Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Leo Schultze Kool, Monique Brink, Gijs Bloemsma, and Maarten Truijers are greatly acknowledged for their help in preparing this chapter.
Conventional contrast-enhanced arteriography is no longer considered the standard imaging modality for vascular disease. As with many technologic advances, however, the process of image creation continues to become more difficult for the average end user to understand. Although the typical vascular surgeon can perform clinical evaluation and can make decisions without an understanding of the basic principles behind computed tomography (CT), these concepts remain important. A better understanding of the imaging process and terminology also aids collaboration between radiologists, radiographers, and the surgeons who use the images to plan interventions. Finally, an understanding of the basic concepts enhances the ability of the surgeon to understand technologic advances as they become the new standard of care.
In the early 1930s, the Dutch radiologist Ziedses des Plantes first devised a technique that reduced the problem of superimposition of structures in basic radiography (X-ray tube and plain film). Physically connecting the X-ray tube and film opposite each other and rotating the combination around a body segment sharpens the image created by the points on the focal plane, whereas the images of points outside the focal plane are blurred, thereby creating less superimposition artifact. This technique was called “planigraphy” or “tomography,” derived from the Greek words tomos , which means “a section” or “a cutting,” and graphein, meaning “to write.” Tomography played an important role in diagnostic radiology until the 1970s, when invention of the transverse axial scanning method together with the availability of minicomputers, which allowed computational reconstruction of images, led to the development of so-called computed axial tomography (CAT scan, or later, CT scan).
Two similar methods for transverse axial scanning and image reconstruction were independently invented by Sir Godfrey Newbold Hounsfield in Hayes, United Kingdom, at Elector-Musical Instruments (EMI) Limited Central Research Laboratories, and Allan McLeod Cormack of Tufts University in Massachusetts. A combination of hardware, mathematical algorithms, and computer software resulted in the cross-sectional images. The first so-called EMI scanner was installed in Atkinson Morley’s Hospital, Wimbledon, England, in 1971. In the United States, the first installation was at the Mayo Clinic in Rochester, Minnesota. These machines would acquire two adjacent brain tomographic sections in about 4 minutes and needed about 7 minutes of computation time per picture. This was such a leap forward in imaging technology that Hounsfield and Cormack shared the Nobel Prize in 1980.
Many of the principles used in this first-generation CT scanner are still in use today and provide a framework for understanding the technology. The fundamental unit for this scanning method consists of an emitter and a detector. The emitter produces a thin X-ray beam. The beam is transmitted through the tissue and detected on the other side. The attenuation (the rate of reduction of X-ray energy recorded at the detector) of multiple X-ray beams traversing the same point in the matrix from different angles is collected and an ingenious method applied to calculate backward to the density or CT number that must be present at each location in the matrix.
The resulting CT number is expressed in Hounsfield units (HU). CT numbers range from the extremes of air (−1000 HU) to dense bone (>1000 HU), but fat (−20 to −100 HU), water (0 HU), and muscle and blood (40 to 60 HU) tend to lie in a much narrower range. Differences in factors such as the energy level of the beam and tissue thickness prevent the density in HU from being absolutely uniform from one CT scan to another, but the ranges are similar. When the range of CT numbers for a scan is determined, it can be broken up into smaller ranges for graphic display by a set of gray-scale values. Each gray-scale data point in the matrix is known as a pixel because it represents a “picture element.” When displayed as a whole, this matrix of gray squares becomes an interpretable image.
A first-generation CT scanner is capable of producing a cross-sectional image with a 160 × 160 matrix. These CT scans were applicable only to parts of the body with limited motion (e.g., the head). To obtain useful scans in areas such as the chest and abdomen, subsequent generations of CT scanners were designed to decrease the time required to obtain a complete cross-sectional image.
With development of a so-called slip-ring gantry, the emitter and detector array can rotate continuously in the same direction, and at the same time, the computer can acquire data continuously. If the table moves in a continuous linear motion through the gantry while the X-ray emitter and detector rotate continuously over 360 degrees, data can be acquired in a single sweep over the entire volume of interest. In this technique the emitter traces out a spiral relative to the patient, which is referred to as a spiral CT or helical CT scan.
