Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Complex regional pain syndrome (CRPS) is an exaggerated response to injury or immobilization, which, at times, leads to devastating chronic pain and disability. It involves an aberrant reaction of multiple aspects of the nervous system that persists long after the initial insult has resolved. Diagnosis is frequently delayed, prolonging morbidity and allowing entrenchment of peripheral and central neuroplastic changes, making treatment all the more difficult. Patients with pain out of proportion to the objective tissue damage or persisting beyond a typical recovery period should be examined for other signs and symptoms of CRPS, including autonomic nervous system dysregulation, and treatment instituted as early as possible. Interventions including physical therapy, desensitization, oral medication focused on pain as well as restoring nervous system homeostasis, sympathetic and peripheral nerve blocks, and implantable nerve stimulators, which have all been used successfully to treat CRPS.
Historically, in the 17th century, the French battlefield surgeon Ambrose Paré first described this syndrome of burning pain after peripheral nerve injury. King Charles IX was treated for smallpox by Paré, who lanced his arm. After treatment, the king suffered from persistent pain, muscle contracture, and inability to flex his arm. In 1864, Mitchell, an American Civil War physician, coined the term causalgia for the burning pain that follows gunshot injuries to the peripheral nerves. In 1900, Sudek observed spotty radiographic osteopenia in affected extremities. A little later in 1916, Leriche implicated the sympathetic nervous system, and subsequently, Livingston in 1943 described the vicious circle of pain. This theory described abnormally firing self-sustaining loops in the dorsal horn. These were thought of as small irritation foci of nerve endings of major trunk nerves, which activated central projecting fibers, giving rise to pain.
CRPS has historically been known by many names including sympathetic trophoneurosis, minor causalgia, Sudeck atrophy, algoneurodystrophy, acute bone atrophy, peripheral trophoneurosis, painful osteoporosis, sympathalgia, and its most well-known former name—reflex sympathetic dystrophy (RSD). The term reflex sympathetic dystrophy (RSD), coined by Evans in 1946, was initially the popular descriptive term and remains part of the vocabulary of many treating physicians. The name complex regional pain syndrome , put forth by the International Association for the Study of Pain (IASP) in 1993, is now the accepted term.
The IASP has standardized nomenclature around the disease to improve the collection of evidence-based information about this condition. According to the IASP, CRPS exists in two forms ( Table 20-1 ). CRPS type I (formerly RSD) develops after an initiating noxious event. It features a triad of sensory, autonomic, and motor symptoms that occur distally in the affected extremity in a generalized distribution. The symptoms are independent of the type and location of the preceding trauma, and frequently the trauma is minor compared to the extent of symptoms. The symptoms are not limited to the distribution of a single peripheral nerve, are routinely disproportionate to the inciting event, and persist despite resolution of the outward signs of tissue damage. CRPS II has similar symptoms but develops after an injury to a specific nerve.
| Category | Description | Prior Name |
|---|---|---|
| CRPS I | A nonspecific and inappropriate pain response occurring after trauma | Reflex Sympathetic Dystrophy (RSD) |
| CRPS II | An inappropriate pain response after an injury to a specific nerve | Causalgia |
To understand this pathologic entity thoroughly, one must become familiar with a new vocabulary of terms used to describe altered sensations ( Box 20-1 ). Acute nociceptive pain is the normal protective physiologic response to adverse chemical, mechanical, or thermal injury and is what most of us think of as “normal pain.” The physical trauma starts a routine cascade of chemical releases, mediated by prostaglandins, converting nociceptors in the region from high to low threshold, leading to peripheral sensitization and hyperalgesia at the damaged site. Repeated unrelieved peripheral firing causes central sensitization and hyperalgesia and allodynia distant to the damaged site. Appropriate analgesic treatment usually halts this morbid progression. In some patients, however, central sensitization continues, neuronal pathways physiologically modify (plastic change), and acute pain is converted to chronic neuropathic pain.
Allodynia: Pain from a stimulus that does not normally evoke pain. Diabetic patients with neuropathy complain of numb extremities, yet their feet hurt when touched by a blanket or sheet.
Anesthesia dolorosa: Sensitivity to touch is absent while severe pain is present in an anesthetic area.
Hyperalgesia: Exaggerated response to a normally painful stimulus.
Dysesthesia: Unpleasant abnormal sensation, either spontaneous or evoked, that is not described as painful.
Hyperpathia: Painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased sensitivity. The pain usually radiates and persists after the stimulus, often with an abnormal delay between stimulus onset and sensation onset.
Hypoalgesia: Diminished pain in response to normally painful stimulus.
Hypoesthesia: Abnormally decreased sensitivity to stimulation, excluding the special senses.
Paresthesia: Abnormal sensation, either spontaneous or evoked.
Nociceptive pain : Pain caused by activity in the neural pathways in response to potentially tissue-damaging stimuli. Some examples are arthritis pain, sickle cell crisis pain, postoperative pain, and mechanical low back pain.
Neuropathic pain : Pain initiated or caused by a primary lesion or dysfunction in the nervous system. Some examples are complex regional pain syndrome, postherpetic neuralgia, and diabetic neuropathy.
The diagnostic criteria for CRPS are outlined in Table 20-2 . Onset may occur immediately (in the recovery room) or days to weeks after the initial trauma but typically is present within 1 month of the noxious event. Classic stages of RSD are no longer used to classify the progressive development of the disease. Unilateral extremity involvement is most common. Inciting causes can include athletic injury (fracture, sprain, dislocation), fasciitis, tendinitis, surgical procedure, peripheral nerve injury, immobilization in a cast or splint, infection, deep venous thrombosis, malignancy, vasculitis, herpes zoster, polymyalgia rheumatica, and cerebral vascular accident.
| EACH OF THE FOLLOWING: |
|
| CATEGORIES: |
|
Pain in CRPS, present in more than 90% of patients, goes beyond the beneficial aspect of pain (fight-or-flight response) and is pathologic. The pain is often deep, spontaneous, burning, and diffuse and can be triggered by minimal stimulation. Patients may actively avoid clinical examination and appreciate having the examination demonstrated in the unaffected limb. They often describe some combination of the skin feeling raw, an electric-like shooting pain, or a deep, dull bone ache. Cutaneous and deep hyperpathia are typically found. Allodynia is present, depending on the time interval from the initiating event. The pain may be sympathetically maintained, sympathetically independent, or a combination of the two.
