Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes two accompanying lecture presentations that have been prepared by the authors: ![]() and .
and .
Fundamental physics behind computed tomography (CT) and magnetic resonance imaging (MRI).
Current roles of CT imaging in the preoperative, postoperative, and follow-up imaging of a neurosurgical patient.
Imaging findings, techniques, and applications of CT and MRI in the evaluation of patients with neoplastic, infectious, traumatic, and epilepsy disorders.
Fundamentals of advanced MRI techniques, including magnetic resonance spectroscopy, perfusion MRI, diffuse tensor imaging, and functional MRI in the evaluation of a neurosurgical patient.
Computed tomography (CT), formerly called computed axial tomography (CAT), was developed in the early 1970s by Sir Geoffrey Hounsfield and his colleagues in England. It was possibly the most important advance in medical imaging after the discovery of x-rays by Professor Wilhelm Röntgen. It represented the first commercially available imaging equipment with which the emerging technological advances in computing were used to generate digital images, displayed in gray scale. Its development revolutionized the evaluation of patients with neurological diseases and enabled noninvasive visualization of the inside of the body, which allowed detection of diseases and abnormalities and played a key role in the diagnosis, management, and treatment of patients on a daily basis in the practice of medicine all over the world. Although its place in imaging of the brain and spine has been somewhat supplanted by another revolutionary technology, magnetic resonance imaging (MRI), CT remains widely used and an important first study of choice in the practice of neurosurgery. Furthermore, because of important advances in CT technology during the last 20 years—such as multidetector configurations in newer CT scanners and the ever-increasing speed of computer technology, which enable very fast CT scanning of a patient in seconds rather than minutes—its use has strongly surged. Such advances have led to the development of CT angiography (CTA) and perfusion CT, which have become important in noninvasive evaluation of cerebrovascular diseases. In addition, portable CT scanners can provide high-quality images for point-of-care imaging in an intensive care unit setting and thereby avoid potential risks associated with transport of critically ill patients.
CT can be performed in various planes that depend on patient position and the CT gantry angle within its limited arc. For example, direct CT imaging of the paranasal sinuses or brain in the coronal plane can be performed with the patient in a supine position with the patient’s head hanging over the edge of the CT scanner table or with the patient in the prone position and the neck hyperextended. Most commonly, however, CT imaging of the brain and spine is performed in the axial plane, with the patient in a supine position on the scanner table and the head and neck in a neutral position. The need for a direct coronal patient position has lessened since the advent of high-resolution multiplanar reconstruction capabilities in newer generation CT scanners. These reconstruction capabilities can generate axial images in 0.5- to 0.6-mm increments, which can then be reformatted into the sagittal, coronal, and oblique planes, with image quality nearly identical to that obtained from direct scanning. ,
A typical routine brain CT scan consists of 5-mm contiguous axial images through the entire brain from the skull base to the vertex, without the intravenous injection of contrast material. This can be followed by another set of 5-mm axial images through the brain after the intravenous administration of a contrast agent: typically 100 mL of iodinated contrast material, injected through an 18- or a 20-gauge intravenous catheter. Scanning intervals are adjusted for clinical need and for indications such as patient age and size; need for higher resolution images of specific anatomy such as the orbits, temporal bone, and skull base; or CTA. With the newer multidetector CT scanners, these images can be reconstructed into submillimeter axial images that can be used to generate two-dimensional (2D) and three-dimensional (3D) reformatted sagittal and coronal images and thus better delineate parenchymal, vascular, and osseous anatomy.
The radiation dose delivered to patients during CT examination has become an object of significant concern because of the possibility of radiation-induced cancers, especially for pediatric patients undergoing repeated examinations. Radiation doses have been reduced 25% to 98% through the use of interactive reconstruction methods. Since the introduction of these methods in 2008, all major CT manufacturers now offer dose reduction as an option. However, dose reduction can have a significant effect on image quality and must be based on the patient and the likelihood of requirements for repeated surveillance examinations.
CT is most often the first study of choice in evaluation of a patient with suspected acute intracranial disease because of its ready availability, ease of use, short acquisition time, and high sensitivity for detection of acute hemorrhage and fractures. It can provide a wealth of information about the brain, including ventricular size, presence of brain edema, mass effect, presence and location of hemorrhage or masses, midline shift, evolving ischemic injuries, fractures, benign and malignant osseous disease, and evaluation of the paranasal sinuses. Its availability and short acquisition time also allow frequent repeat scanning of the brain, which can contribute to the management and follow-up of patients in the acute, subacute, and chronic phases in both inpatient and outpatient settings.
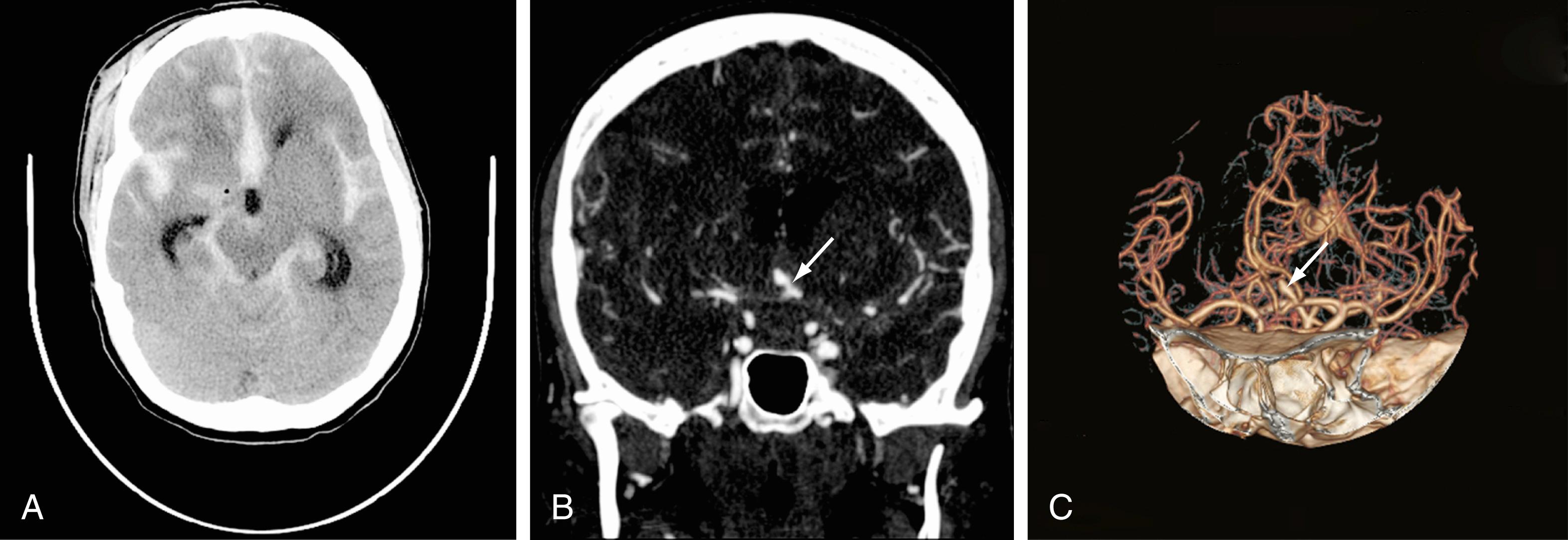
In neurosurgery, CT of the head is used for preoperative and postoperative evaluation for hemorrhage, infarction, hydrocephalus, mass effect, and fracture and for postsurgical assessment. CT is the study of choice in evaluating for acute hemorrhage because it has higher sensitivity and specificity for this indication than MRI does. Intracranial hemorrhage is typically described in terms of its location within the head, such as epidural, subdural, subarachnoid, intraventricular, and parenchymal; each of these different types of hemorrhages have sufficiently distinct appearances and locations. The borders of an epidural hemorrhage have a biconvex contour ( Fig. 10.1A ) in relation to the cranial vault and adjacent brain parenchyma, and this condition is usually the result of acute trauma associated with an acute fracture across branches of meningeal arteries that hemorrhage into the epidural space. Less commonly, rapid venous hemorrhage into the epidural space may cause an epidural hematoma. The extent of an epidural hematoma is usually limited by periosteal dural insertions at the major sutures. However, an epidural hematoma can extend across the midline in the frontal region anterior to the coronal suture because it is not limited by the dural reflections within the anterior interhemispheric fissure ( Fig. 10.1C ).
