Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Complex regional pain syndrome (CRPS) is clinically characterized by pain, abnormal regulation of blood flow and sweating, edema of the skin and subcutaneous tissue, active and passive movement disorders, and trophic changes in the skin, appendages of the skin, and subcutaneous tissue. It is classified into type 1 (previously known as reflex sympathetic dystrophy) and type 2 (previously known as causalgia).
CRPS patients exhibit changes in the somatosensory systems processing noxious, tactile, and thermal information; in the sympathetic systems innervating skin (blood vessels, sweat glands), and in the somatomotor system. This indicates that the central representations of these systems are changed and that CRPS, in particular, type 1, is a systemic disease involving these neuronal systems. Patients with CRPS also demonstrate peripheral changes, such as edema, signs of inflammation, sympathetic–afferent coupling (as a basis for sympathetically maintained pain), and trophic changes, that cannot be explained by but also cannot be seen independent of the central changes. Therefore, CRPS cannot be reduced to one system or to one mechanism only. This view is based on clinical observations, experimentation on humans, and experimentation on animals.
Thus far, limited evidence-based treatment regimens are available for CRPS. Treatment of individual patients is empirical and based on evidence-based techniques that have been proved to be effective for CRPS, as well as for neuropathic pain conditions. Treatment should occur immediately and most importantly be directed toward restoration of full function of the extremity. This objective is best attained in a comprehensive interdisciplinary setting with particular emphasis on pain management and restoration of function. The key question in research is still what the mechanisms are that lead to this complex syndrome.
The key task to be addressed in therapy is to perform controlled multicenter studies that assess the acute in addition to the long-term effects of drug and interventional therapies, as well as physio- and psychotherapy.
During past decades, complex regional pain syndromes (CRPSs) were recognized as poorly defined pain disorders that mostly confused basic researchers, clinicians, and epidemiologists rather than stimulating their scientific activity. This was mainly due to the fact that diagnostic criteria were defined vaguely, underlying pathophysiological mechanisms were unknown, and therapeutic options limited. No data on incidence, prognosis, and prevention were available; research on mechanisms focused primarily on pain; and controlled treatment studies were absent. However, insight into the pathophysiological mechanisms of CRPS has progressed dramatically during the past few years.
Researchers became aware of the fact that CRPS types 1 and 2 are not just neuropathic pain syndromes. In fact, CRPS 1 is unlikely to only be a neuropathic pain syndrome since no obvious nerve lesion is present and all symptoms occur irrespective of the type of preceding lesion. Based on this notion it has become obvious that multiple different pathophysiological mechanisms may occur in different individual patterns ( ). Such mechanisms consist of somatosensory changes (including pain) that interact with changes related to the sympathetic nervous system, peripheral inflammatory changes, and changes in the somatomotor system ( ).
CRPS is a syndrome characterized by continuing (spontaneous and/or evoked) regional pain that is seemingly disproportionate in time or degree to the usual course of pain after trauma or other lesions. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor edema, and/or trophic findings. The syndrome shows variable progression over time.
In CRPS type 1 (reflex sympathetic dystrophy), minor injuries or fractures of a limb precede the onset of symptoms. CRPS type 2 (causalgia) develops after injury to a major peripheral nerve.
The American Civil War physician Weir Mitchell observed that about 10% of patients with traumatic partial peripheral nerve injuries in the distal end of an extremity had a dramatic clinical syndrome that consisted of prominent, distal spontaneous burning pain. In addition, patients reported exquisite hypersensitivity of the skin to light mechanical stimulation. Furthermore, movement, loud noises, or strong emotions could trigger their pain. The distal end of the extremity exhibited considerable swelling, smoothness, mottling, and in some cases, acute arthritis. In most cases the limb was cold and sweaty. Mitchell named this syndrome causalgia. He was emphatic that the sensory and trophic abnormalities spread beyond the innervation territory of the injured peripheral nerve and often occurred remote from the site of injury. The nerve lesions giving rise to this syndrome were always partial; complete transection never caused it. Because of this and the peripheral signs of the disease, he concluded that in addition to pathology in the nerve, some process in the skin or other peripheral tissue was responsible for the pain.