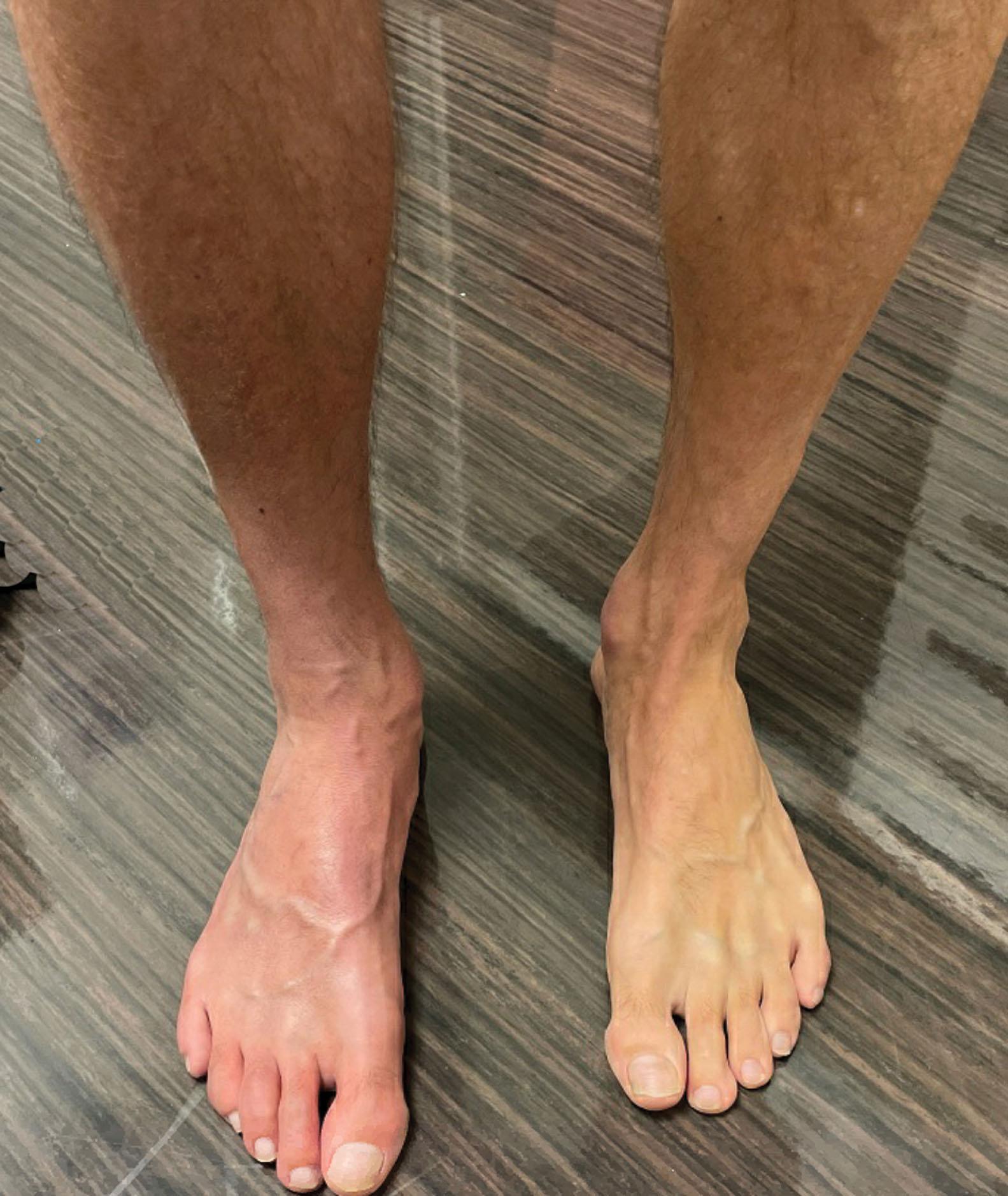
Early autonomic changes consist of swelling (edema), abnormal vasodilation, skin warming, and changes in sweating pattern (e.g., hyperhidrosis or hypohidrosis) (see Fig. 20-1 ). Classic teaching is that early hyperemia gives way to a cool, hypo-perfused limb in chronic CRPS, though this pattern has been disproven. Instead, both vasodilation or vasoconstriction are possible in both the early and the late chronic of CRPS.
Motor changes demonstrate decreased active range of motion, weakness, and increased physiologic tremor (dystonia) of the distal extremity. There may be difficulty in initiating motion.
Sensory changes include hypoesthesia, hyperesthesia, allodynia, and anesthesia dolorosa (see Box 20-1 ).
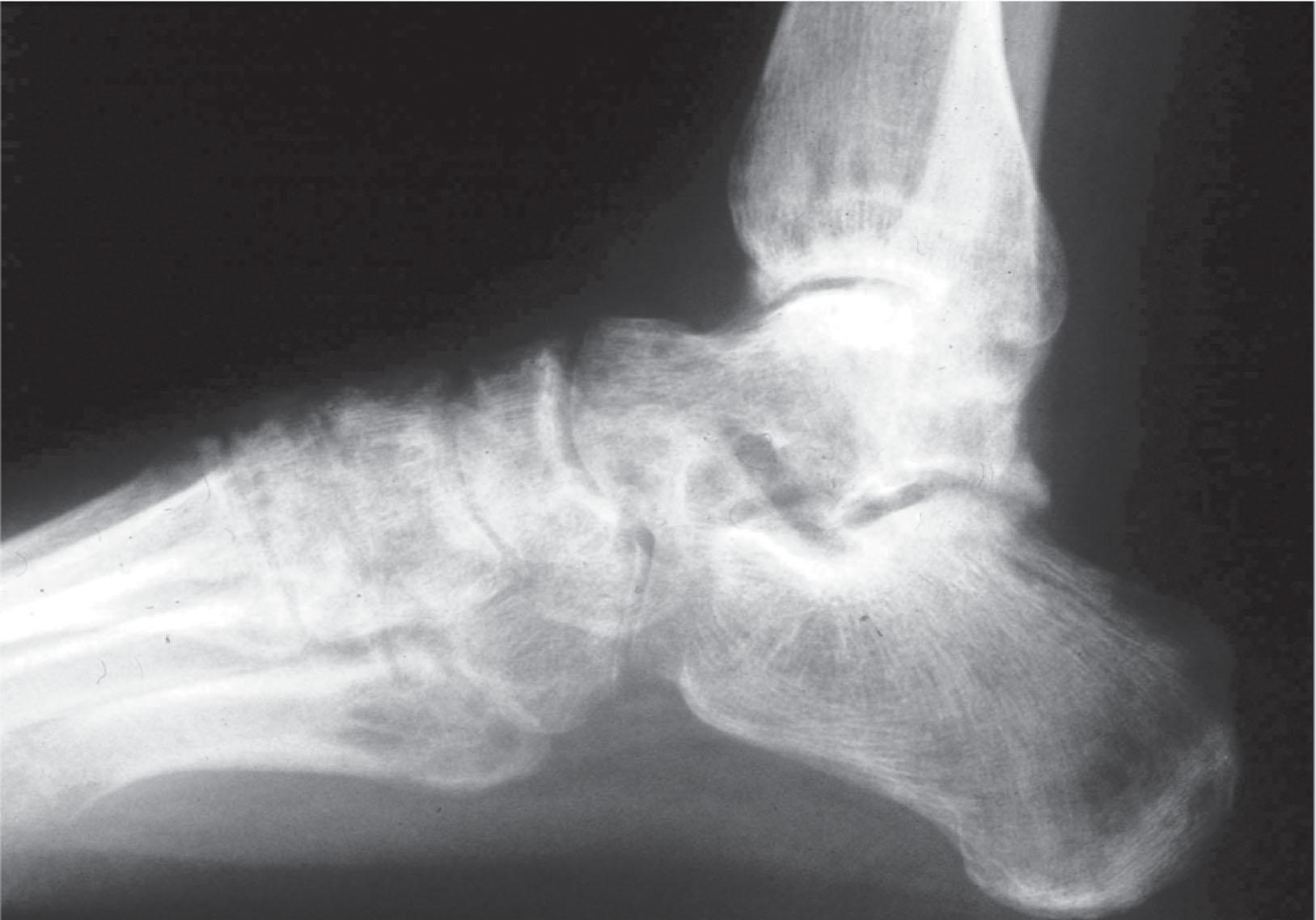
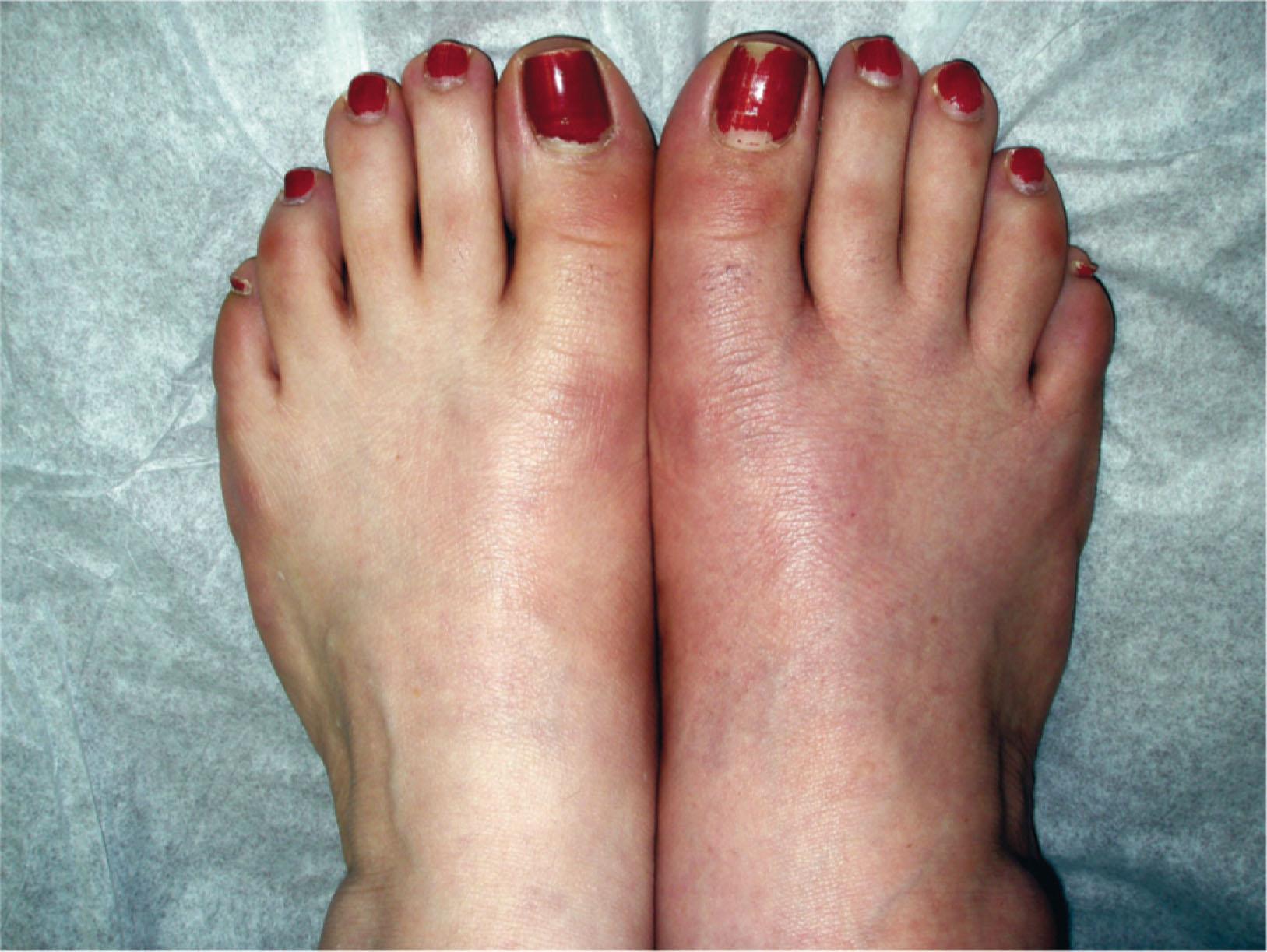
Trophic changes (e.g., shiny skin, nail changes, lack of hair growth, contractures, muscle atrophy, osteoporosis, and cool, pale skin) are late findings (see Fig. 20-2 ). With a full-blown syndrome there is distal extremity pain, diffuse swelling, smooth, shiny skin, and abnormal color and temperature. Allodynia and joint pain are present. Juxtaarticular osteoporosis is seen on radiographs, and increased uptake on a three-phase bone scan may be demonstrated.
Patients may present with varying degrees of these symptoms, suggesting patients with CRPS may be divided into various phenotypes that can help guide treatment. At times, neuropathic pain and sensory abnormalities predominate, while for