be calibrated, and quantitative three-dimensional (3D) imaging is possible with this system. , With this objective, the TSCM has an axial ( z -axis) resolution of approximately 9 μm. The TSCM system is no longer commercially available.
The HRT-RCM is a laser scanning confocal microscope. It operates by scanning a 670-nm laser beam (<1 μm diameter) in a raster pattern over the field of view. The system typically uses a 63× objective lens (0.95 NA) and provides images that are 400 μm × 400 μm in size. The microscope produces images with excellent resolution and contrast and has better axial resolution than the TSCM due to the higher NA objective. ,
The Confoscan 4 is a variable-slit, real-time scanning confocal microscope. , The system uses a 40× objective lens (0.75 NA), and digitized images are 460 μm × 345 μm in size. The scanning slit design allows better light throughput and provides images with a higher signal to noise ratio than the TSCM. However, this is achieved at the expense of axial resolution, which has been measured at approximately 26 μm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here