Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy has been a predominant treatment option for cervical cancer since Marie Curie discovered radium. Radiation therapy is the primary treatment for locally advanced cervical cancer, vulvar carcinoma, and vaginal carcinoma. It is used in the postoperative setting to reduce the incidence of local recurrence in patients with endometrial cancer, vulvar cancer, and cervical cancer who have high risk factors. Different combinations of external beam radiation therapy and brachytherapy are used, depending on the setting in which the radiation therapy is being applied. Cure rates with primary radiation for locally advanced cervical cancer range from 50% to 90% depending on stage and nodal status of the patients.
In a retrospective study of 3489 patients with stage I or II cancer of the cervix who were treated with external beam radiation therapy and brachytherapy, Eifel and colleagues found at 10 years an overall risk of 11.2% for grade 3 or higher complications. There were 3.3% rectal, 4.2% small bowel, and 3.0% bladder complications. The investigators found a correlation between smoking and both bladder and rectal complications. Women who had a body mass index (BMI) below 22 kg/m2 had an increased risk of rectal and small bowel complications, and women with a BMI greater than 31 kg/m2 had an increased risk of bladder complications. In two randomized studies looking at postoperative radiation therapy in patients with endometrial cancer, patients who received external beam radiation therapy had a higher rate of urinary incontinence, diarrhea, and fecal leakage leading to greater limitation in daily activities than patients who underwent observation or vaginal brachytherapy alone.
This chapter is intended to be a guide for the gynecologic oncologist on the most common complications arising from the use of radiation therapy for the treatment of gynecologic malignancies and how to manage them, including both surgical and nonsurgical options depending on the complication and scenario.
Complications from radiation therapy are commonly associated with the dose delivered to the organ from the treatment. Many studies have looked at doses and complications, and there are guidelines for the radiation oncologist regarding what dose is recommended for each organ depending on the type of radiation therapy given (i.e., external beam radiation therapy, brachytherapy, or a combination) and the type of cancer being treated, as well as other factors including history of inflammatory disease, age, and previous surgeries.
Recently, newer techniques have been developed in the field of radiation oncology that may reduce the dose to normal tissues and therefore, it is hoped, decrease both acute and late toxicities of radiation therapy. Intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT) allow for dose painting and beam optimization, which permit a more conformal dose around target areas while limiting the dose to normal organs and tissues, especially the bladder, rectum, sigmoid, and small bowel ( Fig. 23.1 ). Early retrospective studies and as well as phase 2 studies have shown a decrease in both acute and chronic gastrointestinal toxicities with the use of IMRT. Early results of a phase 3 study in which postoperative patients with either cervical or endometrial cancer were randomized to receive IMRT or standard pelvic radiation therapy were recently presented. The results showed that patients treated with IMRT had lower acute gastrointestinal and genitourinary toxicities as well as a better quality of life during treatment than patients treated with standard radiation therapy, but longer follow-up is needed to see if IMRT reduces chronic toxicities.
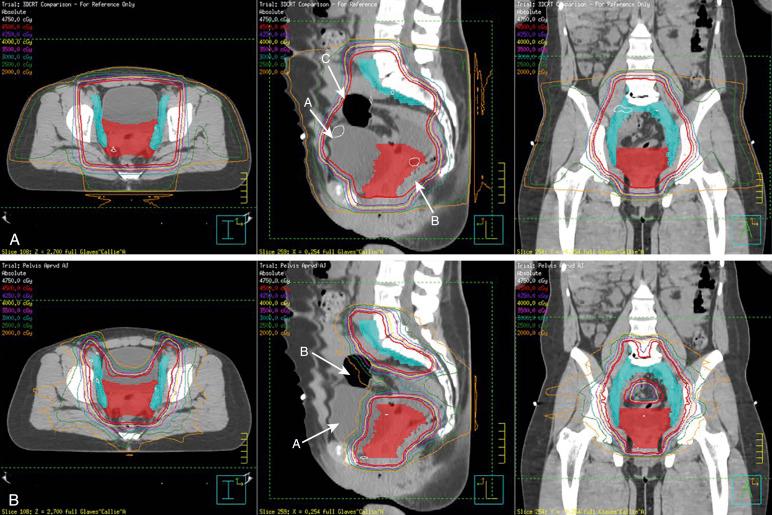
In the field of brachytherapy, there also have been improvements with the advent of image-guided brachytherapy. With image-guided brachytherapy, in which magnetic resonance imaging (MRI) or computed tomography (CT)–based imaging is used during the brachytherapy procedure, the dose is being administered to the cervix and parametrium instead of to points determined on the basis of plain-film radiographs (e.g., point A). Doses to normal tissues such as the rectum and bladder are being accurately calculated instead of, again, administering radiation to points determined with plain-film radiographs. Data from many studies show that use of image-based brachytherapy has increased local control while reducing radiation-related toxicities to the bladder and rectum.