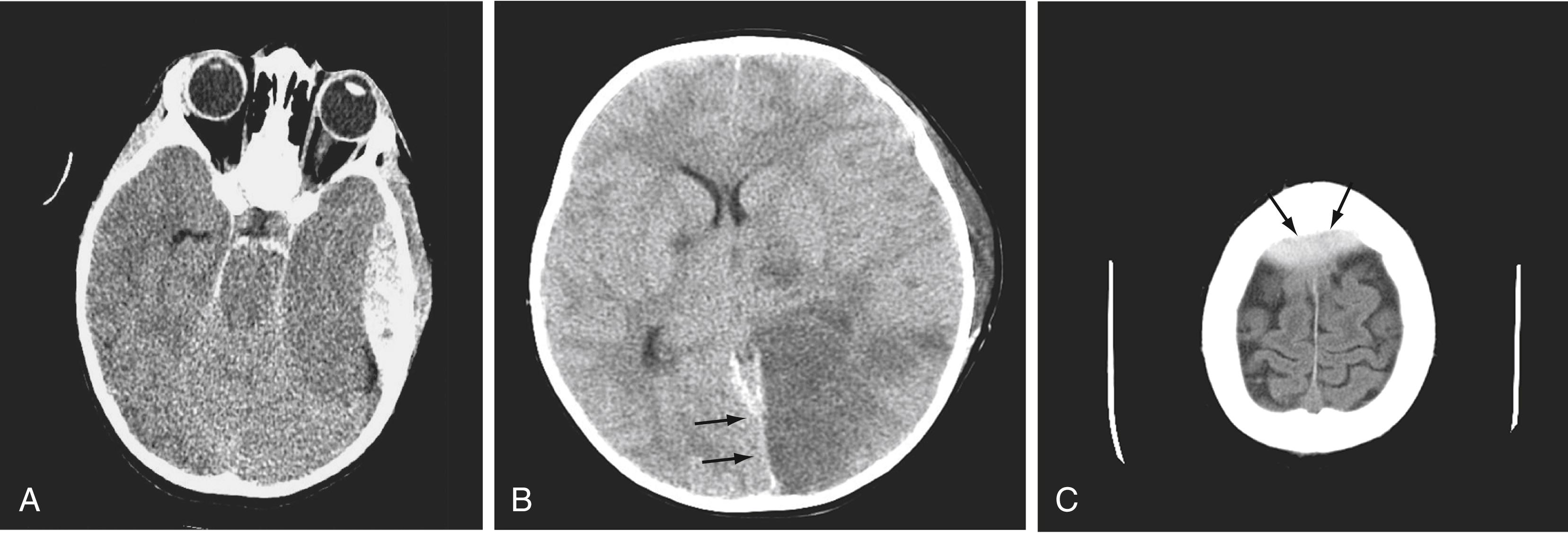
A subdural hematoma (SDH) is more common than an epidural hematoma, particularly in older patients, and is generally associated with acute head trauma, with or without an associated fracture. Its shape is different from that of an epidural hematoma because its deeper border against the brain parenchyma is concave and approximates the contour of the adjacent cerebral hemisphere convexity. An acute SDH is typically a result of venous hemorrhage and is not limited by the periosteal dural insertions at the major sutures. However, it is limited by the midline dural reflections within the interhemispheric fissure. The density of the blood in any type of SDH—whether acute, subacute, or chronic—changes over time from hyperdense to isodense to hypodense ( Fig. 10.2 ). However, a hyperacute SDH or an acute subarachnoid hemorrhage (SAH) in the presence of coagulopathy may sometimes appear isodense or hypodense.
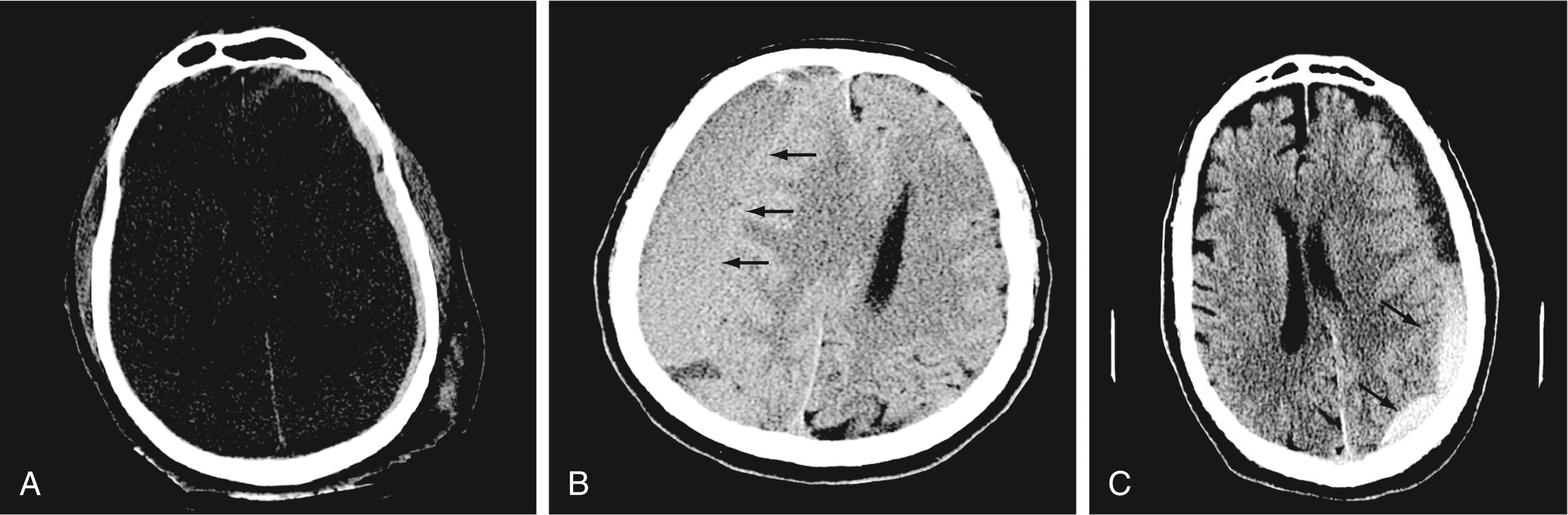
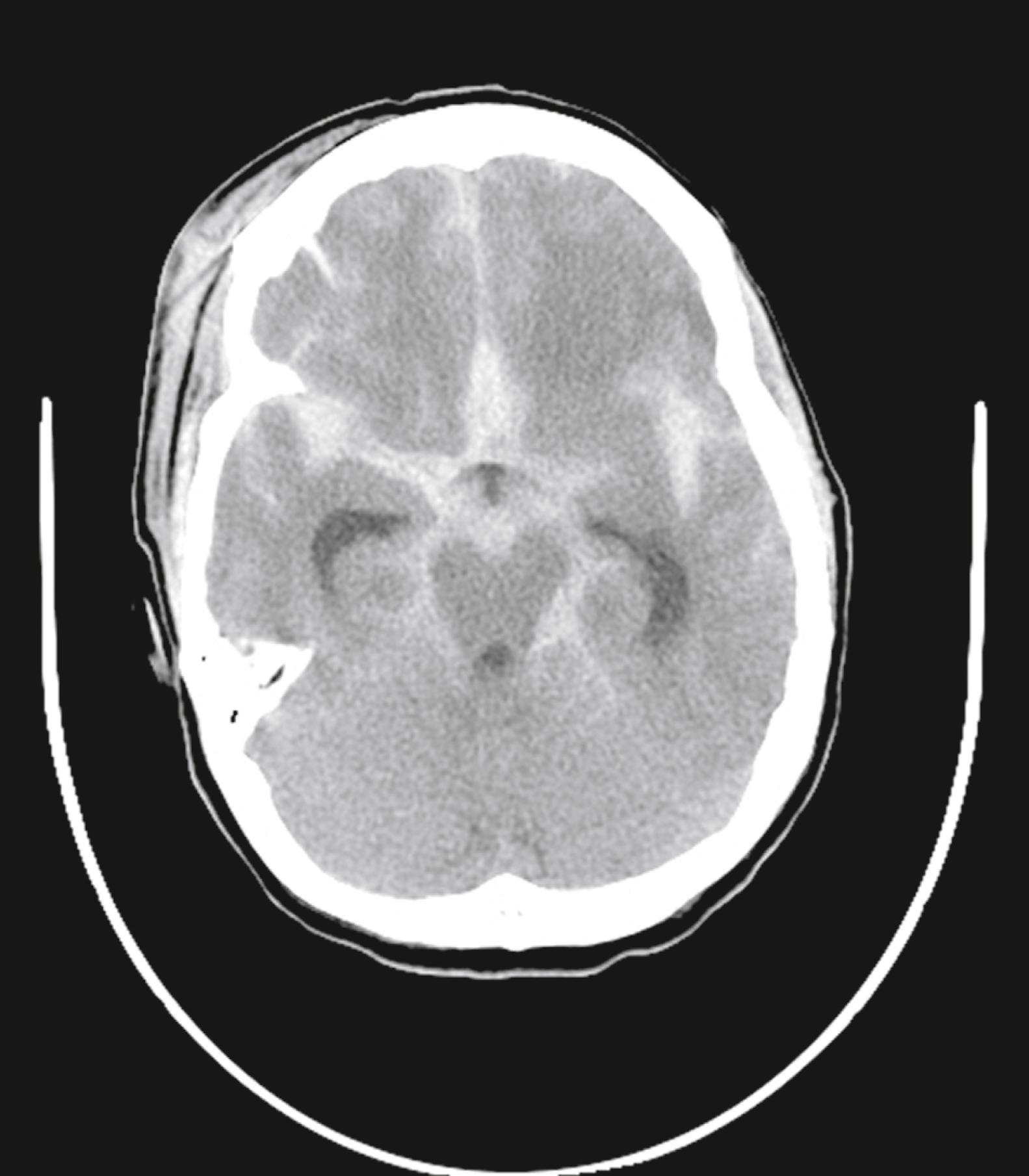
Trauma is the most common cause of SAH, whereas rupture of an intracranial aneurysm is the most common nontraumatic cause of SAH. SAH extends freely within the subarachnoid spaces around the cerebral hemispheres, brainstem, and cerebellum and frequently, by reflux of cerebrospinal fluid (CSF), extends into the intraventricular spaces. It often leads to acute, subacute, or chronic hydrocephalus because the blood products disrupt and obstruct the normal CSF drainage pathways ( Fig. 10.3 ).
