Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clinical hemostasis reflects an integrated interplay between the coagulation system, the fibrinolytic system, platelets, and the vascular endothelium.
The coagulation cascade has four main components, namely the contact activation, the extrinsic, the intrinsic, and the common pathways. There are multipole modulators of these coagulation pathways including thrombin, antithrombin III, protein C, protein S, tissue factor pathway inhibitor, and the von Willebrand factor.
Although heparin is the current preferred anticoagulant for cardiopulmonary bypass, it has multiple limitations.
The probability of heparin-induced thrombocytopenia can be estimated with the 4T score. The diagnosis is confirmed with further testing. The planning of anticoagulation for cardiopulmonary bypass depends on the phase of the disease. The management of acute disease includes anticoagulation with a direct thrombin inhibitor.
Bleeding in cardiac surgery is often multifactorial, including patient factors, cardiopulmonary bypass factors, as well as preoperative exposure to anticoagulants and platelet inhibitors.
Whole blood and its derivatives offer multiple options for transfusion with benefits and risks that must be balanced in perioperative management.
The contemporary approach to perioperative blood management in cardiac surgery includes the institutional stakeholders and encourages a multimodal approach with integrated management of the preoperative, intraoperative, and postoperative phases. Transfusion protocols and point-of-care testing can improve the impact of this approach.
Transfusion is associated with both immunologic and nonimmunologic reactions that can be life-threatening.
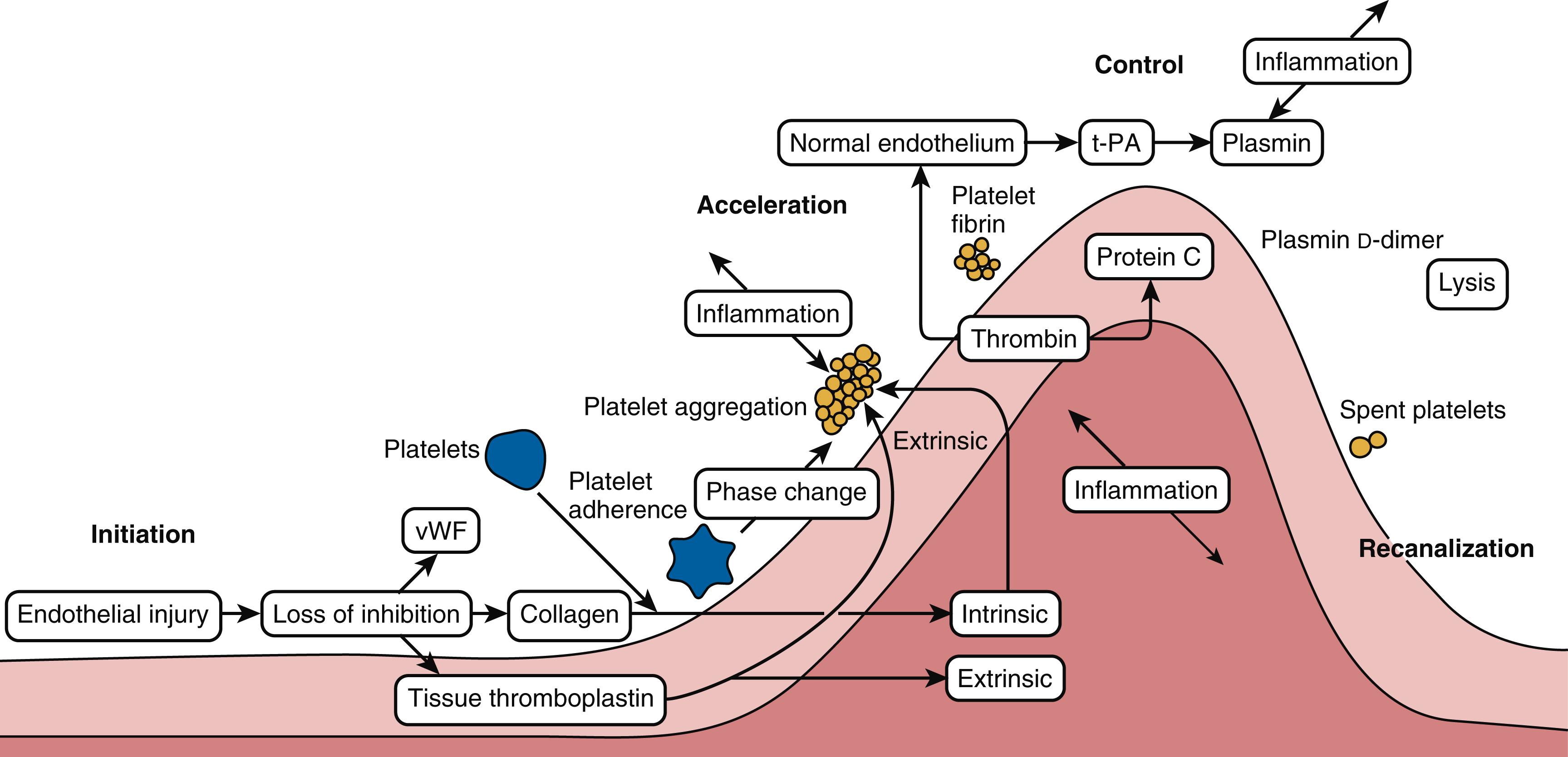