It is hoped that as the field expands, more sophisticated radiation techniques will reduce the incidence of radiation-related toxicities.
Radiation therapy can induce bone damage in a number of ways. The most common presentation is pelvic insufficiency fracture (PIF), but fragility fractures of the femoral neck and vertebrae (if within the irradiated field) can be seen; an even less common but well-known complication is avascular necrosis of the femoral head (incidence, 0.5%–1%). Because the most common presentation is PIF, the rest of this section will discuss this topic ( Fig. 23.2 ).
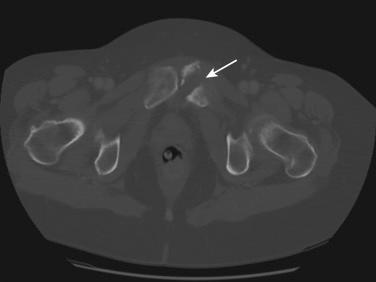
The reported incidence of PIF ranges from 1.7% to 89%. However, the majority of studies show a median incidence range of 10% to 20%, with a median time of occurrence between 6 and 20 months from the completion of radiation therapy. The difference in incidence is due to many reasons, including the method of detection, type of lesion reported (symptomatic or both asymptomatic and symptomatic), population, and dose of radiation therapy. The most common risk factor for increased incidence of PIF after radiation therapy includes older age, postmenopausal status, and low BMI and body weight. Data regarding other risk factors, including decreased bone mineral density before radiation, smoking status, and alcohol intake, are mixed.
There are a number of ways to diagnose PIF; presently, the best imaging study available is MRI or CT ( Fig. 23.2 ). Most patients with PIF develop pelvic pain if the fractures are symptomatic; this usually occurs within the first 2 years after treatment. It is important to perform imaging in these patients to diagnose the cause of the pain, which could include recurrence of the cancer, pathologic fractures, and other conditions, including PIF. The morbidity of PIF can be high, involving pain, time away from work, and definitely a decrease in quality of life. The most common sites of PIF include the sacral ala, sacral body, ilium, acetabulum, pubis, and lumbar spinal vertebrae.
The most common management for PIF is conservative, including bed rest, gentle mobilization, and analgesia, with resolution of symptoms occurring within 3 to 30 months if the fractures are stable. If the fractures are unstable, the patient should be referred to an orthopedic surgeon. For better mobilization, the patient should be referred to a physical therapist. Once a fracture has been diagnosed, it is important to assess the full bone health of the patient, including secondary screens for other bone abnormalities, a bone density test, and vitamin D levels. The vitamin D levels should be treated until greater than 50 nmol/L. Bisphosphonate therapy should be used only if the patient meets the criteria for treatment, which include the confirmed diagnosis of osteoporosis (i.e., a T-score of −2.5 standard deviations or lower), but referral to an endocrinologist is very important because there is currently no evidence that there is any benefit to its use for secondary prevention.
Cementoplasty, particularly sacroplasty, is a treatment option for painful PIFs. Small prospective and retrospective studies have shown improvement in pain with this treatment. The treatment involves percutaneous injection of polymethyl methacrylate (PMMA) into the fractured area. A clinical review of the literature showed that patients treated with sacroplasty have a significant improvement in pain scores ; however, these are early results, and larger prospective trials are needed. Sacroplasty is associated with its own complications including infection, embolization, and movement of the cement.