others, vasomotor disturbances are the predominant symptom. Vasomotor changes may manifest as a warm, edematous, sweaty leg or a cool, pale, or blue-tinged limb. Based on these observations, patients may be grouped into a warm or cool presentation. The phenotypical presentation may help guide treatment based on suspected etiology of the predominant symptoms.
Early diagnosis is possible but not frequent. Diagnosis is frequently delayed for 6 to 12 months despite prolonged symptoms. The patient has frequently seen numerous providers and is understandably frustrated, depressed, or angry. The diagnosis of CRPS is excluded when these symptoms can be explained by another condition.
CRPS I has a very complicated pathophysiology at several integrated levels along the entire neurologic pathway. Changes involve the somatosensory, sympathetic, and sudomotor systems, which result in an imbalance between inhibitory and excitatory nerve function (see Fig. 20-3 ). Nerve biochemistry is altered, rendering them more sensitive and abnormally responsive to stimuli. Normal regulatory mechanisms become impaired. At the periphery, nerves become sensitized, spontaneous ectopic discharges become common, and axonal sprouting and degeneration occur simultaneously. Centrally, there is increased sensitivity, disinhibition, and reorganization of synaptic connectivity. This response is unpredictable and varies in different patients. If left untreated, irreversible anatomic and physiologic changes can occur, and symptoms can continue for years.