Spiral or helical CT technology has several important ramifications beyond a simple decrease in scan time. A spiral CT scan collects data over a continuous volume rather than discontinuous slices ( Fig. 29.1 ). The most obvious advantage of acquiring data over a continuous volume is that thin axial slices can be reconstructed from the digital data set at arbitrarily small intervals without additional radiation exposure. Single-slice sequential CT can produce similar overlapping or adjacent axial slices, but the tradeoff is increased scan time and additional radiation exposure. The advantage of sequential scanning, however, is a lower level of reconstruction artifacts because table movement along the z-axis during scanning does not need to be corrected in the acquired data sets. This is the main reason why sequential scanning is often used for brain imaging.
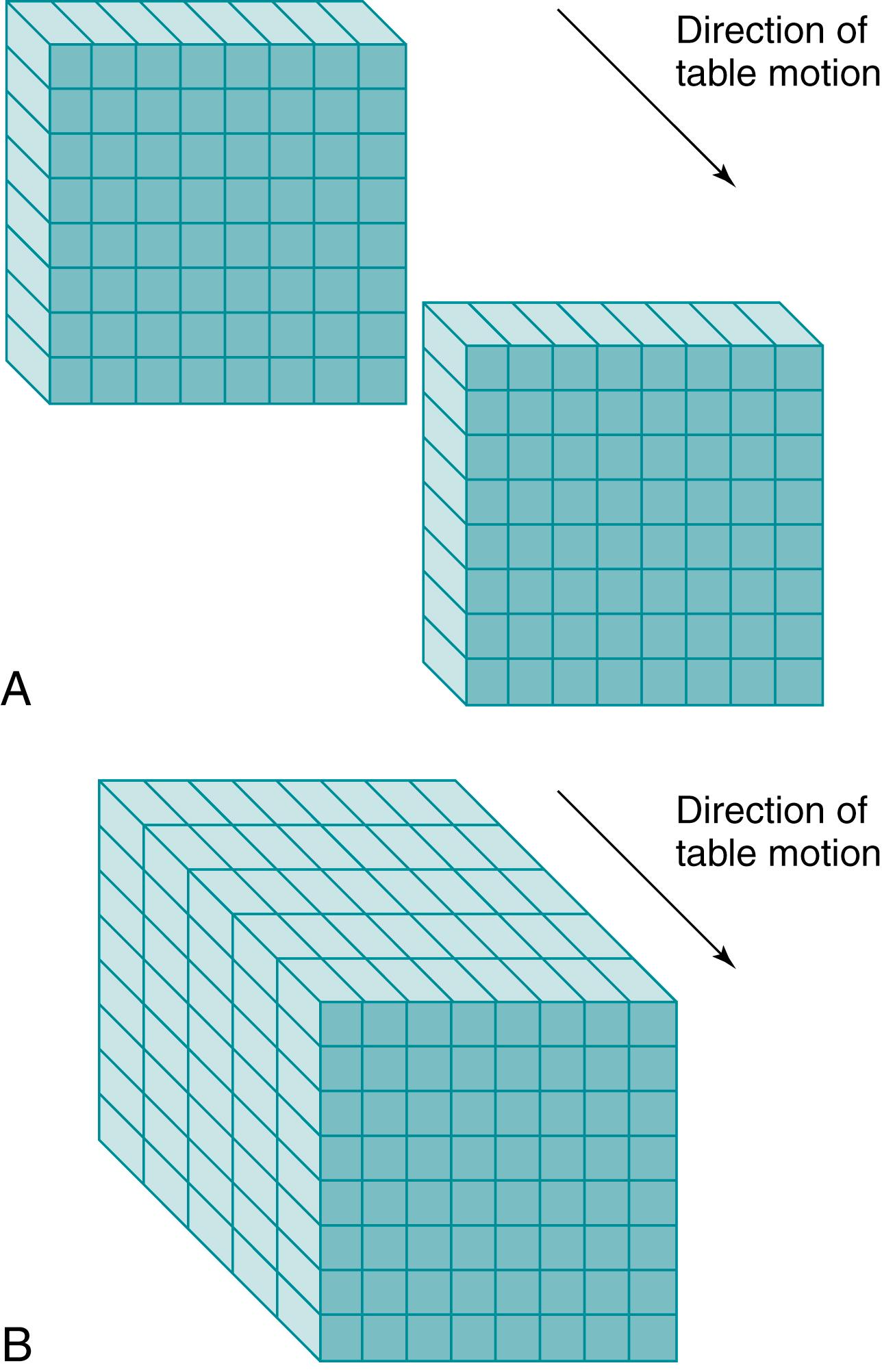
Multidetector scanners have multiple rows of detectors in craniocaudal direction (z-direction), so that the volume to be scanned can be covered more quickly. Whereas a single-slice detector acquires one slice per rotation, multidetector CT scanners are capable of acquiring multiple separate slices or 1 big volume per rotation. Each slice can be acquired at 1 mm or even smaller with rotation times in the range of 0.2 to 0.3 seconds. Complete imaging of the abdominal vasculature can be accomplished in a fraction of the time required with previous-generation scanners. This diminishes or eliminates many artifacts or compromises that must be dealt with in single-row detector scanners, as discussed subsequently. For this reason, multidetector or multirow scanners have almost completely replaced the earlier-generation single-row scanners.
Advances in hardware and computer software technology have also greatly improved the graphic image display despite the reduction in scan times. A first-generation CT scanner produced a cross-sectional image with a 160 × 160 matrix, but current scanners typically generate a 512 × 512 matrix. Each data point in the matrix is mapped to a gray scale for display, so the size of the matrix and the field of view (FOV) have a direct impact on spatial resolution of the display (the smallest distinguishable element). Data points are displayed as a two-dimensional (2D) picture, and each point in the display matrix is a pixel (picture element). Data points are acquired in three dimensions, however, and each data point in the matrix actually represents a voxel (volume element). The size of the voxel is determined by multiple factors, including FOV (x, y direction) and detector design (x, y, z direction).