The activation of the hemostatic system involves an integrated cascade with four main components: platelets, coagulation factors, vascular endothelium, and the fibrinolytic system ( Box 28.1 ). The complex interactions between these components produce the highly controlled process of hemostasis occurring at a tissue injury site. There are four stages in clot formation: initiation, acceleration, control, and lysis ( Fig. 28.1 ). , The initiation phase begins with tissue damage and endothelial cell destruction leading to the simultaneous binding of platelets and protein activation. As platelets adhere, the acceleration phase leads to cellular/protein messaging that gathers cells at the site of injury and that leads to interactions with control proteins. Eventually, the control phase dominates and leads to the lytic phase. A key concept is that hemostasis is part of systemic inflammation. In this context, it is not surprising that the contact of blood with a foreign surface during extracorporeal circulation activates the coagulation cascade and initiates a systemic inflammatory response syndrome (see Chapter 25, Chapter 27 ). During cardiac surgery, the endothelium is disturbed both locally and systemically triggering both hemostasis and inflammation. The hemostatic system is not only activated but also is altered during cardiopulmonary bypass (CPB) (see Chapter 25 ). , Both coagulation proteins and platelets are hemodiluted and consumed. A complex cascade unfolds that can trigger a multifactorial coagulopathy, which requires an integrated approach to coagulation management including evidence-based guidelines, intense monitoring, and algorithm-driven blood management. ,
Phases of clot formation: initiation, acceleration, control, and lysis
Coagulation pathway: contact activation, intrinsic, extrinsic, and common pathways
Modulators of coagulation: thrombin, antithrombin III, protein C, protein S, tissue factor pathway inhibitor, von Willebrand factor