After World War II, Leriche for the first time reported that sympathectomy dramatically relieves causalgia. This notion was supported by several large clinical series, primarily involving wounded soldiers. In 1967 Richards described the clinical features of causalgia and the effect of sympatholytic interventions in hundreds of cases. He repeatedly stressed the dramatic response of causalgia to sympathetic blockade: “One of the outstanding surgical lessons that was learned during World War II was that interruption of the appropriate sympathetic nerve fibers is almost invariably effective in the treatment of causalgia. When the sympathetic chain is blocked by a local anesthetic, complete relief occurs almost immediately if the injection has been correctly placed, and the dramatic change in the patient’s appearance and attitude is remarkable.” The finding that sympatholysis relieves causalgic pain gave rise to the concept of sympathetically maintained pain.
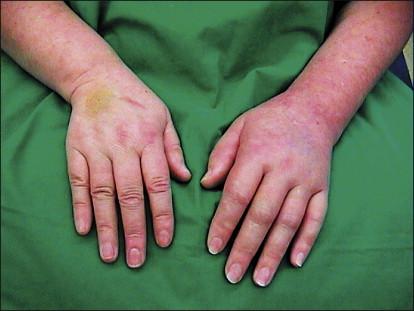
In the years between World Wars I and II, the concept that sympathetic outflow can influence pain was extended to a group of patients without detectable nerve injury. Asymmetrical distal extremity pain and swelling develop in these patients ( Fig. 67-1 ). The disorder had first been described by Paul Sudeck early in the 20th century. Precipitating events include fracture or minor soft tissue trauma, low-grade infection, frostbite or burns, and stroke and myocardial infarction. The swelling and pain often develop at a site remote from the inciting injury, without any obvious local tissue-damaging process at the site of pain and swelling. This syndrome was named reflex sympathetic dystrophy because vasomotor (altered skin color and temperature) and sudomotor (altered sweat production) abnormalities are common, the pain and swelling are often spatially remote from the inciting injury, and patients typically obtain dramatic relief with sympathetic blockade.
In 1994 the terminology changed to CRPS, and thereafter new diagnostic criteria were proposed in 2007 and validated in 2010.
A population-based study of CRPS 1 in the United States calculated an incidence rate of about 5.5 per 100,000 person-years at risk and a prevalence of around 21 per 100,000. An incidence rate of 0.8 per 100,000 person-years at risk plus a prevalence of about 4 per 100,000 was reported for CRPS 2 ( ). A European study using different diagnostic criteria described an incidence of 6.2 per 100,000. CRPS 1 develops more often than CRPS 2. The most common inciting events in CRPS are preceding trauma or surgery. Remarkably, in about 10% of patients CRPS 1 develops after minor trauma and in 5–10% without any known event. The severity of the trauma is not correlated with the clinical characteristics of CRPS ( ). Estimations suggest a 1–37% incidence of CRPS 1 after fractures. The incidence of CRPS 2 after peripheral nerve injury varies from 2–14% in different series, with a mean around 4% ( ). The arm is affected more often than the leg, and females are affected more often than males, with a female-to-male ratio ranging from 2–4:1. CRPS shows a distribution over all ages, with the mean peak in age being 37–50 years.
The most common precipitating event is trauma affecting the distal part of an extremity (~75%), especially fractures, post-surgical conditions, contusions, and strain or sprain. Less common incidents are central nervous system (CNS) lesions such as spinal cord injuries and cerebrovascular accidents.
Asymmetrical distal extremity pain and swelling develop in patients with CRPS 1 without an overt nerve lesion ( Fig. 67-1 , Table 67-1 ). These patients often report a burning spontaneous pain in the distal part of the affected extremity. Characteristically, the pain is disproportionate in intensity to the inciting event. The pain usually increases when the extremity is in a dependent position. Stimulus-evoked pain, including mechanical and thermal allodynia and/or hyperalgesia, is a striking clinical feature. These sensory abnormalities often appear early, are most pronounced distally, and have no consistent spatial relationship to individual nerve territories or to the site of the inciting lesion. Typically, pain can be elicited by movement of and pressure on the joints (deep somatic hyperalgesia), even if they are not directly affected by the inciting lesion. Besides symptoms of pain, sensory loss is frequent. A recent study described the coexistence of gain (e.g., pain, hyperalgesia) and loss (e.g., hypoesthesia) in CRPS patients without detecting a distinct sensory pattern.