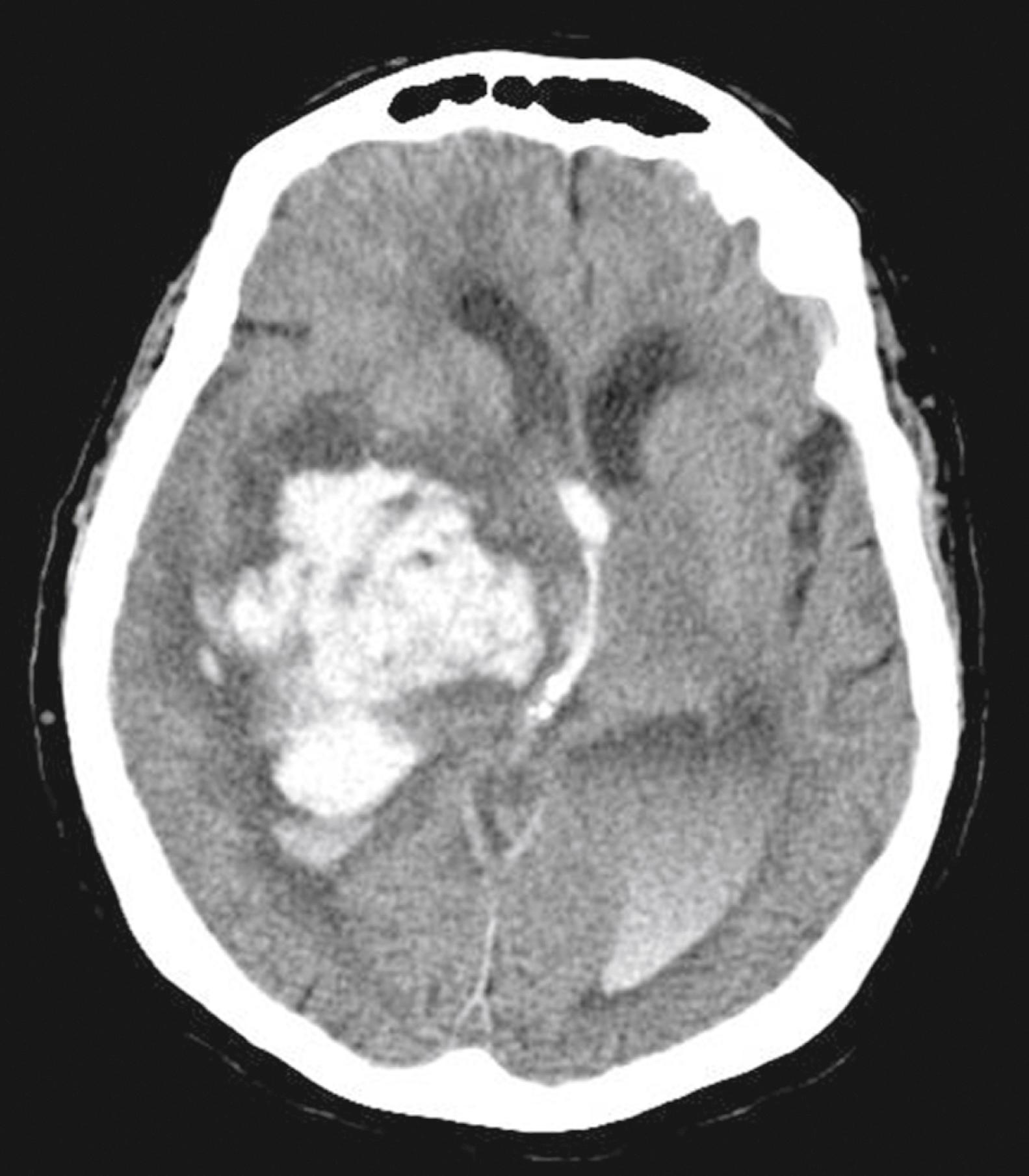
Parenchymal hemorrhages have many causes, including trauma, hypertension, vascular anomalies such as arteriovenous malformation or cavernous malformation, infarction, neoplasm, infection, or vasculitis. They can be small or large, and they can be single or multiple; a patient’s prognosis depends on the cause, number, size, and associated mass effect of the hemorrhage, among other variables ( Fig. 10.4 ).
Advances in CT scanner technology have improved the capacity for higher resolution images in the submillimeter range with shorter acquisition times. Such technological improvements have led to imaging techniques such as CTA, which enables relatively noninvasive imaging of the major arteries and veins of the neck and brain after an intravenous injection of iodinated contrast material, rather than the traditional catheter-based intra-arterial angiogram technique. CTA helps avoid the small risk for complications such as vascular dissection and iatrogenic embolic strokes associated with traditional catheter angiography.
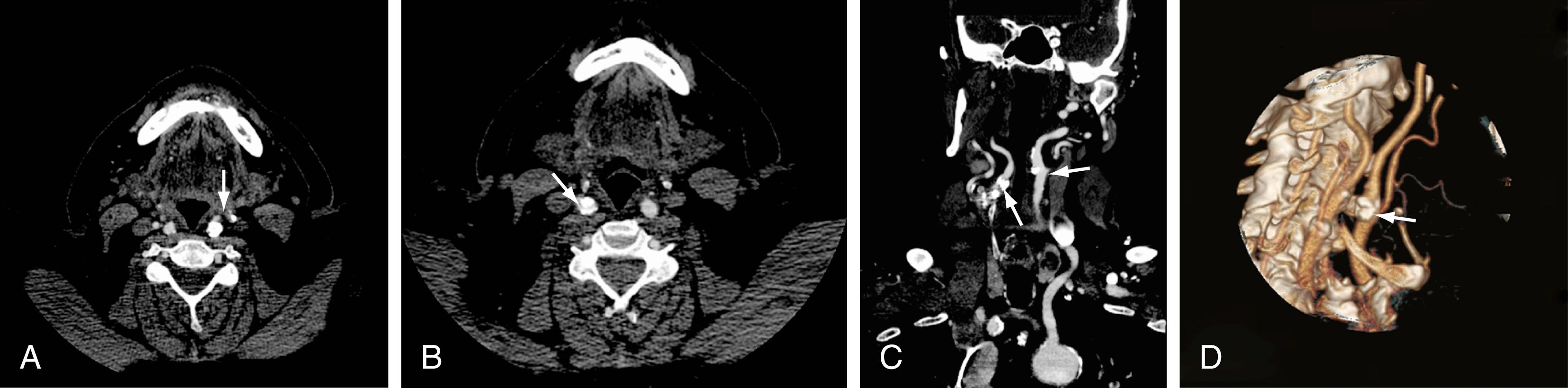
CTA of the neck or brain is performed with a multidetector CT scanner, which enables rapid dynamic imaging of the anatomy of interest. Iodinated contrast material is delivered as a bolus injection through a large-bore (18-gauge) intravenous catheter. Typically, submillimeter axial images are obtained and then reformatted into 2D sagittal and coronal image data sets at 1- to 2-mm intervals. The reconstruction images are usually 3D, but interpretation of the study is based primarily on the original axial data set and the 2D sagittal and coronal reformatted images. The diagnostic sensitivity and specificity of CTA are comparable with those of catheter angiography for both the extracranial and intracranial vasculature. , Although CTA cannot entirely replace traditional catheter angiography, it is a very useful noninvasive screening study for the evaluation, management, and follow-up of patients with definite or possible aneurysms, as well as the evaluation of vasospasm, arteriovenous malformations, traumatic dissection, stroke, and atherosclerotic stenosis of the carotid or vertebral artery ( Figs. 10.5 and 10.6 ).
Perfusion computed tomography (pCT) provides physiologic information in addition to anatomic information. pCT is performed with the latest-generation multidetector CT scanners, which allow very rapid CT imaging of a particular anatomic area, such as the cerebral hemispheres. Iodinated contrast material is delivered in a bolus intravenous injection at a rate of 4 to 5 mL/s, and rapid serial CT images of a chosen volume are obtained in multiple phases over an approximately 1-min period. At the end of this acquisition, multiphase time-density curves corresponding to each voxel are generated within a 2D image of a multilevel image data set. The data from these images are further postprocessed with a mathematical algorithm that allows displays of the data in color maps that represent such physiologic cerebral perfusion parameters as cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT). The CBF, CBV, and MTT maps generated from this perfusion CT technique are, in part, quantitative; that is, the numerical values obtained from these images may be expressed in mL/100 g/min for CBF, mL/100 g for CBV, and seconds for MTT. The pCT technology has been validated against other proven in vivo techniques such as xenon-enhanced perfusion CT and positron emission tomography.