Prevention is probably the most important treatment for PIFs. This includes a full assessment of the patient’s bone health including assessment of fracture risk and a bone density test, as well as measurement of baseline vitamin D level before the start of radiation therapy. It is recommended that all postmenopausal women should be treated empirically with vitamin D to levels greater than 50 nmol/L. There is little evidence that bisphosphonates should be used as primary prevention of PIFs, but if a patient is at high risk for hip or vertebral fragility fractures and meets the criteria for bisphosphonate therapy on this basis, then national guidelines should be followed. Future work is needed in the treatment and prevention of PIFs that occur in patients treated with radiation therapy because it can affect the quality of life of patients who survive cancer.
The most common site of necrosis is at the apex of the vagina, especially in patients treated definitively with radiation therapy for cervical cancer. This usually occurs within 1 month and up to 16 months after treatment. Symptoms include pain and bleeding. The best treatment for vaginal necrosis is conservative, including topical estrogen cream (about 1 g per applicator) two to three times a week and, if possible, the use of a hydrogen peroxide douche (50/50 3% hydrogen peroxide and water), but it is best to start with estrogen cream even in patients with endometrial cancer for a short period of time. The necrosis usually resolves within a month with conservative treatment.
Total vaginal necrosis is a rare complication that manifests with sudden onset of pain (worsened by sitting) that starts 4 to 16 months after the completion of treatment. Clinically, fibrinous changes and later ulceration of the vagina are seen, followed eventually by consolidation with concentric granulation of the vaginal wall. In some patients, the necrosis will proceed to fistula formation between the vagina and the bladder or rectum. It is important to rule out disease progression or recurrence with biopsies, but one also needs to be careful about being too aggressive with biopsies, because these could also lead to fistula formation. Factors contributing to necrosis include total dose of the radiation therapy, fields used in the treatment, medical comorbidities (particularly cardiovascular disease), and smoking. Treatment options include surgical debridement, sitz baths, hydrogen peroxide douches, local and systemic antibiotics, estrogen replacement, and analgesics. Hyperbaric oxygen may be also helpful. Hyperbaric oxygen should be used if conservative measures fail, usually after 4 to 6 months of conservative management. Treatment consists of at least 20 sessions but usually between 20 and 30 sessions of 100% oxygen inhalation at 2.4 atmospheric absolutes (ATAs) for 60 to 90 minutes in a hyperbaric chamber.
Cervical and uterine necrosis after radiation therapy is also a rare event but occurs in 1.75% of all women treated, and the incidence is higher in women who are current smokers (2.76%). Clinical presentation includes vaginal discharge with pelvic or abdominal pain, as well as, in some patients, vaginal bleeding. Mean time for occurrence is 9.3 months (range, 2.2–20.5 months). As with vaginal necrosis, biopsy should be performed to rule out recurrence of disease, but care needs to be taken not to be too aggressive to avoid fistula formation. Treatment should again be conservative initially, including counseling for smoking cessation for all patients, vaginal douching with hydrogen peroxide, consideration of antibiotics (usually metronidazole 500 mg twice a day or three times a day for 10 days) and pain control. Fig. 23.3 shows a patient who had necrosis after treatment and was treated with conservative management as described earlier; within 6 months she had complete resolution of the necrosis and was disease free 3 years later.