| SIGNS AND SYMPTOMS | DURATION OF CRPS | ||
|---|---|---|---|
| 2–6 mo (%) | >12 mo (%) | Total from 0–>2 mo (%) | |
| Pain | 88 | 97 | 93 |
| Increase in complaints after exercise | 95 | 97 | 96 |
| Neurological | |||
| Hyperesthesia/allodynia | 75 | 85 | 76 |
| Coordination deficits | 47 | 61 | 54 |
| Tremor | 44 | 50 | 49 |
| Muscle spasm | 13 | 42 | 25 |
| Paresis | 93 | 97 | 95 |
| Sympathetic | |||
| Hyperhidrosis | 56 | 40 | 47 |
| Color difference | 96 | 84 | 92 |
| Temperature difference | 91 | 91 | 92 |
| Changed growth of hair | 71 | 35 | 55 |
| Changed growth of nails | 60 | 52 | 60 |
| Edema | 80 | 55 | 69 |
| Atrophy | |||
| Skin | 37 | 44 | 40 |
| Nails | 23 | 36 | 27 |
| Muscle | 50 | 67 | 55 |
| Bone (diffuse/spotty osteoporosis on radiography) | 41 | 52 | 38 |
Autonomic abnormalities include swelling and changes in sweating and blood flow in the skin. In the acute stages of CRPS 1 the affected limb is often warmer than the contralateral limb. An initially decreased skin temperature (“cold” CRPS type) is thought to be a predictor of an unfavorable course (see Prognosis, later) and is associated with an increased incidence of, for example, dystonia, sensory loss, and cold-induced pain, whereas in “warm” CRPS, mechanical hyperalgesia occurs frequently. Sweating abnormalities—either hypohidrosis or, more frequently, hyperhidrosis—are present in nearly all CRPS 1 patients. The acute distal swelling of the affected limb depends very critically on aggravating stimuli. Since it may diminish after sympathetic blocks, it is likely that it is maintained by sympathetic activity.
Trophic changes such as abnormal nail growth, increased or decreased hair growth, fibrosis, thin glossy skin, and osteoporosis may be present, particularly in the chronic stages. Restriction of passive movement is often observed in long-standing cases and may be related to both functional motor disturbances and trophic changes in joints and tendons.
Weakness of all muscles of the affected distal extremity is frequently present. Small accurate movements are characteristically impaired. Range of motion may be reduced by edema or, later on, by contractures. Passive movements are less affected than voluntary ones. Findings on nerve conduction and electromyography studies are normal, except in patients in very chronic and advanced stages. About half of patients have a postural or action tremor representing an increased physiological tremor. In about 10% of cases, dystonia of the affected hand or foot develops ( ). Additionally, a neglect-like syndrome impairs motor control. ( ).
Importantly, in the course of the disease the symptoms often spread, sometimes also to the contralateral, primarily unaffected extremity.
The symptoms of CRPS 2 are similar to those of CRPS 1, the only exception being that a lesion of peripheral nerve structures and subsequent focal deficits are mandatory for the diagnosis. The symptoms and signs spread beyond the innervation territory of the injured peripheral nerve and often occur remote from the site of injury, but restriction to the territory is not in conflict with the current definition.
CRPS occurs predominantly in one extremity. Retrospective studies of large cohorts have shown the ratio of upper and lower extremity involvement to be 1–2:1. In 113 retrospectively reviewed cases the symptoms occurred on the right in 47%, on the left side in 51%, and bilaterally in 2%. Multiple extremities were affected in up to 7% of patients ( ).
For therapeutic reasons, effort should be undertaken to diagnose CRPS as early as possible. CRPS mostly starts acutely (i.e., the cardinal symptoms may appear within hours or days). At the onset, the main symptoms of CRPS are spontaneous pain, generalized swelling, and systematic between-side difference in skin temperature. These early symptoms already begin to develop in areas and tissues that were not affected by the preceding lesion. Thus, swelling and pain provide valuable information for an early diagnosis of CRPS: before the onset of CRPS, pain is felt inside the area of the preceding lesion; with the onset of CRPS, the pain becomes diffuse and is felt deep inside the distal extremity and the swelling generalizes, yet the initial pain may have already disappeared.
