Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Colorectal cancer (CRC) development is a complex process. Causal agents and mechanisms include environmental and dietary factors and inherited and somatic mutations. Great progress has been made over the past 30 years toward defining the constellation of molecular alterations contributing to colorectal tumor development. Specific oncogene and tumor suppressor gene defects have been identified in tumors of various stages, with oncogenes being broadly defined as those genes affected by gain-of-function alterations in cancer, and tumor suppressor genes as the genes affected by loss-of-function alterations. Oncogenic activation of proto-oncogenes can result from many different mechanisms, including specific point mutations and rearrangements that alter gene structure and function, as well as from chromosomal rearrangements and gene amplifications that disrupt the regulated expression of the proto-oncogene. Tumor suppressor gene inactivation can also result from many different mechanisms, including localized mutations, such as nonsense or frameshift mutations, or complete loss of the gene. Besides mutational mechanisms, it is now clear that changes solely in the expression but not the structure or sequence of proto-oncogenes and tumor suppressor genes can lead to lead to oncogene activation or tumor suppressor gene inactivation. Although many proto-oncogenes and tumor suppressor genes encode proteins, they can also encode various noncoding RNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). Thus the definitions of oncogene or tumor suppressor gene are essentially agnostic with respect to the function of the gene in the cell, but instead refer to whether the resultant genetic or epigenetic defect in a given gene leads to increased or novel gene function (i.e., oncogene) or loss of gene function (i.e., tumor suppressor gene). Finally, although the vast majority of gene defects found in colorectal cancer are somatic, inherited mutations in selected tumor suppressor genes have a critical role in a number of CRC predisposition syndromes.
The principal objectives of this chapter are to review the following topics: (1) the epidemiology of CRC; (2) the sequence of histopathologic alterations in the course of the progression to malignancy; (3) the genetic basis of various inherited CRC syndromes and the relevance of certain inherited syndromes to sporadic CRC development; (4) somatic oncogene and tumor suppressor gene defects and epigenetic changes in CRC; (5) some of the apparent variations in gene defects associated with different precursor lesions at risk of progressing to CRC; and (6) the potential clinical utility of the genetic alterations in early detection and clinical management of patients and families affected by CRC.
CRC is the second leading cause of cancer deaths in the United States. In 2013, it is anticipated that nearly 143,000 individuals in the United States will be diagnosed with colon or rectal cancer, and about 51,000 will die from the disease. Male and female incidence and survival are very similar. Important differences in prognosis have been observed in different racial groups in the United States. These differences may reflect a combination of factors, including differences in access and adherence to screening tests, tumor location and size at diagnosis, and appropriateness of treatment. At this point, there is less evidence that differences in molecular alterations among tumors (intertumoral genetic and epigenetic heterogeneity) have a major contributing role in accounting for differences in prognosis among racial groups, though further work is needed.
Most cases of CRC are considered to be sporadic, indicating that clear-cut familial or genetic predisposition factors are not readily apparent. However, based in part on family studies (i.e., twin and kindred studies), it is estimated that more than 25% to 30% of CRC cases may have a hereditary component. Less than a fourth of this collection of cases with a presumptive hereditary component (i.e., less than 5% of all cases) occur in a setting with a family history and/or clinical features indicative of a highly penetrant, single-gene-mutation cancer syndrome predisposing to CRC development. Gene alterations or DNA sequence variations with key contributing roles in most familial cases remain to be defined. The familial cases may be a heterogeneous group, in which modest to moderate predisposition to CRC is possibly conferred by an undetermined number of potentially common or rare genetic variations.
In addition to family history, other risk factors for CRC include older age, chronic inflammatory bowel disease, heavy alcohol use, and diets rich in unsaturated fats and red meat and refined starches and low in fruits and vegetables. Evidence for a protective effect against CRC for aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) as well as hormone supplementation in postmenopausal women has been offered, albeit with attendant risks for these agents. An unfortunate reality is that many of the key dietary, lifestyle, and environmental factors contributing to CRC in the United States and other Western countries are uncertain, and the majority of incident CRCs arise in people with no well-defined risk factor. On a more optimistic note, a large fraction of CRC deaths seem to be preventable by early detection. By some estimates, improved adoption and implementation of current screening recommendations for colorectal cancer could save thousands of lives per year.