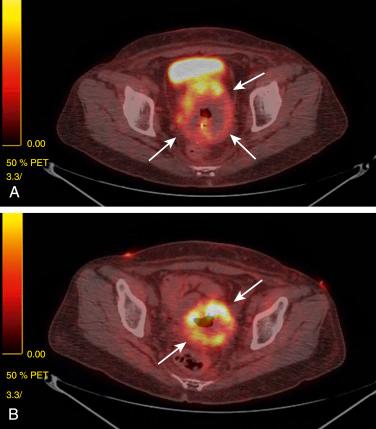
Sometimes, conservative management does not work, and additional treatment may be needed. There are data suggesting that administration of pentoxifylline (PTX) and vitamin E (tocopherol) works for osteoradionecrosis. The usual treatment includes 400 mg of PTX twice a day and tocopherol 1000 IU once a day. A literature review showed that this treatment may be beneficial for colorectal anastomosis and postburn scars, in addition to radiation-induced skin and soft tissue injuries. Fig. 23.4 shows positron emission tomography–computed tomography (PET-CT) images of a patient with stage IIIB cervical cancer. The images include the initial PET-CT scan, a PET-CT scan taken 3 months after radiation therapy, and a scan taken 6 months after treatment with PTX and tocopherol. She was completely disease free 1 year after completion of treatment.
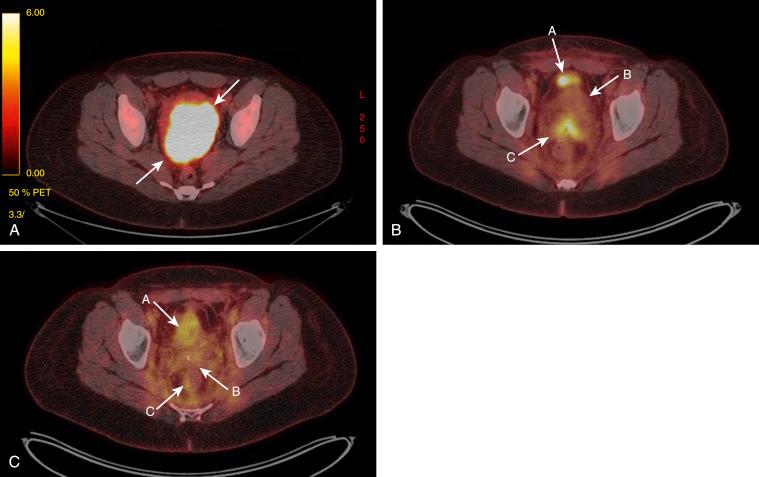
Necrosis usually resolves with conservative management within 4 to 6 months. Hyperbaric oxygen may also be useful if there is no improvement within 4 months. In patients in whom there is no improvement within 6 months and who continue to be symptomatic, surgical debridement and resection should be considered, but this should performed only in a worst-case scenario because it can lead to further complications such as fistula formation.
Radiation proctitis is radiation-induced rectal mucosal injury including loss of mucosa, endothelial swelling in the arterioles, and subsequent fibrosis of connective tissue and arteriolar endarteritis ( Fig. 23.5 ). The incidence rate varies from 2% to 39% and is definitely correlated with the dose the rectum receives during radiation therapy. Data show that the risk of proctitis increases as a function of mean rectal dose, ranging from 2% for patients receiving 50 Gy or less to the rectum to 18% for patients receiving 80 Gy or more to the rectum. Patients with inflammatory bowel disease and acquired immunodeficiency syndrome (AIDS) are at a higher risk of radiation proctitis.
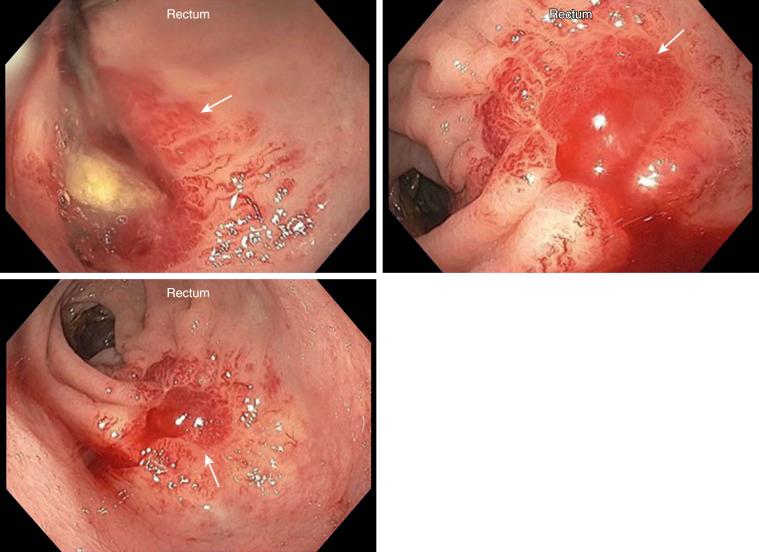
Radiation proctitis has been subdivided into two different phases depending on timing of the symptoms. Acute proctitis occurs during or within 3 months of radiation therapy and is usually transient and self-limiting; patients have diarrhea, urgency, and tenesmus, in general without rectal bleeding. Chronic proctitis either continues from the acute phase or begins after a latent period of at least 90 days, and symptoms include rectal bleeding that can progress to intestinal obstruction from stricture and sepsis.