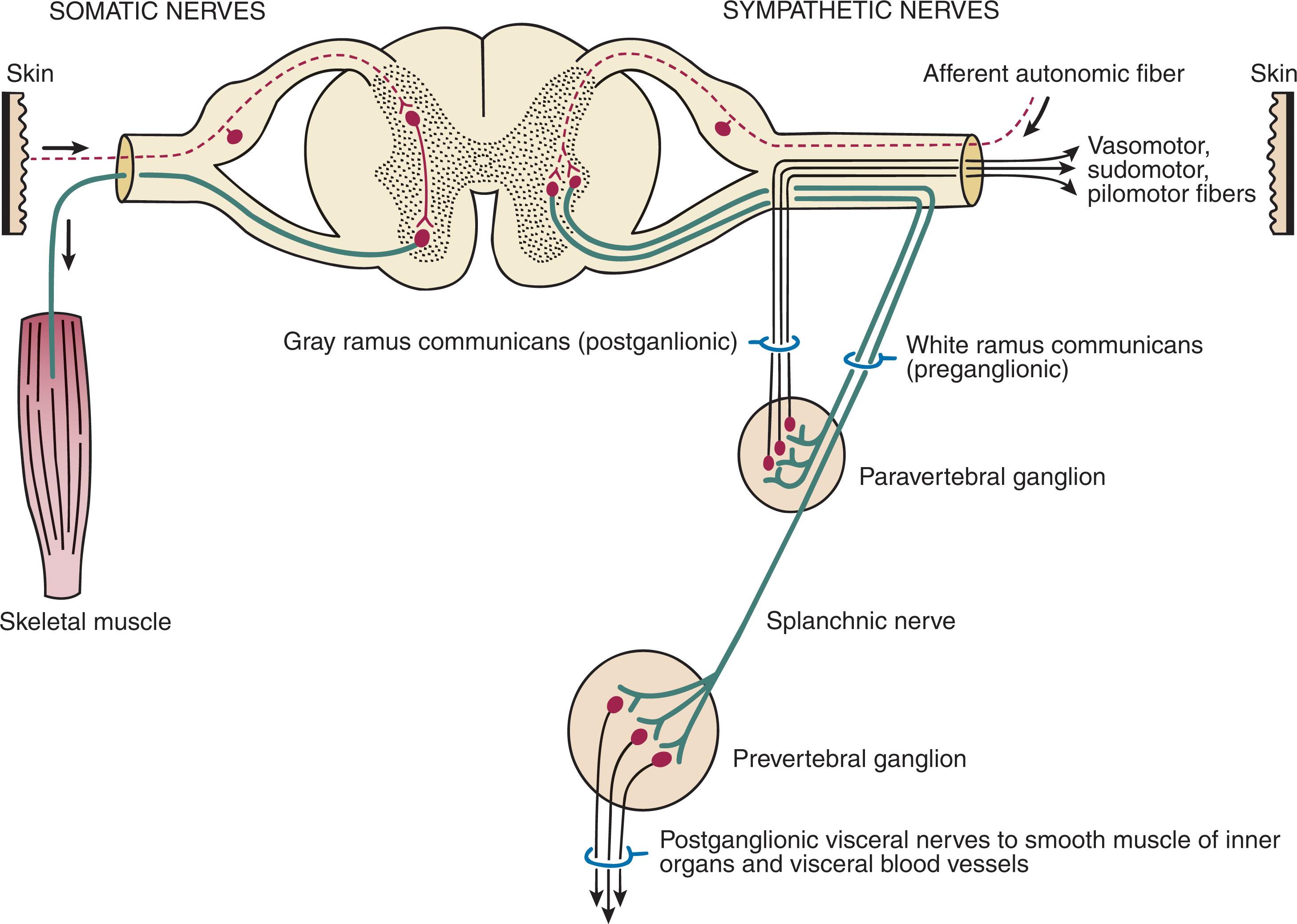
In the neuropathic state, peripheral areas of the nervous system not normally responsible for pain transmission are altered to aberrantly conduct pain. A-β fibers, normally pathways for touch, start to sprout and grow into the C-fiber terminals, becoming fast transmission conduits for pain. Nerves outside the main area of injury may be recruited, thus explaining the late development of pain in peripheral, non-injured anatomy.
With chronic central sensitization, afferent sensory information is relayed to the cerebral cortex via the thalamus. The chemicals released from the damaged tissue (histamine, substance P, bradykinins, prostaglandins, serotonin, acetylcholine) centrally and peripherally remain elevated, and neuronal plasticity develops. At this point, the structure, function, and biochemical profile of nerves actually modify.
In CRPS, the balance of glutamate (an excitatory neurotransmitter) and γ-aminobutyric acid (GABA) (an inhibitory neurotransmitter) becomes unstable. Phosphorylation of the neuropeptide N -methyl- d -aspartate receptor (NMDA) is overactivated by the glutamate and causes increased release of calcium and potassium ions, further sensitizing the NMDA receptor. The α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors also become more active.
In addition, under these circumstances, increased substance P (a vasoactive neuropeptide causing vasodilation and extravasation) is released, and pain is processed at lower thresholds. Upregulation of sodium channels propagates nerve conduction, and the balance between inhibition and excitation is lost. This amplified response to sensory input results in allodynia.
Subsequent to these biomechanical aberrations, abnormal feedback mechanisms develop. Normally dormant genes that encode ion channels, receptors, and neurotransmitters are activated, causing sympathetic tone to persist inappropriately. With chronic pain, cortical reorganization occurs in the central nervous system (CNS), increasing representation of the affected extremity.
The autonomic nervous system controls thermoregulatory and nutritional blood flow. Inappropriate arteriovenous shunting and abnormal receptor input can cause vasomotor, temperature, and trophic changes. A hot, swollen foot and a cold, ischemic, stiff foot can both be explained by this mechanism (see Fig. 20-1 ).
CRPS type II (causalgia) is a pain syndrome that develops after a distinct peripheral nerve injury. The trauma releases neurogenic inflammatory mediators, causing spontaneous pain associated with allodynia. The sensory deficit is usually limited to the territory of the nerve but tends to spread.
Skin blood flow and sweating abnormalities are also usually restricted to the nerve territory. Motor function deficits are explained by disruption of motor axons. Swelling and trophic changes are discrete.
Sympathetically maintained pain (SMP) or sympathetically independent pain (SIP) may be a component of either syndrome, and symptoms of CRPS I and CRPS II may be present in the same patient. Animal research using sympathetic blocks, α-adrenergic receptor agonists, and electrical stimulation suggests that sympathetic nervous activity can be altered to produce pain. In the diabetic patient, the neuropathic painful foot has selective sympathetic denervation, but the neuropathic painless foot does not. The degree of pain is altered by the intensity and ongoing nature of the stimulus, afferent input, efferent modulation, CNS interpretation, and physiologic adaptation.