Visualization of vessels on CT is limited by the similar densities of blood and soft tissue. Administration of iodine intravenous contrast material highly overcomes this problem. However, optimal visualization of the vessels (computed tomographic angiography [CTA]) was not possible until the availability of fast spiral CT: faster scan times and increased numbers of detectors will allow a larger part of the body to be imaged as the contrast bolus passes.
Timing of the initiation of image acquisition relative to injection of the contrast agent is crucial for maximizing opacification of vessels in the scanned volume. Various dedicated bolus tracking algorithms have been developed by the CT scanner manufacturers to detect contrast arrival in the vessel of interest in order to start the scan on an appropriate time. These algorithms are all based on a stationary, continuous scan of a single slice in which contrast density is measured in a region of interest (ROI) in a large vessel marked by the operator. When a certain threshold of Hounsfield units is achieved at the region of interest, the spiral CT is initiated.
The iodine concentration, injection rate, and the volume of the contrast bolus are important parameters. Bolus length should be balanced against the length of the volume to be imaged. In the past, a typical CTA study from the celiac axis down to the external iliac arteries would require 120 to 180 mL of 300 mg/mL nonionic contrast. , For optimal arterial enhancement, fast injection rates are important.
Optimization of scanning and power injector protocols, faster scanning times, and the use of saline push bolus techniques reduced this volume significantly, and often volumes do not exceed 100 mL.
Split-bolus techniques are alternative ways to administer intravenous contrast. These techniques employ multiple phases – for instance, enhancement of venous and arterial structures – in one single scan. This is achieved by splitting the contrast bolus volume into one early bolus of contrast and one late bolus of contrast administration before obtaining the CT scan. This technique decreases ionizing radiation dose since fewer scanning phases are needed per patient.