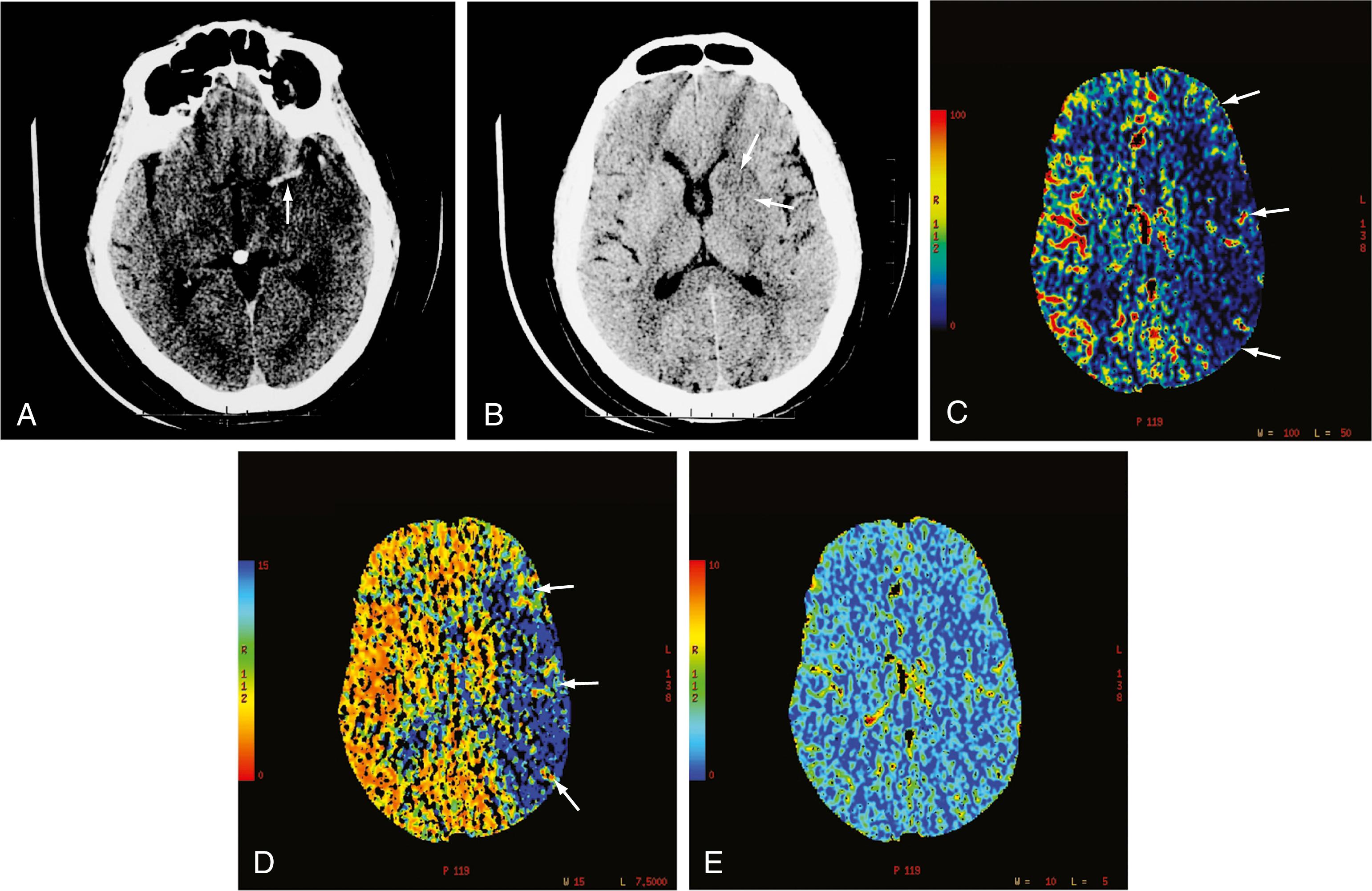
pCT has been used to evaluate acute stroke, central nervous system (CNS) neoplasms, and ischemic sequelae of SAH-related vasospasm. However, the most common use of pCT is for the evaluation of acute large-vessel occlusion stroke in which a patient presents with moderate to high National Institutes of Health Stroke Scale scores (usually 10 or greater). The various color maps of cerebral perfusion help determine the presence of core infarction and salvageable ischemic penumbra after a large-vessel occlusion stroke, which helps to identify those patients who might benefit from an intra-arterial thrombectomy to enable rapid recanalization of occluded large intracranial arteries (e.g., the supraclinoid segment of the internal carotid artery [ICA] or the M1 segment of the middle cerebral artery) ( Fig. 10.7 ). Multiple prospective randomized thrombectomy trials in 2015 and 2018 have demonstrated improved clinical outcome as measured by modified Rankin Scale scores in patients who were treated up to 24 hours from last known normal.
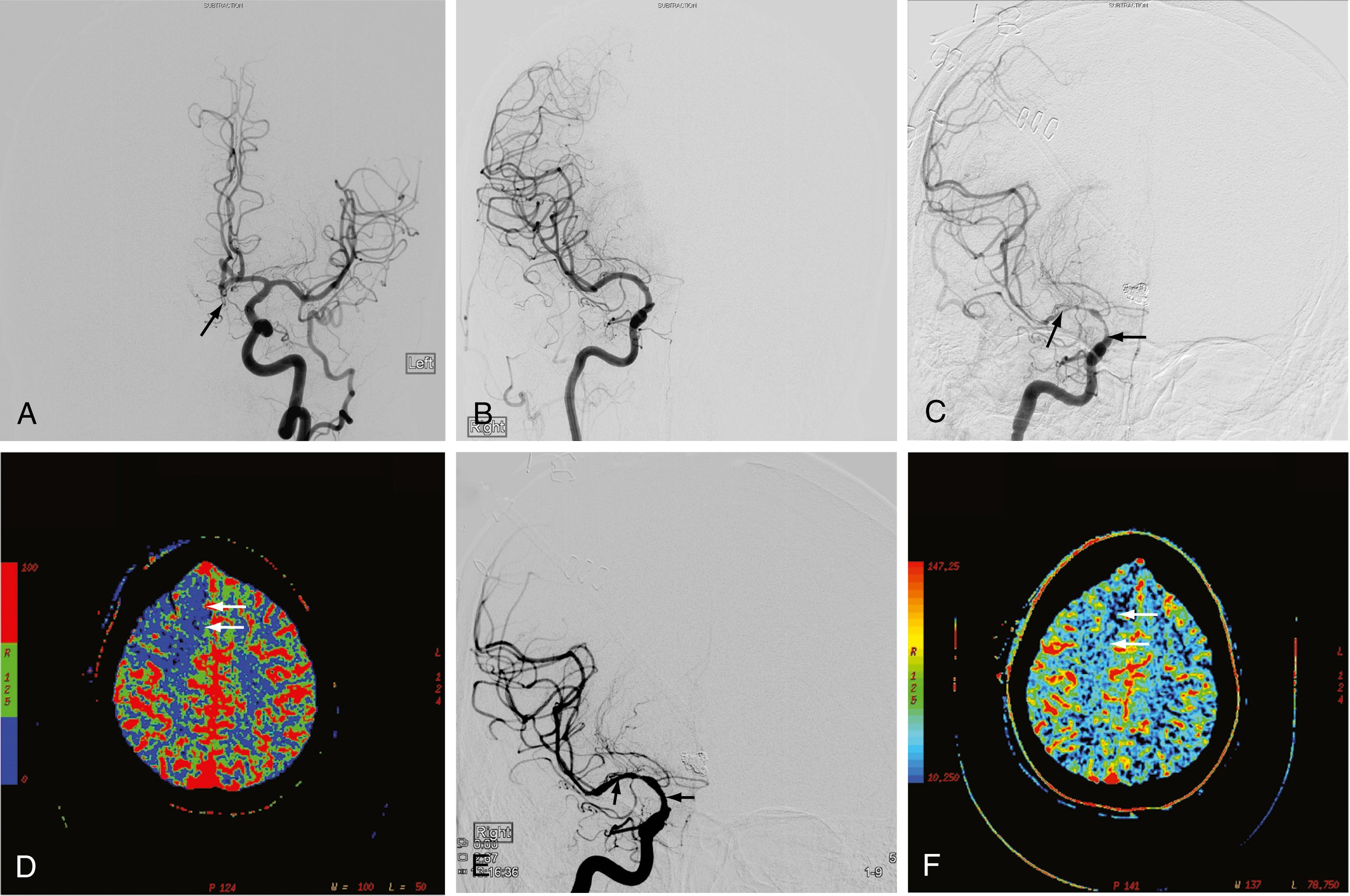
The availability of physiologic data also helps in the diagnosis, management, and treatment of ruptured aneurysm and subsequent vasospasm, which may contribute to acute or subacute ischemic injury. Evaluation of affected patients has typically relied on serial clinical assessment, non–contrast-enhanced head CT, and transcranial Doppler ultrasonography. This evaluation protocol has recognized limitations; in particular, non–contrast-enhanced CT and transcranial Doppler ultrasonography may not accurately reflect the state of cerebral perfusion at an early enough stage to allow successful intervention for reversal of oligemia and ischemia. Baseline and follow-up pCT can demonstrate the size and extent of brain areas at risk for stroke in patients in a neurological intensive care unit, often before symptoms develop and permanent infarction occurs ( Fig. 10.8 ). For some patients, this early detection of at-risk areas may enable earlier medical and catheter-based intervention for vasospasm and thus prevent delayed ischemic injury.
The interaction of the intrinsic magnetic moment of the nucleus with an externally imposed magnetic field results in the phenomenon known as nuclear magnetic resonance (NMR). In 1946, two independent research groups—Bloch and colleagues, working with liquid water, and Purcell and Hansen, working with solid paraffin —detected the hydrogen nucleus resonance in bulk matter. Purcell further described the processes and time constants (T1 and T2) by which the resonance would dissipate. This set the stage for the eventual development of MRI 30 years later. Between 1946 and 1976, NMR became a useful laboratory tool for probing molecular structure. Laboratory NMR instruments had small spaces for the sample, usually a small test tube. Use of NMR for larger objects, such as humans, required the development of larger magnets with larger sample spaces. The term magnetic resonance imaging, rather than nuclear magnetic resonance, is used to allay patient anxiety about a test whose name includes “nuclear.”
Many nuclei bear a magnetic moment. Those relevant to biology include phosphorus 31, carbon 13, and sodium 23. However, all MRI systems use the resonance of the hydrogen nucleus for three reasons. First, it is easy to detect the magnetic resonance signal. The hydrogen nucleus has the largest magnetic moment of any nucleus and is therefore the most detectable. Second, the natural abundance of hydrogen is high: 99.99% for hydrogen 1. In contrast, the natural abundance of carbon 13 is 1.1% (98% of carbon is carbon 12, which has no magnetic moment). Third, hydrogen is contained in water, which is in high concentration in the body; in the brain, the concentration of water is approximately 67% by weight.