The surface of the large intestine is covered by an epithelium, characterized by finger-like invaginations into the underlying stroma; the invaginations are termed crypts . Stem cells near the base of each crypt give rise to distinct cell types required for the intestine’s resorptive, secretory, and endocrine functions. During their differentiation, epithelial cells migrate from the base of the crypt to the surface, and the cells are ultimately shed into the gut lumen. With the exception of the stem cells, the colon epithelium is turned over within a few days.
Among the different types of benign gastrointestinal lesions, a generic term for any localized lesion protruding above the surrounding mucosal surface is polyp . Many polyps, particularly small polyps 5 mm or less in size, are of the hyperplastic type, with characteristic serrated glands and distended goblet (mucus-producing) cells. However, although the role of certain serrated polyp lesions other than hyperplastic polyps in CRC development is discussed later, the adenomatous polyp or adenoma is believed to be the precursor lesion to the vast majority of CRCs. Both gross and histopathologic features can be used to distinguish adenomas. Grossly, the size is measured and the morphology can be described as pedunculated (with a stalk), sessile (flat), or semisessile. Among the histopathologic features, the degree of dysplasia and glandular architecture are used to distinguish lesions, and these features may have value for predicting the likelihood that a lesion contains a focus of cancer or its risk of progression to cancer.
The proposal that many CRCs arise from adenomatous lesions is supported by at least three lines of evidence. First, a few longitudinal studies have assessed the risk of subsequent CRC development in individuals with adenomatous polyps. In these studies it became apparent that patients who did not have their adenomas removed had an approximately eightfold increased risk of CRC compared with the group that had their adenomas removed. Notably, after polypectomy, patients did not show a clear increase in CRC incidence in comparison with a control group without adenomas, suggesting that adenomatous polyp removal had a therapeutic effect. Second, histopathologic studies have shown that foci of carcinoma can often be detected in adenomatous polyps, particularly those with increased size, dysplasia, and villous histopathology. Third, individuals affected by syndromes that strongly predispose to the development of hundreds of adenomas, such as familial adenomatous polyposis (discussed later), invariably develop colorectal cancers by the third to fifth decades of life, if their colons are not removed.
Although adenomatous polyps as a group are well established to have a risk of progression to cancer, some studies have suggested that patients who develop hyperplastic polyps may have an increased risk of adenomas. Also, patients with numerous hyperplastic polyps (e.g., patients with juvenile hyperplastic polyposis syndrome) have a clearly increased risk of CRC. As suggested previously and as discussed further later, it has been increasingly appreciated that certain polypoid lesions—such as “sessile serrated adenomas,” which share some serrated morphologic features with hyperplastic polyps—do indeed show an increased cancer risk. Most carcinomas arising from sessile serrated adenomas seem to be associated with distinct molecular defects that are discussed later (e.g., CpG island methylation phenotype, CIMP).
At least two other pathways leading to CRC and that are not associated with overt development of adenomatous polyps as precursors can be noted: chronic inflammatory bowel diseases (particularly ulcerative colitis [UC] and to a lesser extent Crohn disease) and flat adenoma syndromes. UC is a chronic inflammatory disease of largely unknown etiology. The possible precursor lesions to cancer in patients with UC include dysplasia and flat adenomatous plaques. In a subset of patients with hereditary cancer syndromes, as well as some sporadic cases, colon cancer seems to develop directly from flat adenoma or intraepithelial dysplasia. In these cases the progression from benign lesion to overt carcinoma may be more rapid than in the normal adenoma-carcinoma progression.