Another factor that increases contrast enhancement is lowering of beam energy (kVpeak) which increases iodine density on scans.
A scan protocol consists of a series of settings of acquisition and contrast parameters, reconstruction parameters, technician and patient instructions, and maneuvers to generate a set of images that is optimal for the specific anatomy and indication for the CT scan. The protocol includes the setting of X-ray emitter parameters (i.e., beam energy expressed in kilovolts peak [kVp] and tube current expressed in milliampere-second [mAs]), rotation time, length of helical exposure, pitch, collimation, patient instructions (i.e., breath-hold), dosage of contrast material, delay, volume and infusion rates, reconstruction interval and reconstruction algorithm, parameters of radiation dose optimization tools, and FOV.
Pre-scan and post-scan parameter settings should be distinguished from each other. Various post-scan settings, such as the reconstruction algorithm or interval, can be applied to the raw image data. This is not true for the pre-scan settings. Obviously, an improper pre-scan setting cannot be corrected after the images have been acquired. Particularly in multislice scanners, proper pre-scan settings are important and have a direct impact on the diagnostic value of the CT scan.
Scan parameter settings are also important for optimization of the scan protocol in relation to the clinical question because this may be the only way to limit the radiation dose delivered by the CT scan.
Tube voltage, measured in kVp, is the value that determines the energy level of the X-ray tube. Higher tube voltage renders better tissue penetration but leads to decreasing relative contrast differences between the various tissues. High kVp settings (120 kV) are recommended to allow sufficient photons to reach the detector elements in obese patients. In children or slim patients, low kVp settings (80 kV, or even lower) should suffice and also produce increased contrast differences.
mAs values describe the tube current time product. The mAs value is linearly related to the duration and amount of radiation. Higher mAs represents more radiation delivered by the X-ray tube and leads to a decrease in image noise because more photons will reach the detector. However, this decreased noise is achieved at the expense of an increased radiation dose to the patient. kVp and mAs settings are closely linked, and a change in one may dictate a change in the other.
Collimation is a method of reducing the thickness of the X-ray beam. Collimation has a direct impact on spatial resolution in the z-axis and determines the smallest possible slice thickness.
Pitch (P) is defined as the ratio of table feed (the speed at which the table moves during tube rotation) and collimation. Values above 2 will result in undersampling of the region of interest and might result in artifacts. Values below 1 will result in overlapping scans, which might be beneficial for three-dimensional (3D) reconstructions but will also increase the dose significantly if other scanning parameters are kept constant. For multislice CT scanners, pitch calculations are different, depending on whether single collimation of one detector ring or total collimation of the whole detector array is chosen. An asterisk indicates that total collimation of the detector array is being described; for a four-slice scanner, a pitch of 2 corresponds to a P∗ of 8.
where P = pitch, TF = table feed, N = number of detectors, and SC = single-section collimation.
To give an example for a 64-slice scanner, if pitch is set at 2 and collimation is set at 1 mm, the resulting TF – according to the formula P∗ = TF/(N × SC) – will be 64 mm/s.
Scanning time is the maximum duration of a scan that a certain tube allows while not exceeding the maximum permissible heat capacity. Older scanners are limited to approximately 20 seconds, whereas newer scanners allow scan times of up to 100 seconds.