To begin, the sample is immersed in a strong, constant magnetic field. A magnet that creates the field may be one of three designs. First is the electromagnet, similar in principle to a washing machine solenoid. Bloch and colleagues and Purcell and Hansen used these magnets in their original experiments. However, the maximal field strength that can be achieved is limited in practical applications to approximately 0.4 T (1 T = 10,000 Gy). An electromagnet consumes large amounts of electricity, and its use in MRI has therefore declined.
The second magnet type is a permanent magnet assembled from ferromagnetic material. The field strength of this type of magnet is limited to 0.3 T. Permanent magnets are generally used for small, low-cost open designs. This technology tends to be used in MRI systems that accommodate large (usually >300 lb [136 kg]) or claustrophobic patients.
The third and most widely used magnet type is the superconducting magnet. This design is also similar to that of a washing machine solenoid. However, unlike the solenoid, the superconducting magnet is an alloy that conducts electricity without resistance when kept at temperatures within 15° of absolute zero. An electrical current is slowly driven into the magnet. Once the current reaches the desired level, the ends of the magnet wire are connected, which forces the current to circulate continuously without loss. Magnets of this type can remain at field strength for many years without the addition of electrical current. Field strengths of up to 8 T can be achieved in these magnets, which can accommodate human subjects. Most MRI systems now use superconducting magnets, usually 1.0 to 1.5 T, although an increasing number of hospitals and imaging centers are now using 3-T clinical MRI systems.
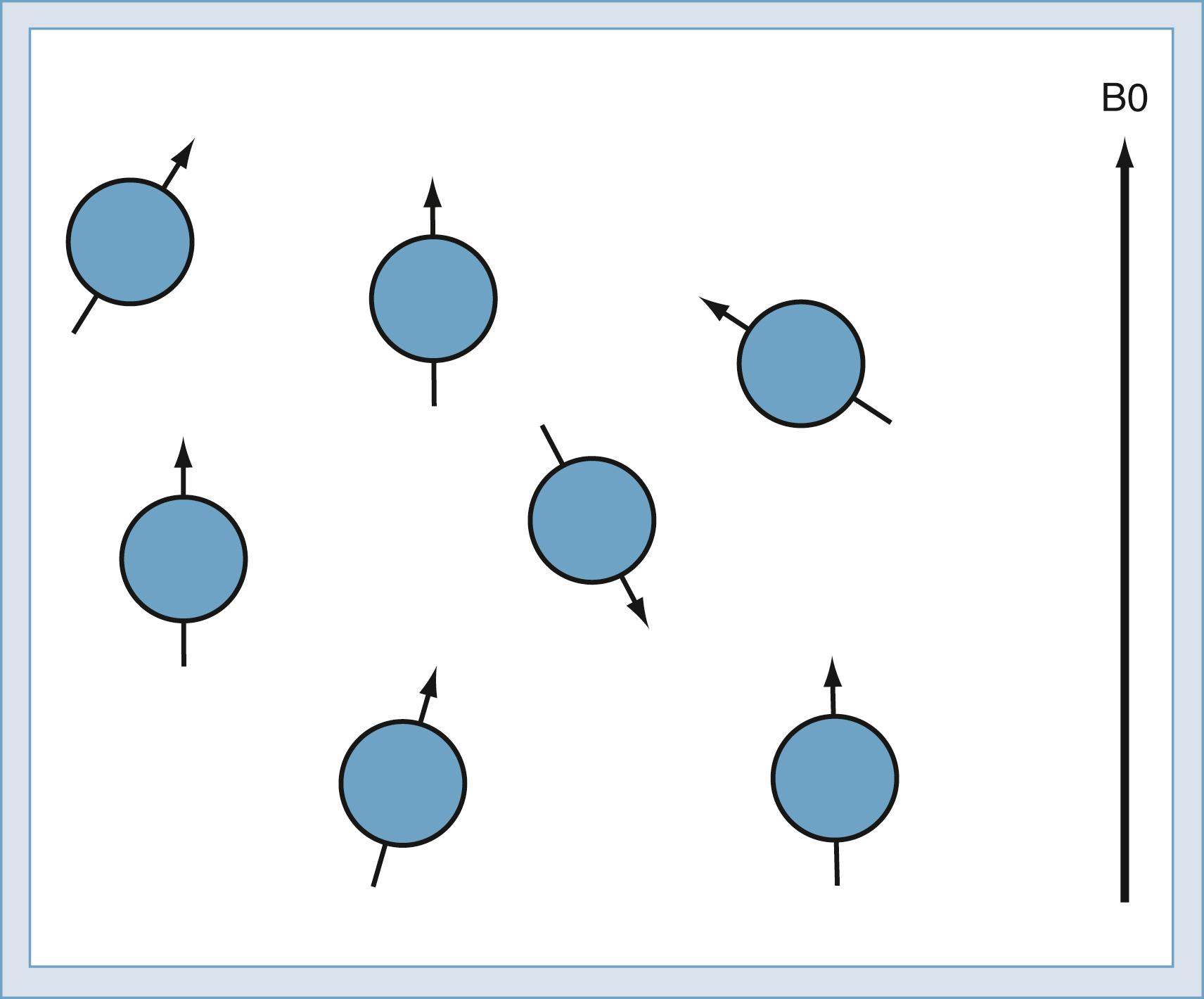
When the sample is immersed in the strong, constant magnetic field, the spins in the sample undergo a slight polarization. This polarization, known as M0, increases with increasing magnetic field. At 1.5 T, this polarization is very slight, approximately 1 × 10 −5 . This means that just 10 in 1 million nuclei are polarized. The polarization competes with the randomizing effect of the thermal vibration ( Fig. 10.9 ). Only the polarized nuclei contribute to the magnetic resonance signal; hence, MRI systems with higher field strength produce better images.