It is now generally accepted that the accumulation of somatic mutations and epigenetic defects drives the initiation and progression of tumor development. The constellation of somatic mutations that commonly accumulate in a clonal fashion in CRCs has largely been defined. However, only a limited subset of this collection of gene defects is usually present in any individual tumor. Thus far, the somatic mutations of greatest initial interest to the field have been those that are recurrent and clonal (i.e., present in all, or nearly all, neoplastic cells of a primary tumor at a given stage, but not present in the normal cells of the patient). It is inferred that such recurrent, clonal mutations are causal in promoting further tumor outgrowth/progression, because somatic mutations can only become clonal by a limited number of mechanisms. The genetic alteration itself could have been selected for because it provided the cell with a growth advantage, allowing it to become the predominant cell type in the tumor (clonal expansion). Alternatively, the specific alteration detected might have arisen coincident with another, perhaps undetected, alteration that was the crucial change underlying clonal outgrowth.
As noted previously, only about 5% of all CRC cases are associated with defined highly penetrant cancer syndromes. The bulk of these cases are attributable to hereditary nonpolyposis colorectal cancer syndromes (HNPCCs), with another significant subset associated with the familial adenomatous polyposis (FAP) syndrome ( Table 34-1 ). A few other syndromes comprise the remaining highly penetrant CRC syndrome cases.
| Syndrome | Features Commonly Seen in Affected Individuals | Gene Defect |
|---|---|---|
| Familial adenomatous polyposis (FAP) | Multiple adenomatous polyps (>100) and carcinomas of the colon and rectum; duodenal polyps and carcinomas; fundic gland polyps in the stomach; congenital hypertrophy of retinal pigment epithelium (CHRPE) | APC |
| Gardner syndrome | Same as FAP; also desmoid tumors and mandibular osteomas | APC |
| Turcot syndrome | Polyposis and colorectal cancer with brain tumors (medulloblastoma) | APC |
| Colorectal cancer and brain tumors | Colorectal cancer without polyposis and brain tumors (glioblastoma) | MLH1, PMS2 |
| Attenuated adenomatous polyposis coli (AAPC) | Fewer than 100 polyps, though marked variation in polyp number (from 5 to >1000 polyps) is seen in mutation carriers within a single family | APC (predominantly 5′ mutations) |
| Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) | Colorectal cancer without polyposis; other cancers include endometrial, ovarian, stomach cancer; urothelial, brain | MSH2, MLH, PMS2, MSH6 |
| Peutz-Jeghers syndrome | Hamartomatous polyps throughout the gastrointestinal tract; mucocutaneous pigmentation; estimated 9-to 13-fold increased risk of gastrointestinal (GI) and non-GI cancers | LKB1/STK11 |
| Cowden disease | Multiple hamartomas involving breast, thyroid, skin, central nervous system (CNS), and GI tract; increased risk of breast, uterus, and thyroid cancer; risk of GI cancer unclear | PTEN |
| Juvenile polyposis syndrome | Multiple hamartomatous/juvenile polyps with predominance in colon and stomach; variable increase in colorectal and stomach cancer risk; facial changes | DPC4 BMPR1A PTEN |
| MYH-associated polyposis (MAP) | Multiple adenomatous gastrointestinal polyps, autosomal recessive often associated with somatic K-Ras mutations | MYH |
| Multiple adenoma and colorectal cancer | Multiple colorectal adenomas and colorectal cancer; endometrial cancer and brain tumors in some individuals and families | POLD1, POLE |
| Hereditary mixed polyp syndrome | Multiple types of colorectal polyps (e.g., Peutz-Jeghers polyps, juvenile polyps, serrated lesions, conventional adenomas) and colorectal cancer | GREM1 |
FAP is an autosomal dominant syndrome affecting about 1 in 8000 individuals in the United States and accounting for about 0.5% of CRCs. Hundreds to thousands of adenomas arise in the large bowel and rectum beginning in the second decade of life. Although only a fraction of the adenomas may progress to cancer, the lifetime incidence of colorectal cancer in untreated FAP patients approaches 100% with a mean age at diagnosis of 39 years, necessitating the prophylactic removal of the patient’s colon early in adult life. The gene that when mutated underlies FAP is the adenomatous polyposis coli (APC) tumor suppressor gene on chromosome 5q21. Germline mutations have been identified in one APC allele of affected individuals in 90% to 95% of families with FAP studied. More than 95% of mutations lead to premature truncation of APC protein synthesis—about two thirds by small insertions or deletions leading to a frameshift, and the remainder by introducing a stop codon ( Figure 34-1 ). Up to 25% of FAP cases seem to be caused by de novo germline mutations and therefore do not show the characteristic autosomal pattern of inheritance. Moreover, about 20% of individuals with a de novo APC mutation display somatic mosaicism for the mutation in their tissues, with the mutant APC allele present in only subsets of cells within a given tissue and/or in germ cells. Some of these cases with somatic mosaicism may manifest milder polyposis phenotypes than individuals carrying inherited germline APC mutations, as the defective APC allele is present in only a fraction of cells in the somatic mosaicism situation.