There are no consistent guidelines or randomized studies on the treatment of symptomatic proctitis, and most patients are treated empirically with individualized treatment. The treatment options are either medical or endoscopic therapies. Medical therapies include antiinflammatory agents that have 5-aminosalicylic acid (5-ASA) as an active ingredient, antioxidants, sucralfate (oral or enema), steroid enemas, and hyperbaric oxygen. Of these medical treatments, the best data are from the use of sucralfate and hyperbaric oxygen. One study showed a benefit of oral sucralfate ; another study showed a benefit of the use of sucralfate enemas, with 92.3% of the patients reporting improved symptoms with no complications. Another study showed that sucralfate enemas were better at preventing and managing acute rectal toxicity than hydrocortisone enemas, and therefore at the present time there is limited use of steroid enemas in the treatment of acute or chronic proctitis.
Hyperbaric oxygen has shown promising results in a large randomized trial involving 120 patients in which there was a significant improvement in the healing responses with better bowel-specific quality of life in patients with refractory radiation proctitis. Another study showed little clinical difference in outcome between hyperbaric oxygen therapy and argon plasma coagulation (APC) for radiation proctitis, with minimal side effects.
Formalin instillation was initially used in 1986 for the treatment of radiation proctitis and is effective in approximately 48% of patients with chronic proctitis. In general, this approach is safe, but bleeding, perforation, and fistulas have been reported. However, in recent years, endoscopic therapies have become the treatment of choice for patients with chronic radiation proctitis with symptomatic bleeding. The goal of endoscopic therapy is the obliteration of the telangiectasis; the options include contact methods, such as heater probe and bipolar electrocautery, and noncontact methods such as laser therapy, APC, radiofrequency ablation, and cryotherapy. Recently, APC has become the preferred first-line endoscopic therapy for hemorrhagic proctitis. APC uses inert argon gas as a conducting medium, and a bipolar diathermy current is delivered. Limited depths of coagulation (0.5–3 mm) and uniform and predictable application are some of the advantages of APC. With the use of APC in one study there was improvement in rectal symptoms such as tenesmus, improvement in diarrhea in 60% to 75% of patients, and alleviation of rectal bleeding in 80% to 90% of patients. Multiple treatment sessions are recommended, up to a total of five, for diffuse lesions. Complication rates vary from study to study, but the most common complication is rectal or anal pain, which usually resolves spontaneously. Rectal ulcers are also common, and the recommendation is to perform pulse treatment to the lesion. Rare complications include urinary retention, necrosis, and arteriovenous fistula; the rate of strictures varies among different studies but ranges from 2% to 13%.
Surgery is the last resort for patients who do not improve with conservative measures, including those with symptoms of refractory bleeding and strictures leading to obstruction. Types of surgical interventions range from simple proximal diversion to formal resection with or without an anastomosis. Rectal proctitis is a common side effect from radiation therapy; it is hoped that as newer and more sophisticated radiation techniques are developed, the incidence of this complication will be reduced. However, until that time, nonsurgical treatments such as APC are the treatment of choice for patients with chronic proctitis with symptomatic bleeding. Early detection and early treatment will minimize the effect of complications. Surgical options should be reserved for refractory symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here