Platelet function: adhesion, activation, and aggregation
Fibrinolysis : extrinsic and intrinsic pathways with plasmin as the key enzyme
Vascular endothelium: secretion of multiple modulators to maintain the balance between clot formation and breakdown
The coagulation cascade leads to the formation of an insoluble clot ( Fig. 28.2 ). , The final stable clot consists of platelets cross-linked by way of insoluble fibrin. The coagulation factors are glycoproteins synthesized in the liver that circulate as inactive molecules termed zymogens . The activation process is sequential and resembles a waterfall. Many reactions require the presence of calcium and/or a phospholipid membrane surface typically on activated platelets or endothelial cells. ,
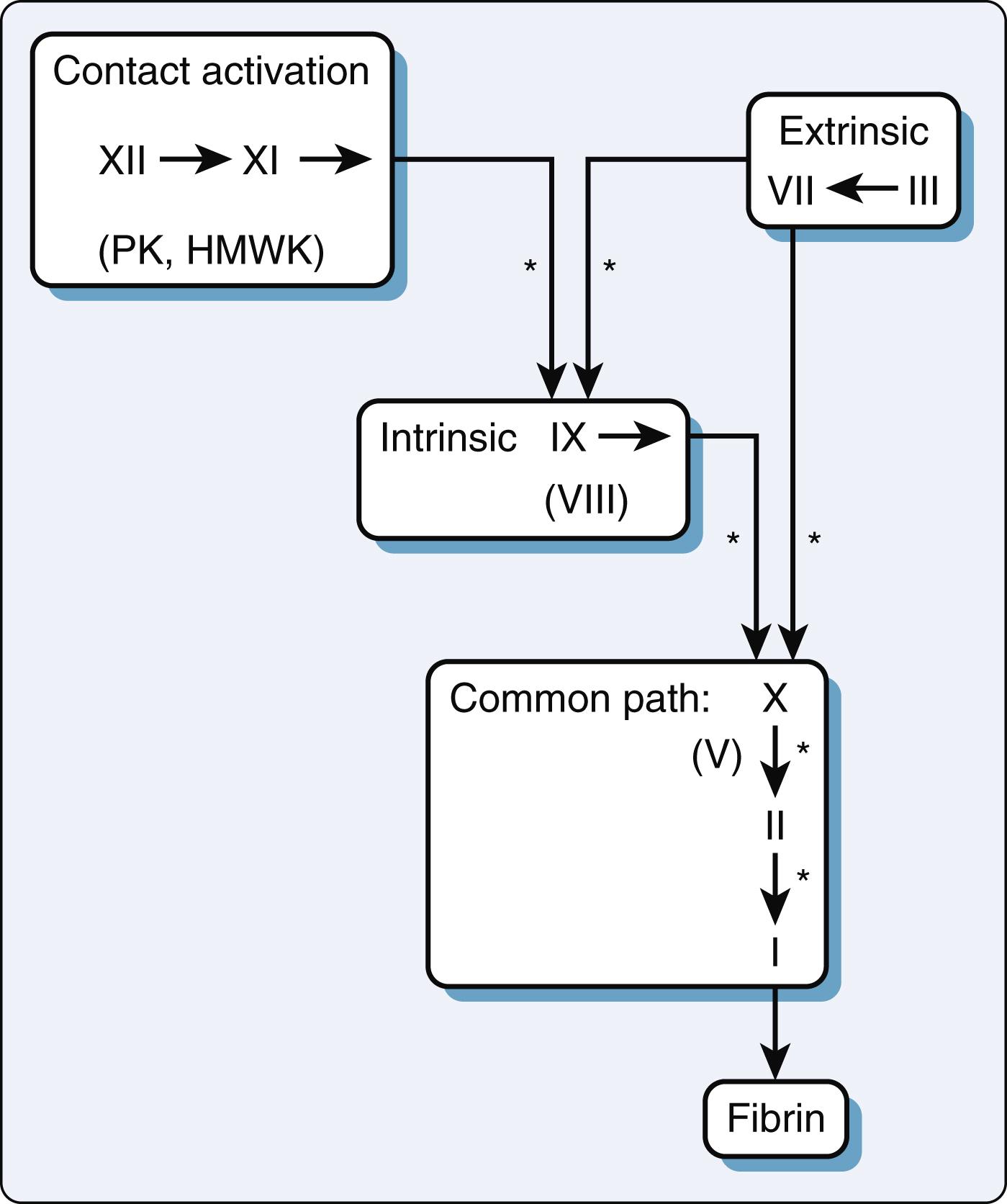
The coagulation factors are organized in four groups: the contact activation, intrinsic, extrinsic, and common pathways. Despite this classification, the factors are all highly interactive, occurring simultaneously on cell surfaces with multiple feedback loops. The contact activation system has a broad role in modulating the intrinsic and extrinsic coagulation cascades as well as fibrinolysis and platelet activation. , Despite the contact activation that occurs in CPB circuits, its overall contribution to coagulopathy in cardiac surgery has been reduced in the era of biocompatible and streamlined extracorporeal circuits (see Chapter 25, Chapter 27 ). Consequently, surface activation is not the driving force behind the severe coagulopathy associated with cardiac surgery. Even though decreased amounts of factor XII may occur, this deficiency is rarely associated with significant clinical bleeding, suggesting it can be accommodated by activation of platelets and other signaling mechanisms (see section on Rarer Bleeding Disorders).