To some extent the tendency of symptoms to generalize may be a physiological phenomenon in post-traumatic states that will disappear without any treatment. Exact differentiation of these physiologically diffuse, post-traumatic reactions and the development of “real“ CRPS is not possible at the present time.
The sequential progression of untreated CRPS has repeatedly been described, each stage of which (usually three are proposed) differs in patterns of signs and symptoms. Nevertheless, this concept has come into question in the past few years. In 2002 the clinical validity of this concept was tested in 113 patients by Bruehl and colleagues. Using cluster analysis, three subgroups of patients were identified who could be differentiated by their symptoms and signs regardless of duration of the disease. The sequential concept relies on the course of untreated CRPS; however, thus far all studies performed to test its clinical validity investigated patients already under treatment. Furthermore, vascular disturbances and skin temperature measurements indicated different thermoregulatory types, depending on time.
In conclusion, it is questionable whether staging of CRPS is appropriate. It is much more practical, with a direct implication on therapy, that CRPS be graded according to the intensity of the sensory, autonomic, motor, and trophic changes as being mild, moderate, or severe (see later).
Most patients with CRPS exhibit significant psychological distress, most commonly depression and anxiety. Many patients become overwhelmed by the pain and associated symptoms and, without adequate psychosocial support, may develop maladaptive coping skills. Based on these symptoms there is a tendency to ascribe the etiology of CRPS to emotional causes, and it has been proposed that CRPS is a psychiatric illness. In fact, it is sometimes difficult to recognize the organic nature of the symptoms. However, when describing the clinical picture in the 1940s, Livingston was convinced: “The ultimate source of this dysfunction is not known but its organic nature is obvious and no one seems to doubt that these classical pain syndromes are real.” drew several conclusions on psychological factors in CRPS:
No evidence has been found to support the theory that CRPS is a psychogenic condition.
Because anxiety and stress increase nociception, relaxation and antidepressive treatment are helpful.
The pain associated with CRPS is the cause of psychiatric problems and not the converse.
Maladaptive behavior by patients, such as volitional or inadvertent actions, is due mostly to fear, regression, or misinformation and does not indicate psychopathology.
CRPS has been diagnosed incorrectly in some patients with conversion disorders and factitious diseases.
In summary, the author concludes that the widely proposed “CRPS personality” is clearly unsubstantiated.
According to this view, an even distribution of childhood trauma, pain intensity, and psychological distress was confirmed in patients with CRPS as opposed to those with other neuropathic pain and chronic back pain ( ). Further studies demonstrated high psychiatric co-morbidity, especially depression, anxiety, and personality disorders, in CRPS patients. These findings are also present in other chronic pain patients and are more likely a result of the long and severe pain and disease ( ). When compared with patients with low back pain, CRPS patients exhibited a higher tendency toward somatization but did not have any other psychological differences ( ). In 145 patients, 42% reported stressful life events in close relation to the onset of CRPS, and 41% had a previous history of chronic pain ( ). Thus, stressful life events could be risk factors for the development of CRPS. However, a recent review did not identify any psychological predisposition, and a prospective study did not find any psychological factors to be associated with the occurrence of CRPS.
One of the unsolved features in human pain diseases is the fact that chronic pain develops in only a minority of patients after seemingly identical inciting events. Similarly, in certain nerve lesion animal models, differences in pain susceptibility were found to be due to genetic factors. The clinical importance of genetic factors in CRPS is not clear. Even though familial occurrence has been described, mendelian genetics does not seem to have any impact on the incidence and prevalence of CRPS. However, although the importance of the findings reported later is not clear, they suggest some genetic influence on the development and maintenance of CRPS.