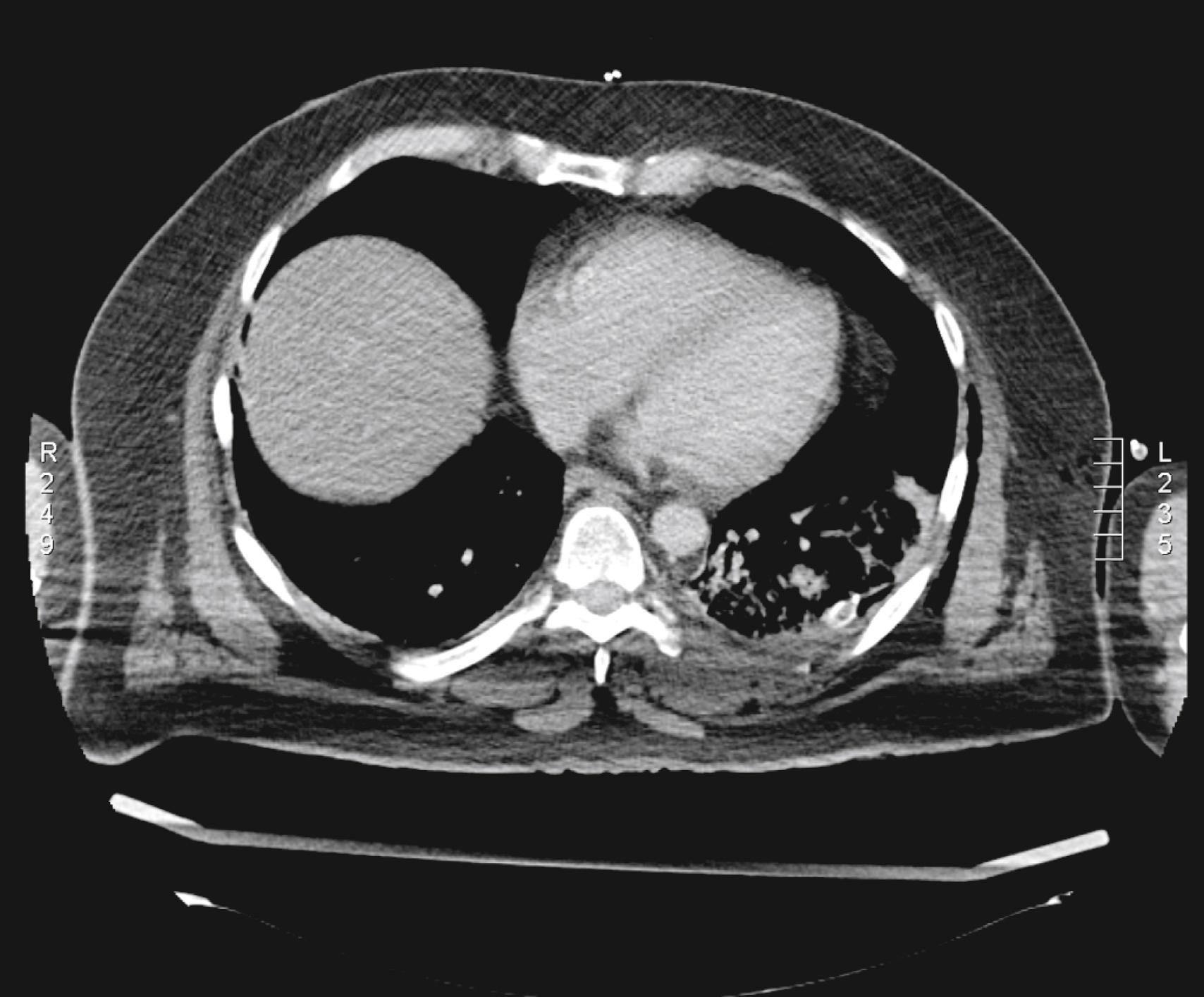
Proper, central positioning of the patient in the gantry can significantly improve image quality and decrease radiation dose. A clear example is raising the arms of the patient when scanning the upper part of the chest ( Fig. 29.2 ).
With the newer scanner types, good timing of the administration of contrast material has become an essential part of the examination. Depending on the indication and parameter settings (pitch), optimization of total volume of contrast, contrast iodine density, volume, and speed of injection is required.
Dual energy/source CT was introduced as a novel technique in 2006. Instead of one X-ray tube, the system was equipped with two X-ray tubes each working at a different kVp. Dual energy/source CT has not found broad application for vascular imaging. The use of two different X-ray tubes has inherent hardware design issues and similar advantages might be acquired by smart software algorithms with a single source CT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here