Evidence indicates that true CRPS I or II is not psychogenic. No personality disorder is specific to CRPS. Severe pain from the disease complex is the direct cause of the psychologic suffering, not the converse. One can often see anxiety, hopelessness, anger, and depression in the patient. Other factors, such as fatigue, emotional state, stress, gender, and culture, also affect pain transmission.
The response to CRPS, however, is attenuated or enhanced by psychosocial factors. Pain catastrophizing is associated with poor outcomes. In particular, a higher pain catastrophizing score is associated with greater disability, greater pain at rest (PAR), and greater pain with movement.
In our litigious society, it is also imperative to determine whether legal issues or vocational disability are involved. Obviously, there are situations in which litigation and workers compensation claims influences the way a patient responds to treatment or documents symptoms.
CRPS is still a diagnosis of exclusion, and the clinician must maintain a high index of suspicion for underlying sources of pain. Such patients are often difficult to deal with and usually try the clinician’s patience. The evaluation is intense; the patient is often anxious, frustrated, and occasionally angry. The importance of a complete history and physical examination cannot be underscored but understandably stretches the time constraints of a busy clinical practice.
Florid cases are easy to diagnose, but most cases are confusing; many patients present with only one or two of the classic symptoms involving pain of unknown cause. No two people with this disease state have the exact same disorder. Even with the assistance of myriad scientific tests, the physician’s role here is classic: listening, reassuring, and relieving suffering.
The prevalence of CPRS is incompletely understood because the process is often misdiagnosed, and many cases go unreported. It has been reported to be between 5.5 and 26.2 per 100,000 people. Demo-graphic studies indicate the frequency of CRPS is three times greater in women than men.
It is strongly associated with a history of cigarette smoking, suggesting a possible presensitization of receptor sites. It has been suggested that the incidence of CRPS I is 1% to 2% after fracture and 2% to 5% after peripheral nerve injuries. CRPS diagnosis by strict criteria was found in 0.3% of 306 prospective followed ankle fractures in a multi-center study, though it was noted many more patients displayed some symptoms. CRPS II (causalgia) has an incidence of 1% to 5% after injury to a peripheral nerve. It appears in all age groups but is much less common in children younger than 10 years.
Genetics also might play a role, with the possibility that CRPS is a neuroimmune disorder linked to multiple sclerosis, rheumatoid arthritis, and narcolepsy. Prompt recognition can prevent unnecessary investigations and lead to treatment that will shorten the course of the disease.
The differential diagnosis is shown in Box 20-2 . Erythromelalgia is a poorly understood clinical syndrome manifesting as hot, red, painful extremities, usually affecting the lower limbs. It is classified as primary (idiopathic) or secondary, following myeloproliferative syndrome–related thrombocytopenia. Cold, elevation, and a daily dose of aspirin usually provide relief (see Fig. 20-4 ).
Chronic pain syndrome
Compartment syndrome
Diabetic neuropathy
Erythromelalgia
Fibromyalgia
Hysteria
Ischemic monomelic neuropathy
Malingering, worker’s compensation
Myofascial pain
Lyme disease neuropathy
Medicolegal status of the condition
Personal family or sexual conflict
Spasticity
Tarsal tunnel syndrome
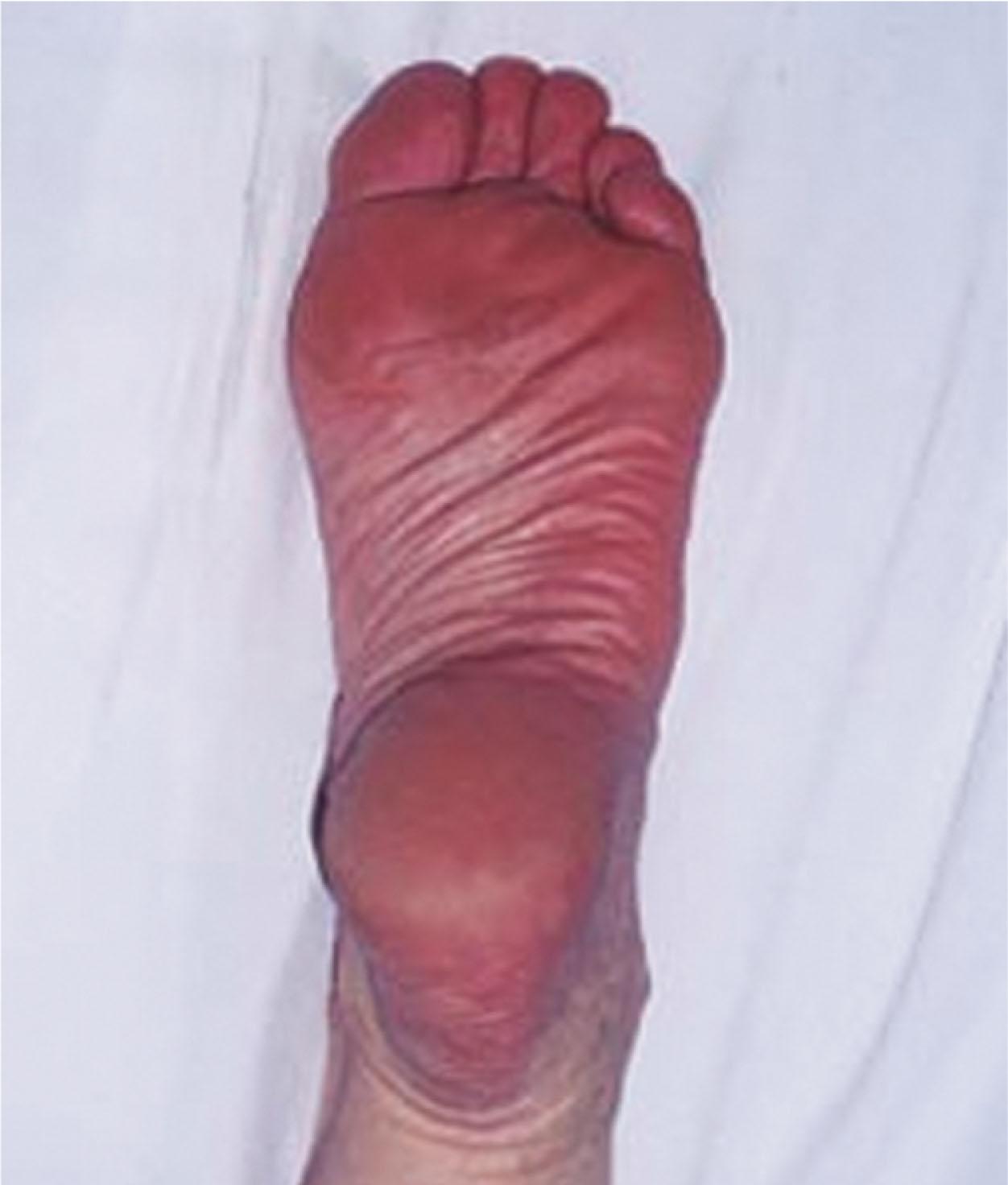
The clinician should always consider CRPS when the injury seems minor and the physical exam findings benign, despite pain so intense that a patient may be erroneously judged to be hypochondriacal or emotional. This will cue one to examine the patient more closely for signs of CRPS such as autonomic dysregulation.