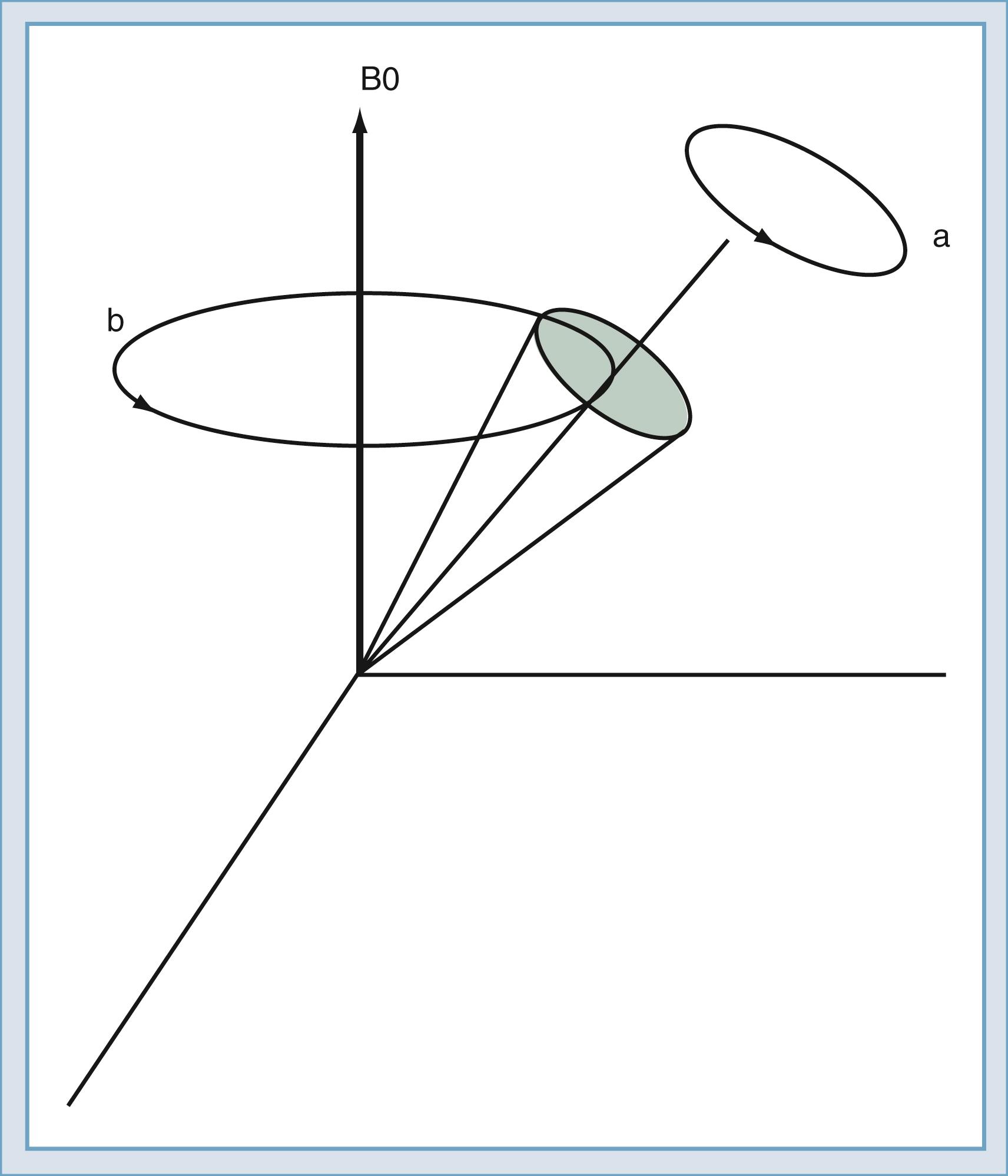
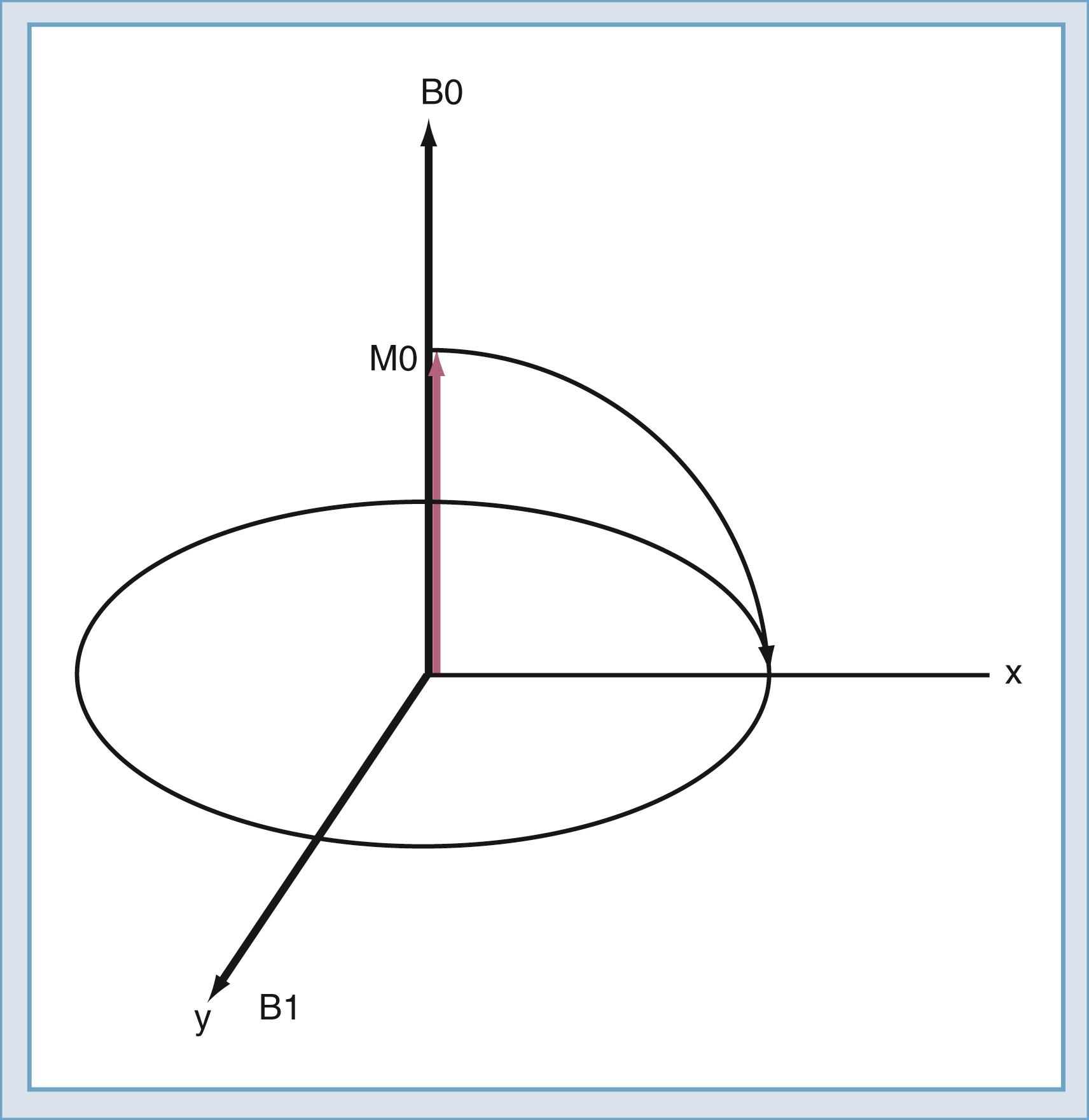
The classic model created by Bloch helps describe the motion of the spins. Spins that align with the main field precess around the direction of the main field in a manner similar to the spinning of a toy top ( Fig. 10.10 ). The rate of this precession is a product of the intrinsic magnetic moment (the gyromagnetic ratio) of the spin and the strength of the main magnetic field. The rate of precession is known as the Larmor (or resonant ) frequency. The spins aligned along the B0 magnetic field are rotated into a plane transverse to the direction of the main magnetic field by the action of a time-varying magnetic field called B1 ( Fig. 10.11 ). The B1 field is created by the radiofrequency (RF) transmitter and the antenna, known as the RF coil. The frequency of the B1 field matches the Larmor frequency of the spins. The B1 field is of brief duration, typically 1 to 10 ms, and is thus referred to as an RF pulse. The angle through which the spin is rotated is called the flip angle. The flip angle depends on the duration and amplitude of the RF pulse.
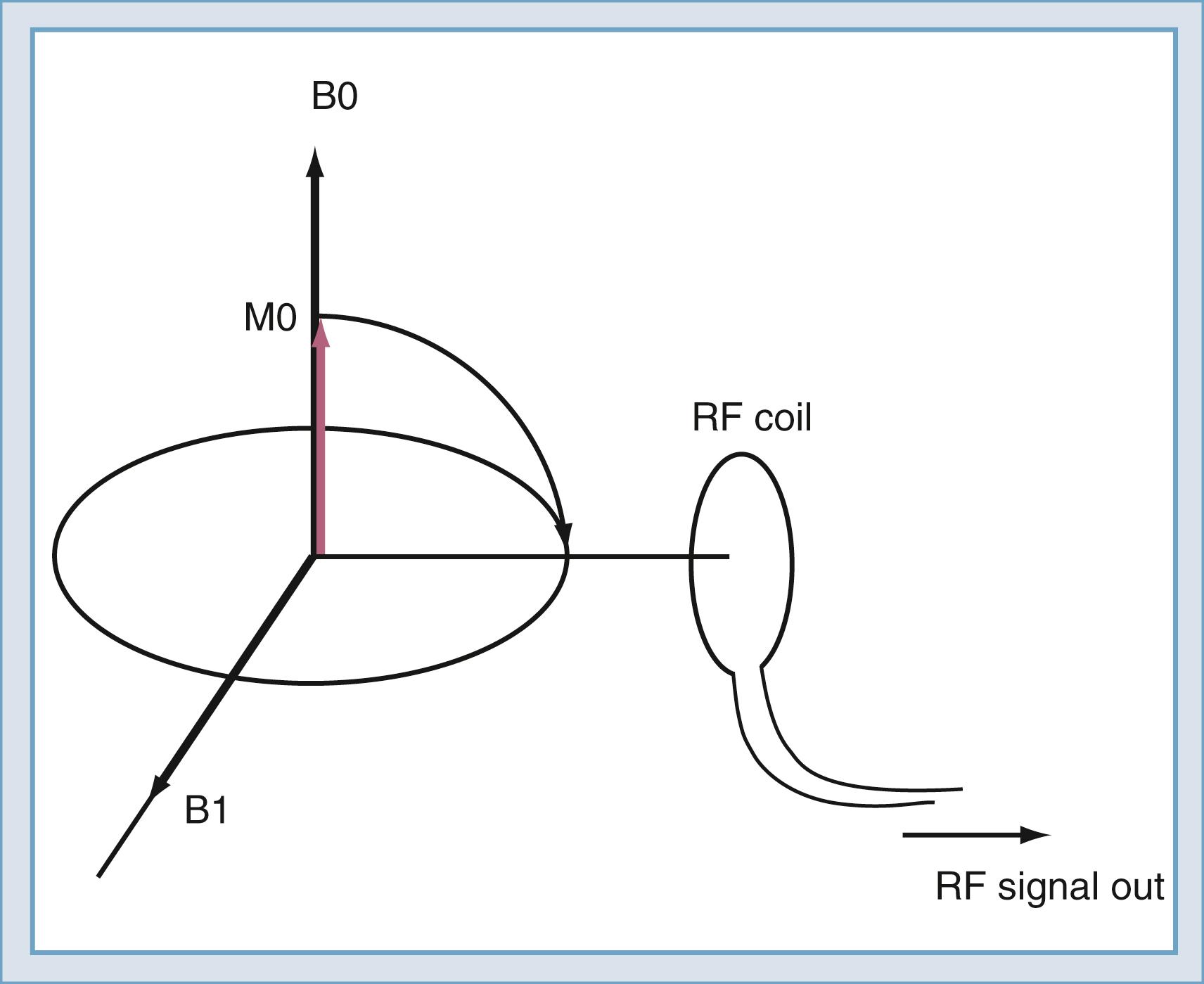
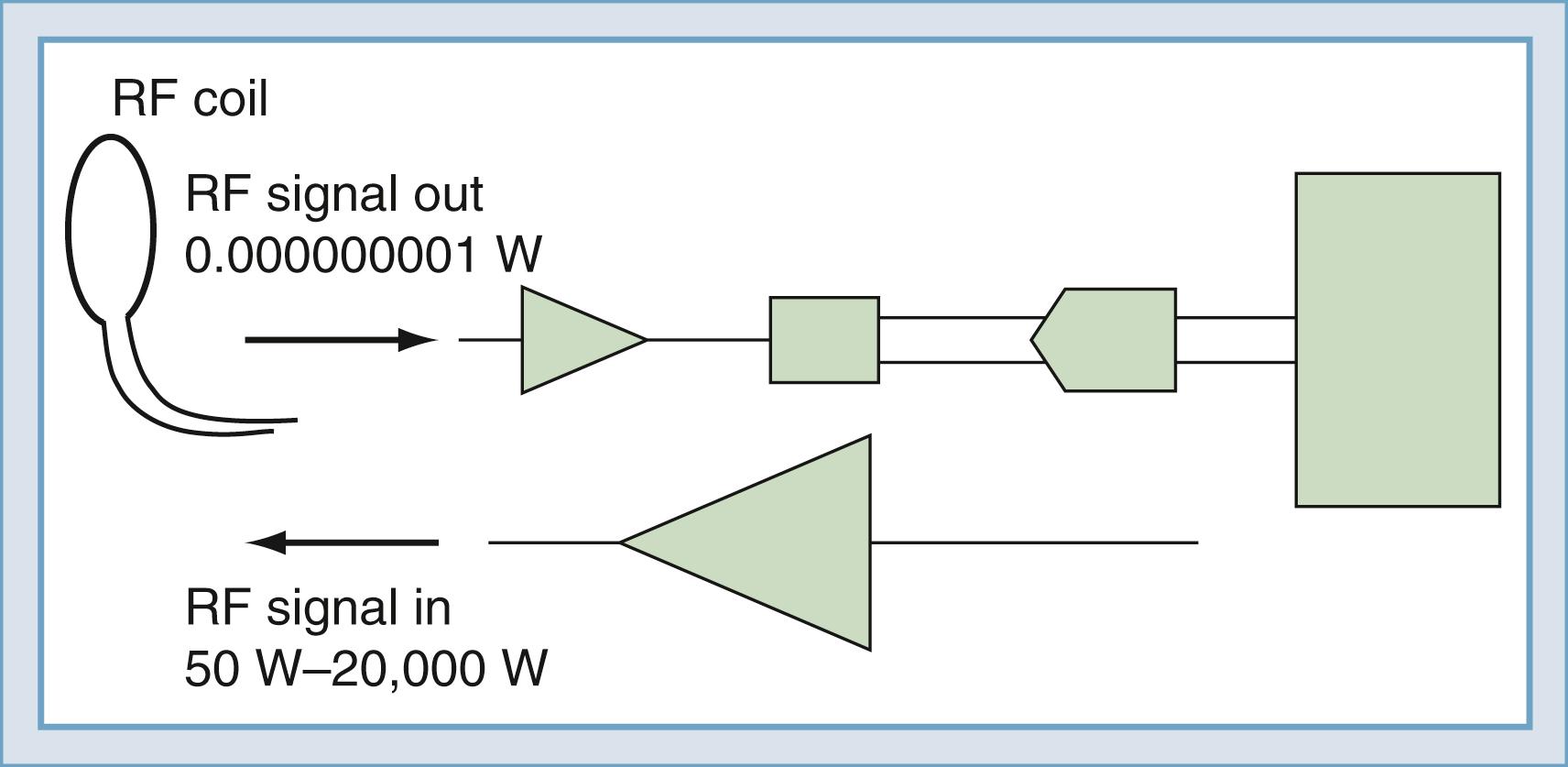
To detect the magnetic resonance signal, an RF coil is placed as shown in Fig. 10.12 . This may be the same coil used to transmit the B1 pulse. A time-varying magnetic field is created at the coil by the magnetic field of the precessing spins (rotated in the transverse plane) as they pass by the RF coil. Magnetic induction (the Faraday law) causes the RF coil to produce an electrical current, which can then be amplified and detected ( Fig. 10.13 ). An interesting and important disparity should be noted. The amount of power used to produce the RF pulse is in the range of 100 to 20,000 W. The signal that is received from the object is approximately 10 −12 W. This received signal is no greater than the signals from radio or television stations, among other sources. To prevent interference from these external sources, MRI systems are enclosed in electrically shielded rooms, which are six-sided copper boxes.