The intrinsic pathway shares factor XII with the contact pathway and also includes factor XI and factor IX. Once activated, the cascade proceeds with cofactors such as calcium, a phospholipid surface, platelet factor 3, and activated factor VIII. Activated factor X then feeds into the common pathway shared by both the intrinsic and extrinsic pathways. The extrinsic pathway begins with tissue factor release from injured endothelial cells. Tissue factor acts as a cofactor to activated factor VII and ultimately factor X. Events such as ischemia-reperfusion, sepsis, and/or cytokine release can all stimulate tissue factor production to trigger coagulation. The multimodal activation of tissue factor likely plays an important role in the pathogenesis of the coagulopathy associated with cardiac surgery. , The extrinsic and intrinsic coagulation pathways are further linked through the activation of factor IX by activated factor VII.
The common pathway entails the formation of thrombin (activated factor II) by activated factor X in the presence of calcium and activated factor V ( Fig. 28.2 ). The combination of activated factors X and V with calcium is termed the prothrombinase complex . Once thrombin is formed, it cleaves the fibrinogen molecule (factor I) to form soluble fibrin monomer. Fibrin monomers then associate to form a soluble fibrin matrix. Factor XIII, activated by thrombin, cross-links these fibrin strands to form an insoluble clot. Patients with factor XIII deficiency are at risk for significant bleeding after cardiac surgery. Furthermore, those factors that require calcium (factors II, VII, IX, X) also depend on vitamin K for adequate carboxylation. Calcium tethers the negatively charged carboxyl groups to the platelet surface to facilitate their roles in the coagulation cascade.
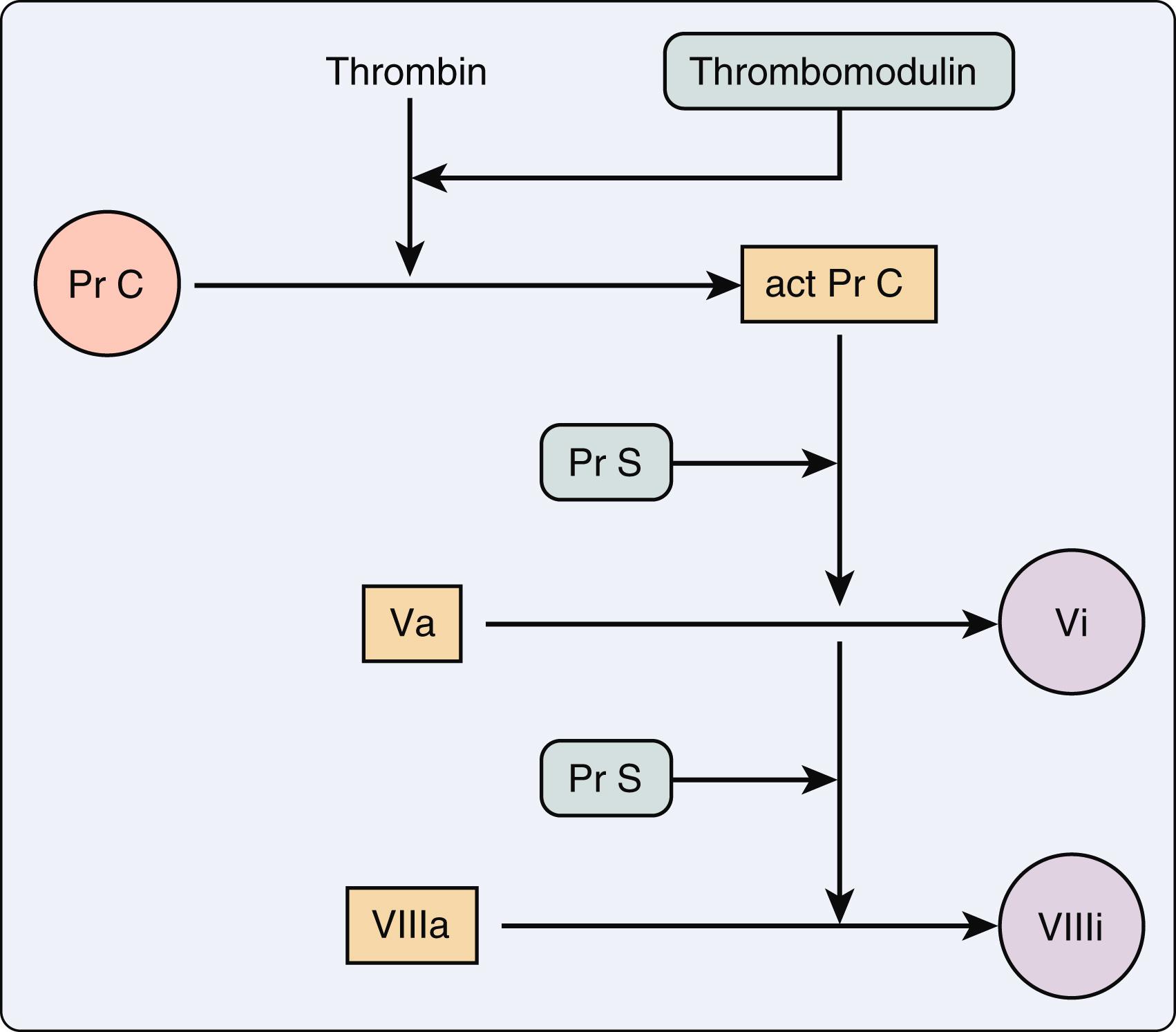
Thrombin is a central modulator of multiple coagulation processes. , It activates factors V, VIII, and XIII, and cleaves fibrinogen to fibrin. Thrombin also activates platelets, stimulates chemotaxis of leukocytes, and with thrombomodulin, activates protein C, to dampen coagulation ( Fig. 28.3 ). , Endothelial injury triggers the coagulation pathways with significant amplification due to these multiple effects of thrombin. Platelets adhere to a site of injury and, along with the coagulation cascade, create a thrombin burst that leads to clot formation (see Figs. 28.1 and 28.2 ). , CPB may affect the amplitude of the thrombin burst due to quantitative and qualitative effects on platelets and coagulation proteins (see Chapter 25 ). The formation of clot is, therefore, a balance between the platelet-driven thrombin burst and the intrinsic inhibitors of anticoagulation such as protein C, protein S, and antithrombin III. Antithrombin III is a potent inhibitor of blood coagulation. It binds to the active site of thrombin, thus inhibiting action of thrombin. , It also has