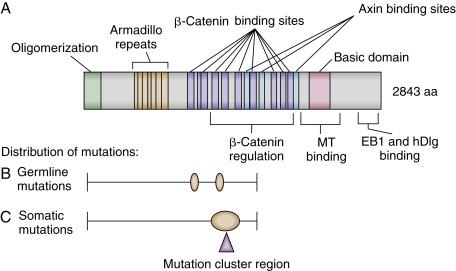
Several extracolonic tumors and symptoms are associated with FAP (see Table 34-1 ). The combination of polyposis with brain tumors (in particular, medulloblastoma in pediatric cases) has been termed Turcot syndrome . Gardner syndrome comprises extensive polyposis with epidermoid cysts, desmoid tumors, and osteomas and seems to be correlated with mutations between APC codons 1403 and 1578. Upper gastrointestinal polyps are responsible for a large proportion of morbidity and mortality in FAP patients after prophylactic total colectomy. This is particularly true for the duodenal cancers that develop in 4% to 12% of patients. Benign gastric fundic gland polyps and gastric adenomas, potential premalignant precursor lesions of gastric cancer, are observed with increased frequency, ultimately leading to stomach cancer in 0.5% of cases. In only a small percentage of FAP cases, thyroid cancer, bile duct cancer, hepatoblastoma (pediatric), and central nervous system tumors such as medulloblastoma are observed. In addition to FAP, rare germline APC variants appear to play a role in other familial CRC cases. Epidemiologic studies have revealed that the APC I1307K (isoleucine-to-lysine substitution at codon 1307) allele is present only in individuals of Ashkenazi Jewish origin, and those who carried the I1307K allele had a roughly twofold increased risk of developing CRC. The mechanism underlying increased risk of adenomas and CRC in carriers of the APC I1307K allele is that the allele appears to be more susceptible than the wild-type APC allele to somatic mutations—particularly insertion and deletion of one or a few nucleotides near the homopolymeric adenine tract created by the lysine 1307 codon substitution, resulting in frameshift mutations.
Notwithstanding the critical role of the APC gene in FAP and related variant syndromes, the APC gene has an even more prominent role in sporadic colorectal tumors, as about 80% of sporadic colorectal adenomas and carcinomas have somatic mutations inactivating APC , the overwhelming majority of which lead to premature truncation of the APC protein. APC mutations have been found in a number of the earliest sporadic lesions analyzed, including microscopic adenomas composed of only a few dysplastic glands. As predicted by Knudson’s two-hit model for tumor suppressor genes, both APC alleles appear to be inactivated in the majority of colorectal adenomas and carcinoma, as a result of localized mutations in both alleles or localized mutation in one allele and chromosomal loss of the remaining allele.