Sensory examination is important to assess the location and distribution of the neuropathic pain and to determine if it involves a single or multiple dermatomes. One must initially establish if there is allodynia (mechanical and thermal), hyperalgesia (mechanical and thermal), paresthesias, or any other pathologic neurologic signs (see Fig. 20-5 ).
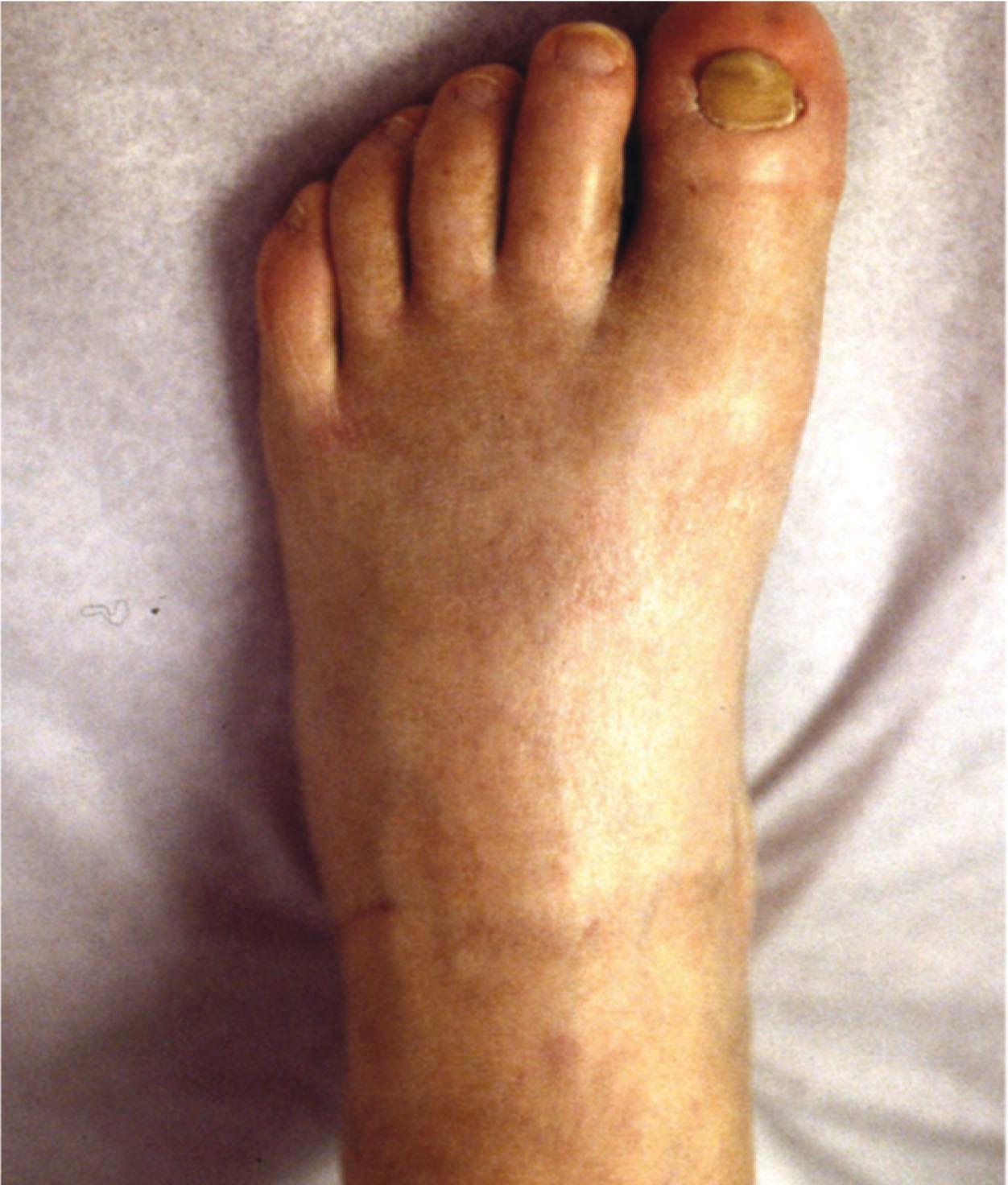
Motor evaluation must assess atrophy, muscle strength, joint motion and stability, gait, and coordination. Some patients have edema with decreased range of motion, and the involved extremity may be warmer or colder, with or without hyperhidrosis. Changes in nail and hair growth patterns are commonly observed and should be looked for. Asymmetry of these symptoms should be noted by examining the opposite limb. In one study of patients with CRPS, discoloration of the skin was reported in 91%, altered skin temperature in 92%, edema in 69%, and decreased range of motion in 88% of patients (see Fig. 20-6 ).
The area of the body that has been exposed to chronic pain will gradually decondition. Normal symmetric coordinated motion ceases. Immobility leads to obesity, which further overloads the painful site. To compensate, the patient overuses adjacent normal areas. Further arthropathies and myopathies develop. A progression from cane to walker to wheelchair is not uncommon.