inhibitory effects on inflammation and fibrinolysis. Thrombin bound to fibrin is protected from the action of antithrombin, thus partially explaining the limited efficacy of heparin in treating an existing thrombus. , Antithrombin III binds to a unique pentasaccharide sequence on the wall of endothelial cells known as heparan; the same active sequence that is present in heparin. Activated antithrombin III is active against unbound thrombin, and does not interact with prothrombin. Activated thrombin is typically bound to platelets and/or fibrin, and, thus remains protected from inhibition from antithrombin III. ,
CPB results in substantial depletion of antithrombin III due to dilution and binding with activated thrombin. Furthermore, patients may also have preoperative depletion of this important inhibitor. , The level of deficiency in the postoperative setting has been shown to affect the risk of thrombotic events. The administration of antithrombin III concentrate has been recommended to improve heparin sensitivity during CPB and extracorporeal membrane oxygenation (ECMO). , Both human concentrate (harvested from multiple plasma donors) and a pharmaceutically engineered antithrombin III are commercially available. , Congenital deficiency of antithrombin III can be encountered. Homozygous mutations are frequently fatal in utero, but heterozygous mutations are tolerated into adulthood with about 40% to 60% of normal activity and a particularly high risk for deep vein thrombosis. ,
Protein C and its cofactor protein S are vitamin K–dependent proteins that dampen the coagulation pathway in the initiation, acceleration, control, and lytic phases (see Figs. 28.1 and 28.3 ). , Genetic variants of protein C and S lead to increased risk for deep vein thrombosis and pulmonary artery embolism. , Activated protein C also can promote fibrinolysis. Regulation of the extrinsic limb of the coagulation pathway occurs via tissue factor pathway inhibitor. It inhibits the activity of the factor VII-tissue factor complex on factor X activation. Both vascular endothelium and platelets appear to produce this inhibitor. , Although this inhibitor has been evaluated in adults during CPB, its significance as a risk factor for bleeding and transfusion after cardiac surgery requires further evaluation. ,
The von Willebrand factor (vWF) is a massive peptide that associates with factor VIII in plasma, protecting it from proteolytic enzymes. It circulates in the plasma in its coiled inactive form, but in the setting of endothelial injury, binds to the platelet membrane and slows the movement of platelets along the endothelial brush border. The increased shear forces begin the process of platelet activation that is then amplified to initiate the coagulation cascade. Qualitative and quantitative deficiencies of this important modulator of coagulation confer significant risk for bleeding and transfusion after cardiac surgery.