Association of human leukocyte antigen (HLA) with different phenotypes has shown an increase in A3, B7, and DR2 major histocompatibility complex (MHC) antigens in a small group of CRPS patients in whom resistance to treatment correlated with positivity of DR2. Class I and II MHC antigens were typed in a cohort of 52 CRPS patients. The frequency of HLA-DQ1 was found to be significantly increased in comparison to the frequency in controls ( ). In patients with CRPS who progressed to multifocal or generalized dystonia, an association with HLA-DR13 was reported, and in those with fixed dystonia, an association with HLA-B62 and HLA-DQ8 was noted ( ). Furthermore, a different locus, centromeric in HLA class I, was found to be correlated with the spontaneous development of CRPS, thus suggesting an interaction between trauma severity and genetic factors that influence susceptibility to CRPS ( ). So far no associations have been detected for different cytokines, neuropeptides, neuronal endopeptides, dystonia-associated genes ( DYT ), and the Na v 1.7 sodium channel gain-of-function mutation within the SCNA9 gene.
Based on numerous animal experimental findings, spontaneous pain and various forms of hyperalgesia at the distal end of the extremity are thought to be generated by the processes of peripheral and central sensitization. Quantitative sensory testing (QST) has revealed heat and cold hyperalgesia in combination with warm and cold hypoesthesia in acute CRPS 1, whereas in chronic CRPS 1, the thermal hyperalgesia declined and thermal hypoesthesia increased. Moreover, similar subtle signs were present in the contralateral extremity. In addition to the positive sensory phenomena of pain, in up to 50% of patients with chronic CRPS 1 hypoesthesia and hypoalgesia develop in the entire half of the body or in the upper quadrant ipsilateral to the affected extremity. Systematic QST has shown that patients with generalized hypoesthesia have increased thresholds to mechanical, cold, warmth, and heat stimuli when compared with the responses generated from the corresponding contralateral healthy side of the body. Patients with these extended sensory deficits have a longer disease duration, greater pain intensity, a higher frequency of mechanical allodynia, and a higher tendency for the development of changes in the somatomotor system than do patients with spatially restricted sensory deficits.
These changed somatosensory perceptions are probably due to functional changes in the central representation of somatosensory sensations. Central sensitization and disinhibition are the two key mechanisms in CRPS that can assist in understanding the sensory changes in CRPS. Accordingly, early positron emission tomography studies demonstrated adaptive changes in the thalamus during the course of the disease ( ). Furthermore, several studies using magnetoencephalography (MEG) and functional magnetic resonance imaging (fMRI) demonstrated a pain network involving nociceptive, motor, and attentional processing that resulted in changed central sensory processing, such as a shortened distance between the little finger and thumb representations in the primary somatosensory (SI) cortex on the painful side ( , 2005; ). The cerebral responses noted with fMRI and MEG were increased on the affected side, thus indicating processes of central disinhibition (i.e., sensitization; e.g., ) ( Fig. 67-2 ). These findings are in line with the clinical findings of glove-like patterns, hemisensory signs, and referred sensations; they resemble the results of imaging studies demonstrating motor circuit disinhibition.
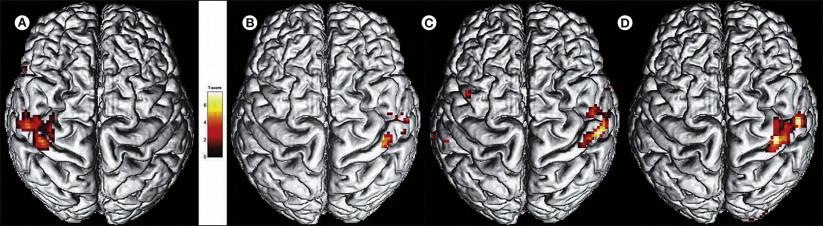
These central changes depend on continuous nociceptive input from the affected extremity, are associated with spontaneous pain and mechanical hyperalgesia, are not specific to CRPS, and are reversible after successful treatment of the pain ( ; ). However, Lebel and co-workers (2008) could show that the altered CNS reactivity to sensory stimuli persisted after recovery from CRPS.
The relevance of structural changes within the peripheral nervous system, such as small-fiber neuropathy or myopathy, is still under debate.
A partial nerve lesion is the important preceding event in the development of CRPS 2. Therefore, it has generally been assumed that abnormalities in skin blood flow within the territory of the lesioned nerve are due to peripheral impairment of sympathetic function and sympathetic denervation. During the first weeks after transection of vasoconstrictor fibers, vasodilatation is present within the denervated area. Later, increased sensitivity to circulating catecholamines may develop in the vasculature, probably because of up-regulation of adrenoceptors.