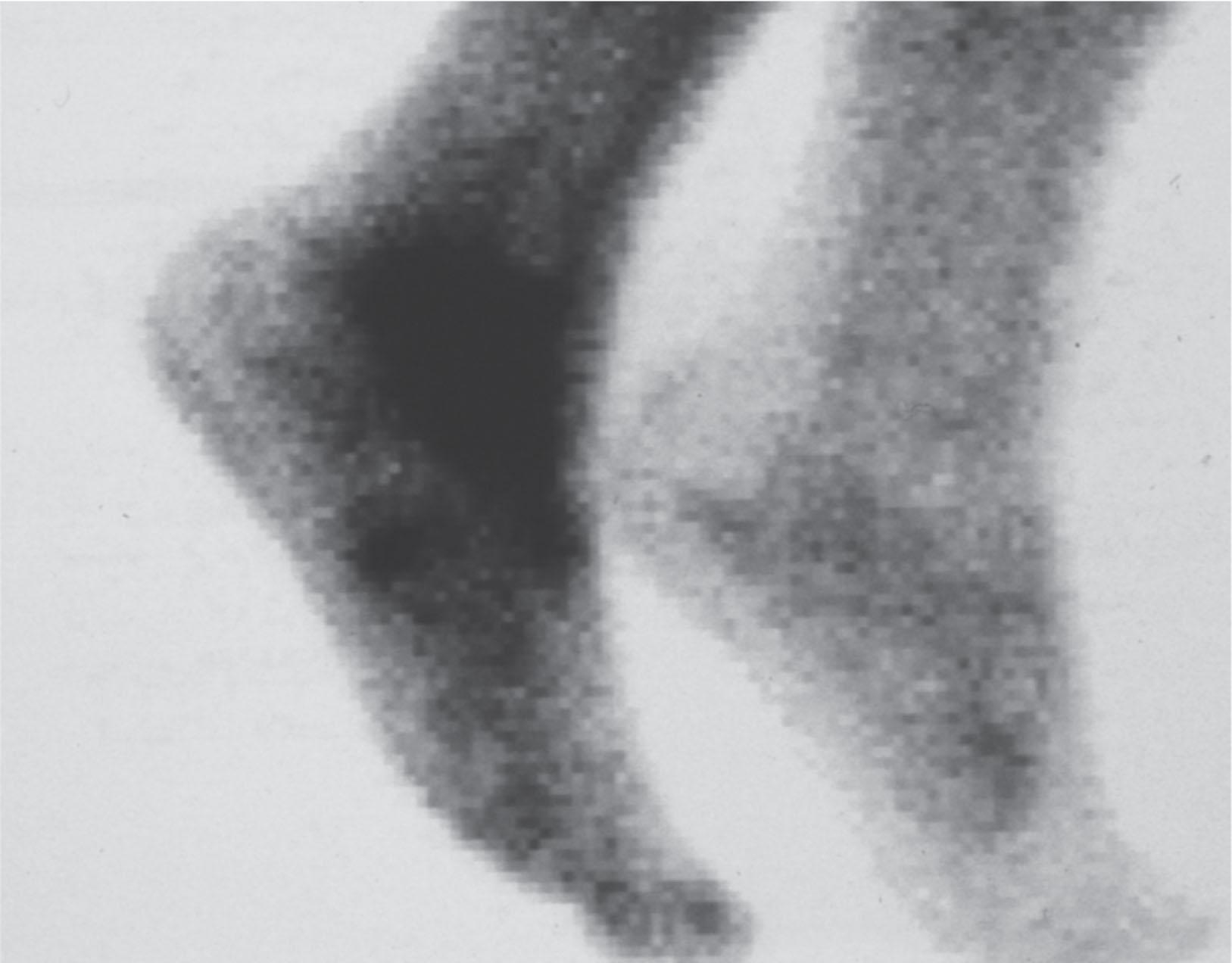
Although clinical evaluation is the mainstay of diagnosis, a three-phase technetium bone scan may be the best imaging modality for the disease. Diffuse increased tracer uptake in the delayed phase (phase 3) is said to demonstrate increased bone metabolism (see Fig. 20-7 ), while phases 1 and 2 are not specific for CRPS. Bone scan is reported to be 96% sensitive and 98% specific; other studies have shown it to be only 44% sensitive. Localized uptake might give clues to an underlying precipitating injury. For CRPS type I, the test seems to be more reliable when done within 6 months of the onset of symptoms and in patients older than 50 years. A negative bone scan in the early phases of the disease is quite common.
More recently, [18 F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) scan in association with magnetic resonance imaging (MRI) scan have been used to increased metabolic activity, likely related to inflammatory response associated with CRPS, which is not seen on MRI alone.
While there are no laboratory markers specific for CRPS, generalized inflammation and immune system activation may be followed. Serum levels of soluble IL-2 receptor (sIL-2R), which is a surrogate marker for T-cell activation, are used by some to assess the contribution of immune dysregulation to CRPS, as well as to track response to treatment with immune modulatory agents.
Heat, cold, and touch sensitivity can be assessed with a quantitative thermal stimulator, von Frey hairs, or electrical stimulation ( Table 20-3 ).
| Symptom | Technique |
|---|---|
| Dynamic mechanical allodynia | Stroking with brush, gauze, or cotton applicator; hair movement; vibration by tuning fork |
| Static mechanical allodynia | Mechanical light pressure |
| Mechanical hyperalgesia |
|
| Mechanical summation | Apply dynamic stimuli every 2–3 sec, 3–6 times |
| Heat allodynia | Contact with objects kept immersed in 40°C–42°C (104°F–107.6° F) water |
| Heat hyperalgesia | Contact with objects kept immersed in 45°C–47°C (113°F–116.6° F) water (feels weakly painful to the clinician) |
| Cold allodynia | Contact with room-temperature metal object, with refrigerated object, and with coolants |
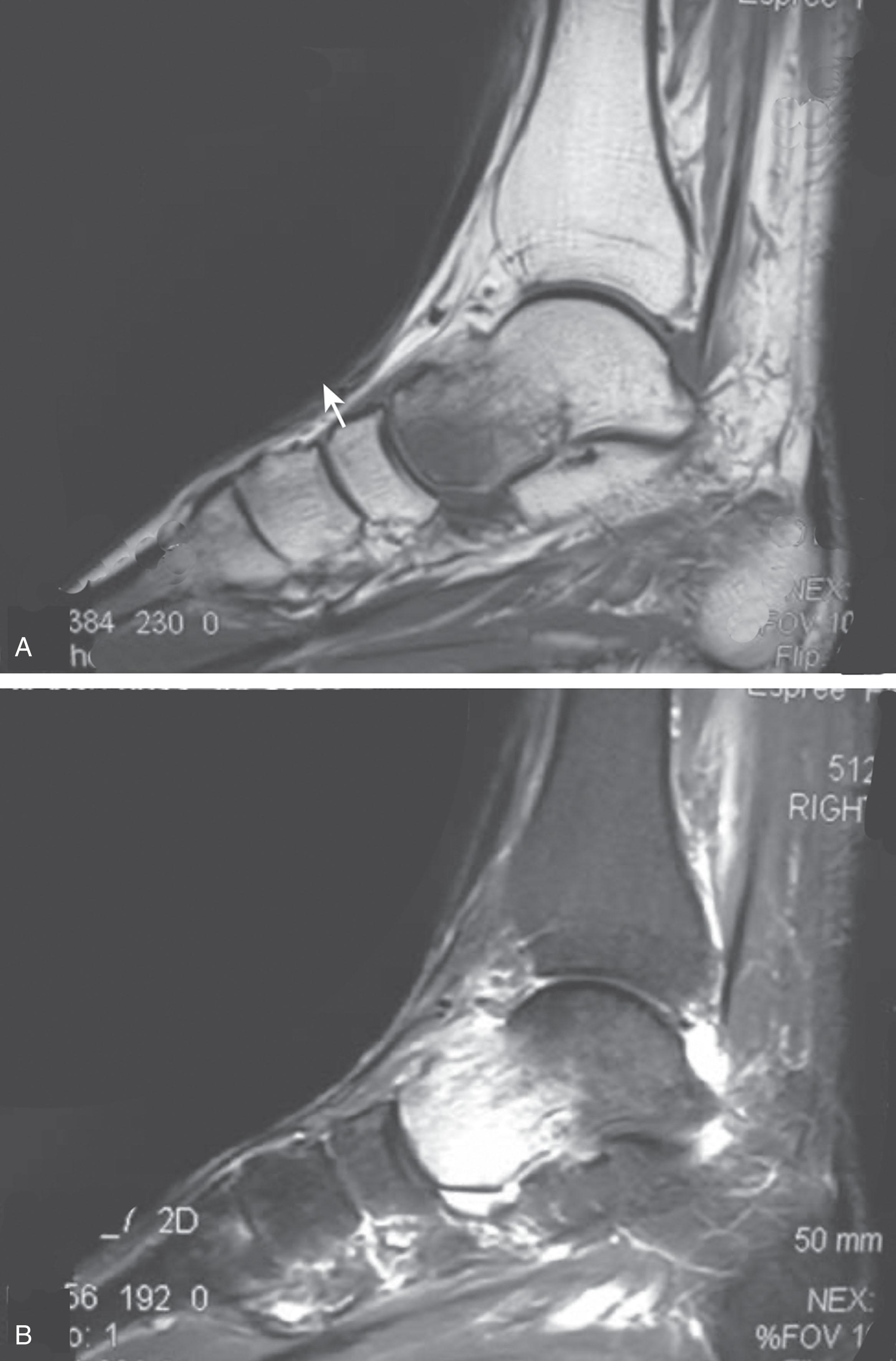
Plain radiographs and computed tomography (CT) are not specific. Plain radiographs can show patchy demineralization at 3 weeks, usually more than would be expected from disuse alone, perhaps related to autonomic dysfunction and increase blood flow. MRI can show bone marrow stress reaction earlier than the bone scan (see Fig. 20-8 ).