Platelets are central contributors to hemostasis. Platelets provide membrane phospholipid for coagulation factor reactions, release coagulation factors, and secrete microvesicles that have important roles in hemostasis. , Their cellular signaling is highly regulated, with many different types of membrane receptors that participate in the coagulation process. The hemostatic response depends on three critical platelet functions: adhesion, activation, and aggregation. Platelet adhesion is triggered by the increased shear stress at sites of endothelial injury sites. The platelets then bind to the exposed subendothelium through multiple interactions on their surface membranes that also include the vWF, as previously described. Turbulent vascular flow associated with mechanical devices, atherosclerosis, and/or valvular stenosis also increases shear stress that can trigger platelet adhesion. After initial adhesion, additional amplification mechanisms occur including glycoprotein release, expression of tissue factor, and receptor activation with the glycoprotein IIb/IIIa receptor. Platelet adhesion starts rapidly after endothelial injury, and due to amplification, the exposed subendothelium is covered within 30 minutes. ,
Platelet activation has multiple triggers including contact with collagen. The activated platelets then release their granular contents and undergo conformational changes that expose the glycoprotein IIb/IIIa receptor that has an important role in platelet aggregation. , Released adenosine diphosphate recruits additional platelets to the site of injury and stimulates platelet G protein, which in turn, activates membrane phospholipase. This results in the formation of arachidonate, which platelet cyclooxygenase converts to thromboxane A2 for further platelet activation and aggregation. Further agonists of platelet adhesion include thrombin and serotonin. Thrombin remains by far the most potent platelet agonist. The presence of sufficient agonists results in platelet aggregation that occurs through the formation of protein bridges between the surface glycoprotein IIb/IIIa receptors of adjacent platelets.
Fibrinolysis is an important part of the hemostatic cycle and involves fibrin breakdown at the vicinity of a clot. This important enzymatic pathway remodels formed clot and removes thrombus when endothelium heals. , Plasmin is the principal enzyme of fibrinolysis that has an extrinsic and intrinsic system. Plasma normally contains minimal circulating plasmin because a scavenging protein, α2-antiplasmin, consumes any plasmin formed from localized fibrinolysis. In extrinsic fibrinolysis, endothelial cells synthesize and release tissue plasminogen activator for localized clearance of clot. , Intrinsic fibrinolysis occurs through cleavage of plasminogen to plasmin that is mediated by activated factor XII. , The plasmin then facilitates additional cleavage of plasminogen by activated factor XII, forming a positive feedback loop. Further activators of fibrinolysis include kallikrein, streptokinase, urokinase, and recombinant tissue plasminogen activator, which is relatively fibrin-specific and has clinical application in acute myocardial infarction and stroke.
The cells that form the intima of vessels provide an excellent nonthrombogenic surface through multiple mechanisms, including the release of prostacyclin, nitric oxide, adenosine, and protease inhibitors. The vascular endothelium maintains tight control over the delicate balance between coagulation and fibrinolysis, as well as playing a central role in the regulation of vascular tone, inflammation, and immune responses. Biochemical mediators work through specific mechanisms. Nitric oxide not only vasodilates blood vessels but also inhibits platelets through activation of guanylate cyclase and intracellular uptake of calcium. Prostacyclin also possesses powerful vasodilator and antiplatelet properties to counteract the vasoconstrictor effects of platelet-produced thromboxane A2. , Prostacyclin also inhibits platelet aggregation through increases in intracellular cyclic adenosine monophosphate. The vascular endothelium also secretes thromboxane, endothelins, and angiotensin for further fine control of local vascular tone. The endothelium cell has a central role in coagulation factor activation including the release of thrombospondin to enhance platelet aggregation. , This local mediator also binds plasminogen to decrease the amount of local plasmin to dampen fibrin breakdown. , A healthy vascular endothelium is essential for the maintenance of hemostasis.