Sympathetic denervation and denervation hypersensitivity cannot completely account for the vasomotor and sudomotor abnormalities in CRPS. First, in CRPS 1 there is no overt nerve lesion, and second, in CRPS 2 the autonomic symptoms spread beyond the territory of the lesioned nerve. In fact, there is direct evidence of reorganization of central autonomic control in these syndromes ( ).
Hyperhidrosis, for example, is found in many patients with CRPS. Resting sweat output, as well as thermoregulatory and axon reflex sweating, is increased in CRPS 1 patients ( ). The increased sweat production cannot be explained by a peripheral mechanism only since unlike blood vessels, denervation supersensitivity does not develop in sweat glands. However, the inflammatory release of calcitonin gene–related peptide (CGRP) has been shown to enhance sweat gland activation.
To study cutaneous sympathetic vasoconstrictor innervation in CRPS 1 patients we analyzed central sympathetic reflexes induced by thermoregulatory (whole-body warming, cooling) and respiratory stimuli ( ; ). Sympathetic effector organ function (i.e., skin temperature and skin blood flow) was measured bilaterally in the extremities by infrared thermometry and laser Doppler flowmetry. Under normal conditions these reflexes do not show between-side differences.
In CRPS patients three distinct vascular regulation patterns related to the duration of the disorder were identified:
In the warm regulation type (acute stage, <6 months), the affected limb was warmer and skin perfusion values were higher than contralaterally during the entire spectrum of sympathetic activity. Even massive body cooling failed to activate sympathetic vasoconstrictor neurons ( ). Consistently, direct measurements of norepinephrine levels from the venous effluent above the area of pain have shown a reduction in the affected extremity ( ).
In the intermediate type, temperature and perfusion were either elevated or reduced, depending on the degree of sympathetic activity.
In the cold type (chronic stage), temperature and perfusion were lower on the affected side during the entire spectrum of sympathetic activity. Norepinephrine levels, however, were still lower on the affected side ( ).
These data support the idea that CRPS 1 is associated with pathological unilateral inhibition of cutaneous sympathetic vasoconstrictor neurons leading to a warmer affected limb in the acute stage ( ). The locus of pathophysiological changes underlying such disturbed reflex activity must be in the CNS. Additional changes in neurovascular transmission may induce disturbed neurovascular transmission with severe vasoconstriction and cold skin in chronic CRPS ( ). Accordingly, α-adrenoceptor density has been reported to be increased in skin biopsy specimens from patients with CRPS 1 ( ). Furthermore, skin lactate was increased in CRPS patients, indicative of enhanced anaerobic glycolysis, probably as a result of vasoconstriction and chronic tissue hypoxia ( ). The few microneurographic studies of small sympathetic nerve fascicles that have been performed thus far in patients with CRPS, however, have not confirmed the presence of reflex abnormalities; the average skin sympathetic activity (i.e., a combination of vasoconstrictor and sudomotor activity) was not different on the two sides ( ). Moreover, changes in the endothelium may contribute to the vasomotor symptoms and signs. Dysfunction leads to lower release of nitric oxide and thereby to vasoconstriction and has been shown in patients with cold-type CRPS 1. It is unknown whether these findings are primary or secondary.
Some of the clinical features of CRPS, particularly in its early phase, could be explained by an inflammatory process ( ). Consistent with this idea, corticosteroids are often used successfully for acute CRPS (see Treatment, later). Neurogenic inflammation is caused by the release of inflammatory neuropeptides, such as CGRP and substance P, from sensory neuron terminals. Additionally, a nerve injury can sensitize nociceptive neurons and thereby start a vicious cycle of inflammation and pain.
Evidence is increasing that localized neurogenic inflammation is involved in the generation of reddening, acute edema, and vasodilatation. The mechanisms are thought to be impaired neuropeptide inactivation and increased receptor availability.
Scintigraphic investigations with radiolabeled immunoglobulins have demonstrated extensive plasma extravasation in patients with acute CRPS 1 ( ). Analysis of joint fluid and synovial biopsy samples in CRPS patients has shown an increase in protein concentration and synovial hypervascularity. Furthermore, synovial effusion is enhanced in affected joints as measured with MRI.