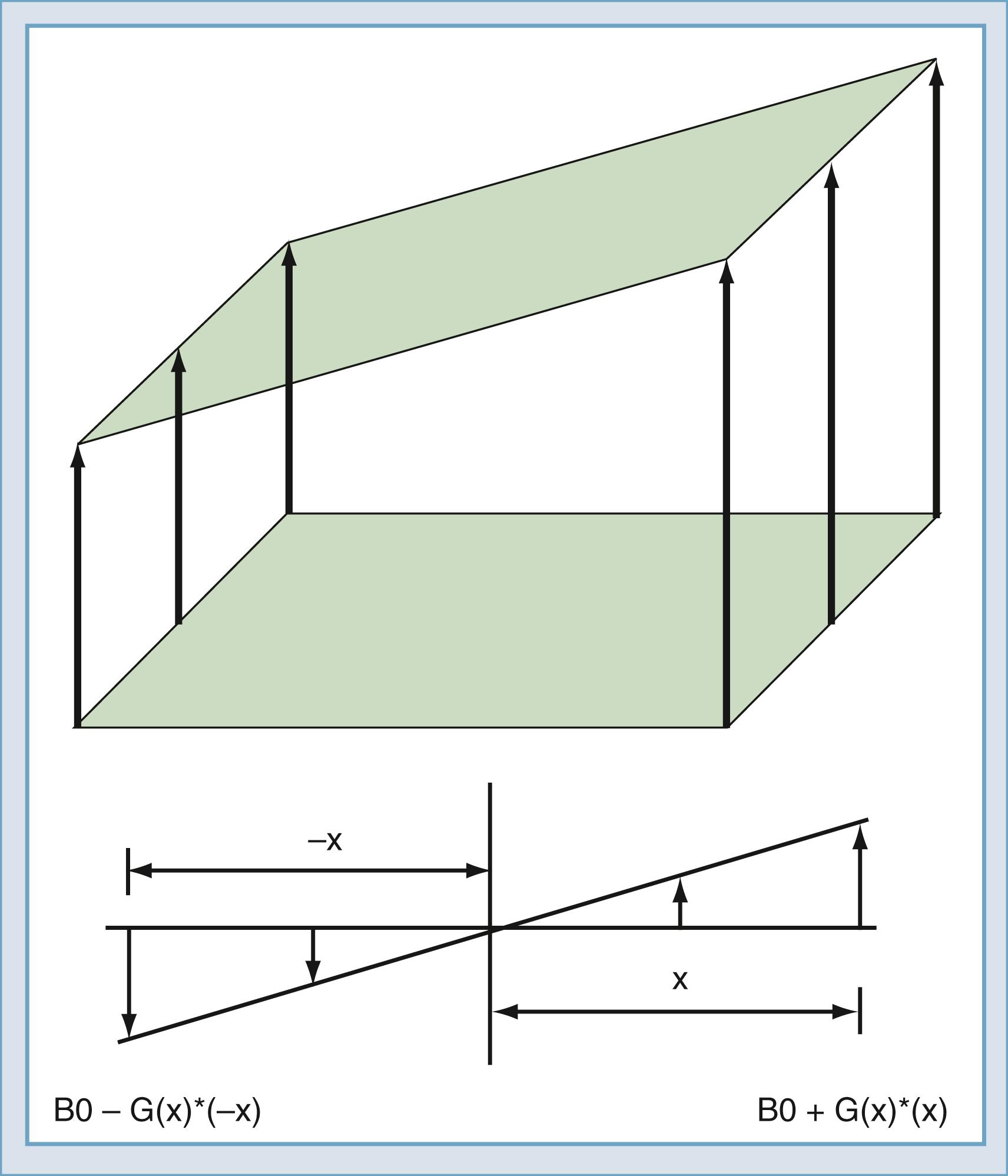
Up to this point, the sample has been polarized and excited and a signal detected, but the location of the spins that created the signal remains unknown. Suppose that two objects are in the magnetic field, both of which create a signal. To force each object to give off a unique signal, the magnetic field can be modified to vary as a function of position along the x -axis ( Fig. 10.14 ). The resonant frequency of the spins is a function only of the magnetic field at that point in space. Thus the spatial origin of the signal can be determined by the frequency of the received signal. In practice, to determine this, a linear gradient is created that adds to or subtracts from the main field as a linear function of offset from the origin. An MRI system has three gradients: x, y, and z. The gradient system serves two purposes in the MRI system. The first is to limit the excitation to one plane or slab ( Fig. 10.15 ). If an RF pulse is transmitted during the time that a gradient is applied, excitation will affect only one slice or slab, the thickness of which depends on the amplitude of the gradient and the bandwidth of the RF pulse. The second purpose is to encode the spatial location of spins to form the magnetic resonance image. In a slice, the two directions of encoding required are frequency and phase. To establish location along the frequency-encoding axis, a gradient is applied during the signal readout time. To encode the orthogonal axis, a gradient is applied somewhere between the time of excitation and reception and is called phase encoding. One unique value of phase encoding is that it is applied every time that the readout gradient is applied. Thus the excitation and readout must be repeated to make an image. The rate at which the excitation is repeated is the repetition time (TR).