The APC tumor suppressor gene encodes a large protein of approximately 300 kDa (see Figure 34-1 ). The APC protein likely has multiple functions in the cell, but the best understood function of APC is as a major binding partner and regulator of the β-catenin protein. β-Catenin was first identified because of its role in linking the cytoplasmic domain of the E-cadherin cell-cell adhesion molecule to the cortical actin cytoskeleton, via binding to the adaptor molecule α-catenin. Based on studies from many different investigators, a model has been developed to explain the biologic significance of APC’s interaction with β-catenin. The model proposes that in the absence of an activating Wnt ligand signal, APC binds to and collaborates with the scaffold protein Axin to promote sequential phosphorylation by casein kinase I and glycogen synthase kinase- 3 β (GSK3β) of several conserved serine/threonine residues in the N-terminal region of β-catenin, thereby targeting β-catenin for ubiquitination and proteasomal degradation ( Figure 34-2 ). In a physiologic setting, the activating Wnt ligands inhibit degradation of β-catenin via binding to their cognate receptor complex of a Frizzled and an LRP5/6 protein.
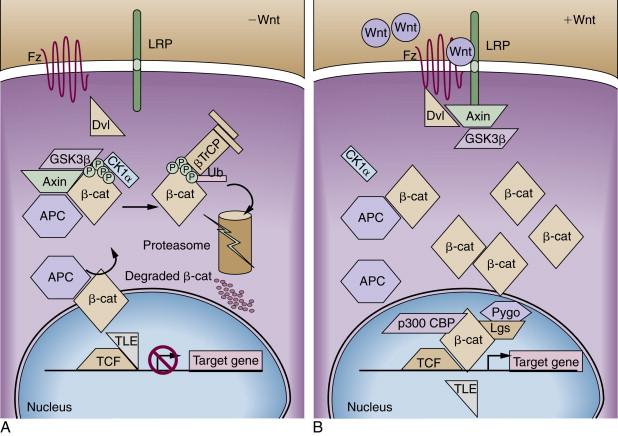
In the roughly 80% of CRCs where both APC alleles are defective, the coordinated destruction of β-catenin is severely impaired, essentially mimicking constitutive activation of Wnt ligand-mediated signaling (see Figure 34-2 ). As a result, β-catenin accumulates in the cytoplasm, complexes with DNA binding proteins of the TCF (T-cell factor family)/Lef (lymphoid enhancer family) family, and translocates to the nucleus. Once there, β-catenin functions as a transcriptional co-activator, activating the expression of TCF-regulated genes. In a subset of the cancers that lack mutations in APC, somatic mutations in β-catenin have been found. A critical net consequence of β-catenin stabilization by APC inactivation or other somatic mutations in CRCs is that the constitutive activation of β-catenin/TCF transcription appears to promote a stem or progenitor cell phenotype in the affected epithelial cells independent of their position in the crypt compartment. Normally, β-catenin/TCF activation is restricted to the crypt base, especially in the so-called crypt base columnar stem cells that are characterized by expression of the Wnt-regulated Lgr5 gene and protein.
Further work has implicated β-catenin stabilization not only in the establishment of a crypt progenitor program but also in the spatial organization and migratory pattern of the cells in the continuous renewal of crypt. Strikingly, feedback inhibitors functioning in the Wnt/β-catenin/TCF pathway are prominent among the critical proteins encoded by β-catenin/TCF-regulated genes, such as the AXIN2, DKK1, NKD1, APCDD1, and WIF-1 proteins, all of which function in some fashion to antagonize Wnt signaling. In CRCs with APC mutations, the ability of the induced feedback regulator proteins to interfere with stabilized β-catenin is largely or entirely abrogated, because the induced Wnt-pathway feedback regulators function upstream of or at the level of the APC protein in the pathway. Interestingly, some of the induced Wnt pathway feedback regulators that are highly expressed in CRCs with APC mutations, such as AXIN2, may play important contributing roles in CRC progression, such as through the ability of AXIN2 to interact with other proteins and pathways in the promotion of epithelial-mesenchymal transition and invasive phenotypes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here