Attempts to block α-adrenergic receptor activity are not entirely reliable or reproducible and may be fraught with the problems of placebo effect and false-positive results. Despite these limitations, response to the block indicates that SMP is involved. Use of blocks may therefore be useful for establishing the diagnosis of CRPS and planning treatment ( Table 20-4 ).
| Technique | Advantages | Disadvantages |
|---|---|---|
| Lumbar ganglion block |
|
|
| Epidural local anesthetic | Allows access to torso innervation |
|
The IASP and others no longer regard differential spinal blockade to be reliable. At best, it can be considered a screening test of sympathetic function. Nerve blocks must be evaluated in terms of the entire clinical picture (e.g., decreased pain, temperature increase of 3° F or more, venous engorgement, change in skin color). Therefore sympatholysis, whether pharmacologic or by nerve conduction block, is useful in disease determination. Pain conditions without any response to sympathetic block are defined, by contrast, as SIP states.
Sudomotor asymmetry (resting or evoked) is thought to be significant. The quantitative sudomotor axon reflex test assesses for sweating abnormalities.
Isolated cold stress testing (cold stressor test), combined with thermographic imaging, can measure total digital (great toe) blood flow and microvascular perfusion. The procedure combines a stressor (refrigerated environment) with a measurement technique and can provide accurate assessment of autonomic and vasomotor stability and the effectiveness of sympathetic blocks. Limb surface temperature differential of 1° F or more is thought to be significant (see Fig. 20-9 ).
Laser Doppler flowmetry, with appropriate stressors, can monitor background segmental vasomotor control. It is fast, noninvasive, painless, and relatively free of artifact interference.
Electromyographic (EMG) and nerve conduction velocity (NCV) testing should be done together if an underlying discrete nerve lesion is suspected. This is critical to the diagnosis of CRPS II. Unfortunately, insurers might rule out this diagnosis because results of these studies are negative. These tests measure large-fiber disease. The critical distinction to make is that much of neuropathic pain is small-fiber (C-fiber)–mediated pain and that small-fiber abnormalities are not well tested with EMG. Therefore EMG/NCV testing has some internal inconsistencies.
Quantitative sensory testing is used to objectively quantify perception thresholds. Precise reproducible stimuli allow comparisons between symptomatic and asymptomatic areas. Skin biopsies are being performed at selected laboratories around the country that actually show damage to the neural elements themselves in affected patients. Their clinical role is unclear.
The main goals of patients undergoing treatment for CRPS are to diminish the exaggerated pain symptoms, reduce reliance on medication, and normalize the functioning of the extremity. Early recognition and urgent, assertive treatment are critical, yet undertreatment of CRPS still persists. Estimates are that half of the patients untreated in the first year have significant residual effects and risk chronicity.
Initially, CRPS I and CRPS II can manifest with the same symptoms. Physical therapy, sympathetic blocks, pharmacologic agents, and repeated careful clinical examinations are the foundations of treatment. A clinician treating this condition must take control of care management, beware of the patient’s tendency to doctor shop, look out for many different physicians prescribing medications concurrently, and look carefully at the literature.
Initial treatment typically consists of physical therapy, desensitization modalities, acetaminophen with or without nonsteroidal antiinflammatories, and possibly a low dose opiate. Second-line treatments to consider are based on the patient’s phenotypical presentation and may consist of tricyclic medications and/or gabapentin or pregabalin for nerve dysregulation, baclofen for muscle dysregulation, and calcium channel blockers for vasomotor dysregulation. Referral to a pain management center specializing in CRPS may be considered for sympathetic blockade or higher risk medical or interventional treatments.
If CRPS is diagnosed early, intense physical therapy is initiated (desensitization; heat; whirlpool, gentle progressive range of motion), in conjunction with nonsteroidal antiinflammatory drugs (NSAIDs) and mild analgesics. The goal of pain reduction is to facilitate function and improve the quality of life. These patients usually have intolerance to cold, and moist heat is effective.
When using physiotherapeutic maneuvers, it is best to apply joint movement in the affected region judiciously to reduce the mechanoreceptor barrage and its increase in perceived pain. The therapist should begin with gentle gliding exercises. One should emphasize to the therapists, even by including it in the prescription, that forcing patients beyond their tolerance can worsen the syndrome.
Recreational therapy should also be part of the program. Have the patient take part in previously lost or new pleasurable activity. This increases mobility, while the socialization helps work on the often-associated depression.
Desensitization uses a variety of stimulation methods to encourage appropriate firing of nerves, downregulating sensitivity, reducing aberrant nerve activation, and retraining the nerves to respond appropriately to nonnoxious stimuli. Graded exposure to stimulation and activity reduces pain, pain-related fear, disability, and improves psychological tolerance. Combinations of light touch, deep pressure, heat, and cold, using intensities that would be pleasing to the unaffected limb, are applied to the affected limb. Capsaicin cream can be used to stimulate pain receptors using the same premise.
The patient with CRPS frequently avoids the affected limb, at times not even wanting to look at it. Using desensitization and graded exposure strategies encourages the patient to re-engage with their limb in a constructive and productive way, regaining some control over an otherwise hopeless situation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here