In patients with acute untreated CRPS 1, neurogenic inflammation was elicited by strong transcutaneous electrical stimulation via intradermal microdialysis. Simultaneous assessment of protein extravasation with the microdialysis system revealed that the extravasation was provoked only on the affected extremity. Furthermore, axon reflex vasodilatation was increased significantly. The time course of electrically induced protein extravasation in patients resembled that observed following the application of exogenous substance P ( ). As further support of a neurogenic inflammatory process, systemic tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-8 levels were found to be increased in acute CRPS but not in the chronic stages ( ). In the fluid of artificially produced skin blisters and in venous blood, significantly higher levels of IL-6 and TNF-α were observed in the involved extremity than in the uninvolved extremity, a finding that changes only marginally in patients with chronic CRPS ( ). Comparison of serum and systemic levels did show higher TNF-α in tissue, thus pointing to a localized inflammatory response. These findings become clinically relevant since the presence of or increase in pro-inflammatory cytokines is correlated with the presence of mechanical hyperalgesia as a marker of central sensitization (Maihofner et al 2005). Nonetheless, no specific prognostic cytokine profiles have been determined.
Recent studies have indicated a role of the immune system in CRPS. Antibodies against sympathetic and mesenteric neurons and adrenergic and muscarinergic receptors have been described in up to one-third of CRPS patients. Interestingly, injection of IgG antibodies from CRPS patients into mice produced symptoms that partially resembled CRPS. Since CRPS is clinically a localized syndrome, a generalized antibody-related autoimmune disorder seems to be unlikely thus far.
Hence, evidence indicates that inflammatory processes are involved in the pathogenesis of CRPS. One further question is whether the sympathetic nervous system may contribute to the early inflammatory state. De novo expression of adrenoreceptors on macrophages after experimental nerve lesions supports this idea. However, this concept has yet to be proved in patients with CRPS. Figure 67-3 illustrates the possible interactions between sympathetic fibers, afferent fibers, blood vessels, and non-neural cells related to the immune system (e.g., macrophages) that could theoretically lead to the inflammatory changes observed in CRPS patients.
![Figure 67-3, A, The micromilieu of nociceptors. The microenvironment of primary afferents is thought to affect the properties of the receptive endings of myelinated (A) and unmyelinated (C) afferent fibers. This has been documented in particular for inflammatory processes, but one may speculate that pathological changes in the direct surroundings of primary afferents may contribute to other pain states as well. The vascular bed consists of arterioles (directly innervated by sympathetic and afferent fibers), capillaries (not innervated and not influenced by nerve fibers), and venules (not directly innervated but influenced by nerve fibers). The micromilieu depends on several interacting components: neural activity in the post-ganglionic noradrenergic fibers (1) supplying blood vessels (3) and release of noradrenaline (NA) and possibly other substances that cause vasoconstriction. Excitation of primary afferents (Aδ and C fibers) (2) causes vasodilatation in precapillary arterioles and plasma extravasation in post-capillary venules (C fibers only) by the release of substance P (SP) and other vasoactive compounds (e.g., calcitonin gene–related peptide [CGRP]). Some of these effects may be mediated by non-neuronal cells such as mast cells and macrophages (4). Other factors that affect control of the microcirculation are the myogenic properties of arterioles (3) and more global environmental influences such as a change in temperature and metabolic state of the tissue. B, Hypothetical relationship between sympathetic noradrenergic nerve fibers (1), peptidergic afferent nerve fibers (2), blood vessels (BV) (3), and macrophages (MP) (4). The activated and sensitized afferent nerve fibers activate macrophages, possibly via release of SP. The immune cells start to release cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), which further activate afferent fibers. SP (and CGRP) released from afferent nerve fibers reacts with neurokinin 1 (NK1) receptors in blood vessels (arteriolar vasodilatation, venular plasma extravasation, neurogenic inflammation). The sympathetic nerve fibers interact with this system on three levels: (1) via adrenoceptors (mainly α) on the blood vessels (vasoconstriction), (2) via adrenoceptors (mainly β) on macrophages (further release of cytokines), and (3) via adrenoceptors (mainly α) on afferent blood vessels (further sensitization of these fibers). Figure 67-3, A, The micromilieu of nociceptors. The microenvironment of primary afferents is thought to affect the properties of the receptive endings of myelinated (A) and unmyelinated (C) afferent fibers. This has been documented in particular for inflammatory processes, but one may speculate that pathological changes in the direct surroundings of primary afferents may contribute to other pain states as well. The vascular bed consists of arterioles (directly innervated by sympathetic and afferent fibers), capillaries (not innervated and not influenced by nerve fibers), and venules (not directly innervated but influenced by nerve fibers). The micromilieu depends on several interacting components: neural activity in the post-ganglionic noradrenergic fibers (1) supplying blood vessels (3) and release of noradrenaline (NA) and possibly other substances that cause vasoconstriction. Excitation of primary afferents (Aδ and C fibers) (2) causes vasodilatation in precapillary arterioles and plasma extravasation in post-capillary venules (C fibers only) by the release of substance P (SP) and other vasoactive compounds (e.g., calcitonin gene–related peptide [CGRP]). Some of these effects may be mediated by non-neuronal cells such as mast cells and macrophages (4). Other factors that affect control of the microcirculation are the myogenic properties of arterioles (3) and more global environmental influences such as a change in temperature and metabolic state of the tissue. B, Hypothetical relationship between sympathetic noradrenergic nerve fibers (1), peptidergic afferent nerve fibers (2), blood vessels (BV) (3), and macrophages (MP) (4). The activated and sensitized afferent nerve fibers activate macrophages, possibly via release of SP. The immune cells start to release cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), which further activate afferent fibers. SP (and CGRP) released from afferent nerve fibers reacts with neurokinin 1 (NK1) receptors in blood vessels (arteriolar vasodilatation, venular plasma extravasation, neurogenic inflammation). The sympathetic nerve fibers interact with this system on three levels: (1) via adrenoceptors (mainly α) on the blood vessels (vasoconstriction), (2) via adrenoceptors (mainly β) on macrophages (further release of cytokines), and (3) via adrenoceptors (mainly α) on afferent blood vessels (further sensitization of these fibers).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ComplexRegionalPainSyndromes/2_3s20B978070204059700067X.jpg)
Motor deficits are common findings in CRPS: about 50% of patients with CRPS have decreased active range of motion, an increased amplitude of physiological tremor, and reduced active motor force of the affected extremity. In about 10% of cases, dystonia of the affected hand or foot develops with a delayed onset. It is unlikely that these motor changes are related to a peripheral process (e.g., influence of the sympathetic nervous system on neuromuscular transmission and/or contractility of skeletal muscle). These somatomotor changes are more likely generated by changes in activity in the motor neurons and maladaptive central neuronal plasticity.
These hypotheses have been confirmed by several functional imaging and behavioral studies: kinematic analysis of target reaching, as well as grip force analysis, to quantitatively assess motor deficits in CRPS patients ( ). These results pointed to abnormalities in cerebral motor processing. Pathological sensorimotor integration in the parietal cortex was found that may induce abnormal central programming and processing of motor tasks, as confirmed by fMRI. Interestingly, motor performance is also slightly impaired on the contralateral unaffected side ( ). These early findings have been substantiated by electrophysiological and imaging studies demonstrating widespread central disinhibition of motor processing circuits.
According to this view of central maladaptation, a neglect-like syndrome was clinically described as being responsible for disuse of the extremity and change in body perception ( ). Patients describe their affected limb as being larger, with a change in shape and posture; demonstrate impaired recognition of laterality; and often report an “alien limb,” which explains the occasional desire for limb amputation. However, the neglect-like syndrome in CRPS differs from the neglect after stroke since patients are usually able to report the altered limb perceptions. The complex pathomechanism is the result of an implicit mechanism of pain avoidance and altered central limb representation caused by functional and not structural changes.
In line with these concepts, emerging evidence supports an incongruence between central motor output and sensory input as an underlying mechanism in CRPS. As an example, in the method of mirror visual feedback the visual input from a moving unaffected limb to the brain was able to re-establish the pain-free relationship between sensory feedback and motor execution. After 6 weeks of therapy, pain and function were improved in comparison to the control group ( ). Furthermore, combining laterality recognition training, imagination of movements, and mirror movements was efficacious in reducing pain and restoring function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here