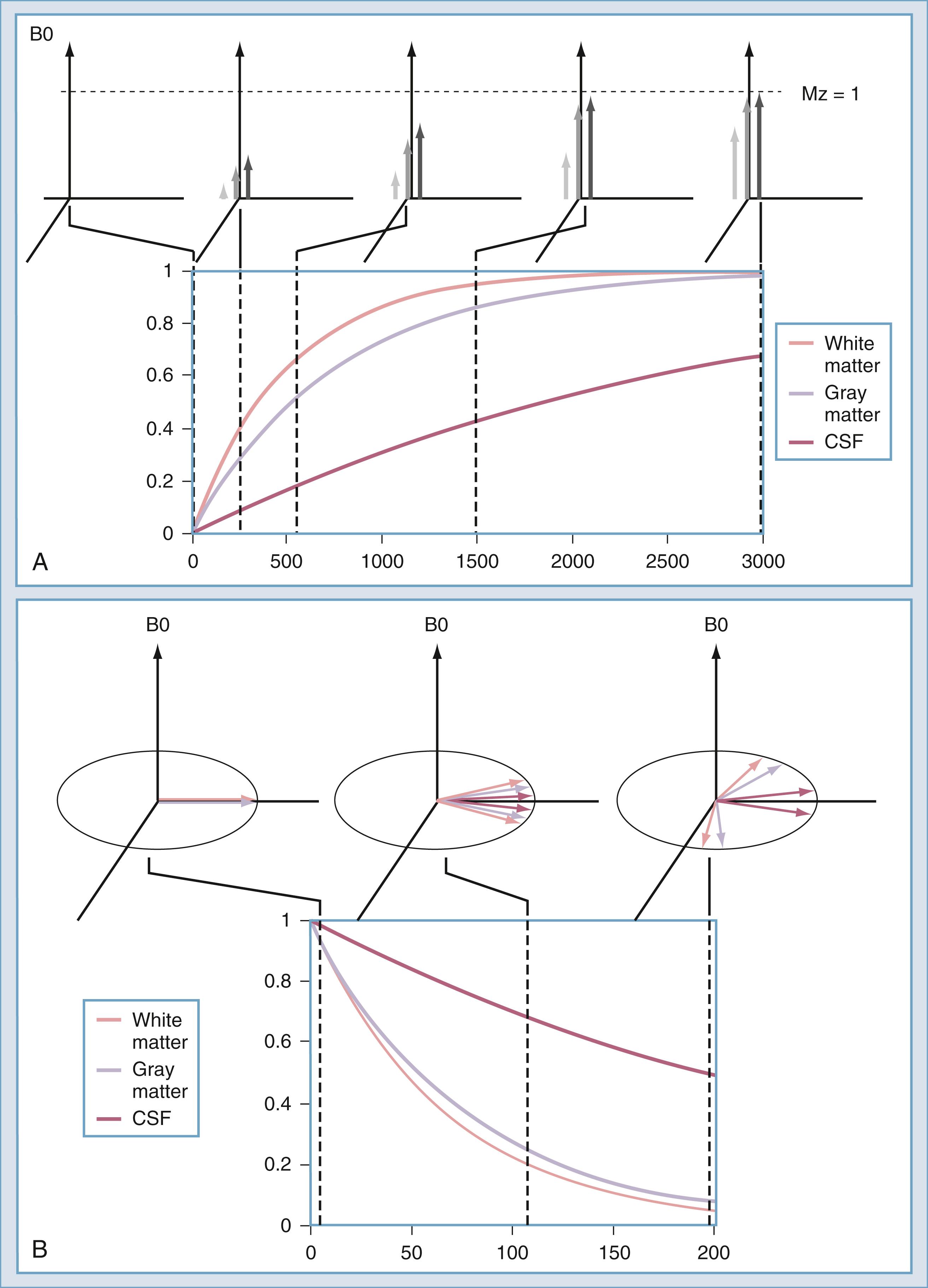
The intensity of a voxel in an image arises from three principal factors. The first is the number of protons in the voxel, known as proton density. If this were the only image mechanism, MRI would be little more than CT. However, intensity in the voxel also depends on the relaxation rates of the spins. One such rate is the longitudinal relaxation rate: T1 ( Fig. 10.16A ). After excitation, the magnetization in the slice returns along the axis of the main magnetic field by interaction with other nonmoving hydrogen spins, typically those attached to large molecules. The magnetization returns along B0 as an exponential function of the ratio of T1 and the rate at which the excitation is repeated: TR. The transverse relaxation rate, T2 ( Fig. 10.16B ), is the rate at which spins that have been excited into the transverse plane lose coherence. Each spin precesses at a rate that is determined by the magnetic field at the location of that spin. Macroscopic and microscopic field gradients, created by differences in the magnetic susceptibility of tissue, cause some of the excited spins to precess faster and some slower. Eventually, the spins are spread out uniformly, which produces no signal in the RF coil used to detect the spins.
Three principal image intensity factors—proton density, T1, and T2—influence the appearance of every voxel on MRI. For example, cortical bone appears hypointense on MRI because the proton density (of mobile spins) is quite low. Lung parenchyma usually appears hypointense because the T2 of lung tissue is so low that the signal is gone before it can be sampled. The long T1 of liquid water causes CSF to appear hypointense on sequences that use short TR times.
An MRI scan user has a choice of pulse sequence parameters such as echo time and TR. Maximal contrast between structures of interest may be achieved by the appropriate choice of sequence parameters. Conversely, inappropriate choices may result in failure to detect a lesion. Other endogenous sources of contrast include blood flow (on magnetic resonance angiography [MRA]) and water-macromolecular T1-based interactions (magnetization transfer).
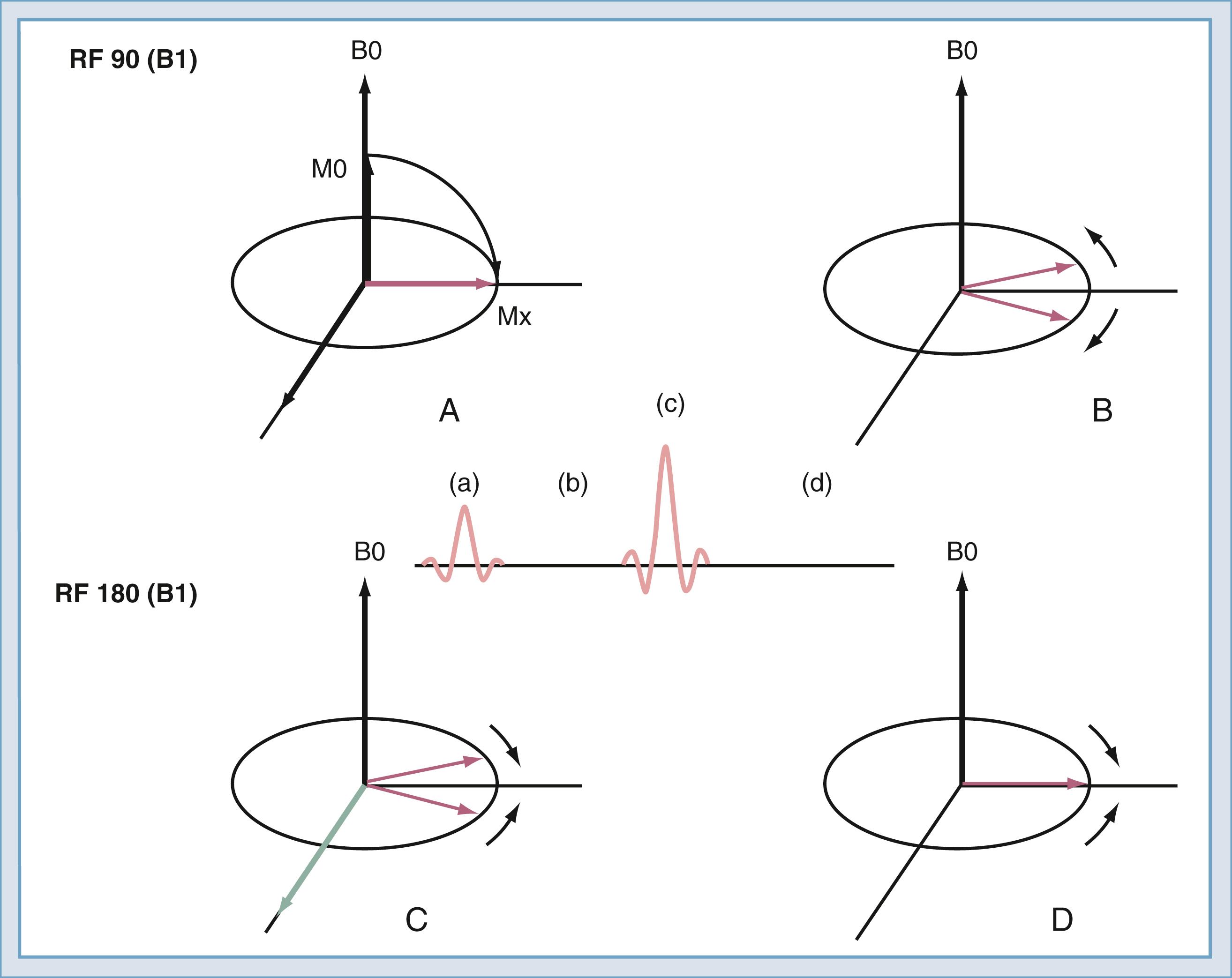
After the spins have been rotated into the transverse plane by the initial 90-degree RF pulse ( Fig. 10.17A ), they begin to lose coherence because of the effects of local inhomogeneities (contributed by changes in tissue), inhomogeneities secondary to imperfections in the B0 field, and diffusion of water molecules ( Fig. 10.17B ). If a second RF pulse is transmitted at twice the amplitude of the first pulse, the relative direction of the spins can be reversed ( Fig. 10.17C ). Then, at the echo time, the spins will have nearly regained coherence ( Fig. 10.17D ). The effects of magnetic field inhomogeneities are thus canceled out. One loss, that caused by diffusion, cannot be reversed but is usually negligible in routine imaging. The resulting coherence produces the spin echo as described by Hahn in 1950. If the 180-degree pulse is not used, the signal decreases with increasing echo time; the rate of decrease is referred to as T2∗. With the 180-degree pulse included, the decrease is T2, with T2 always being greater than T2∗.
The spin echo sequence is the mainstay of routine clinical imaging. By adjusting echo time and TR, the technician can select proton density–weighted images, T1-weighted images, or T2-weighted images ( Table 10.1 ).
| Repetition Time (TR) | Echo Time (TE) | |
|---|---|---|
| Short TE | Long TE | |
| Short TR | T1-weighted image | Mixed contrast—do not use |
| Long TR | Proton density–weighted image | T2-weighted image |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here