In 1916, while investigating the procoagulant properties of cephalin, a medical student, Jay McLean, instead discovered a fat-soluble compound extracted from dog liver that actually prolonged coagulation. His mentor, William Howell, later named this substance heparin (after hepatic for “liver”). Over a century later, heparin remains one of the safest, and most effective drugs in clinical practice, including its widespread application as the most common anticoagulant in cardiac surgery with or without CPB. ,
Heparin is a naturally occurring polysaccharide that belongs to a family of glycosaminoglycans stored in the granules of mast cells. Unfractionated heparin (UFH) has an average molecular weight of 15,000 to 19,000 kDa with variations in both length and side-chain composition yielding a range of molecular weights from 5000 to 30,000 kDa. , Abundant sources of heparin include mammalian tissues rich in mast cells, such as lungs, intestines, and liver. , While heparin could be isolated from bovine intestine or bovine lung, most commercially available preparations of UFH primarily come from porcine intestine. , On average, approximately 180 to 260 mg of heparin is extracted per animal after an extensive purification procedure for purity and degree of sulfation to ensure appropriate anticoagulant activity and to prevent unwanted effects such as hypotension and death. In the past, some adverse reactions were attributed to an oversulfated chondroitin sulfate contaminant in heparin. Alternative sources of heparin, including nonmammalian sources with a lower risk of contamination, are being investigated. ,
Heparin potency is determined by comparing a test specimen against a known standard’s ability to prolong coagulation. , Determination of the manufacturing standards for heparin are made by organizations such as the United States Pharmacopeia. Given that assays can vary, updates against this standard allow for a more universal reference.
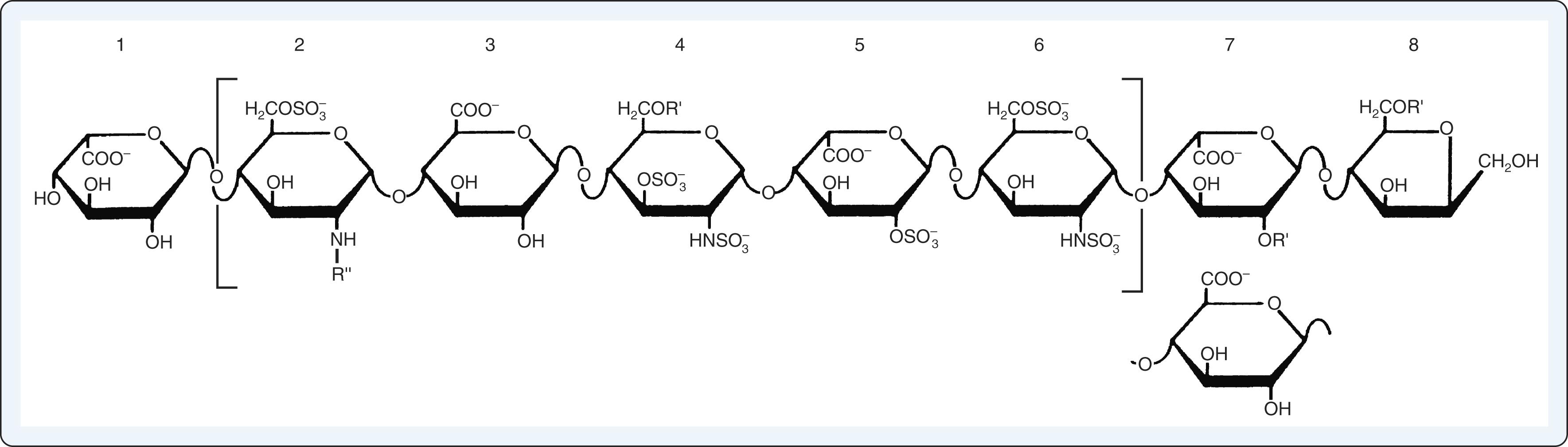
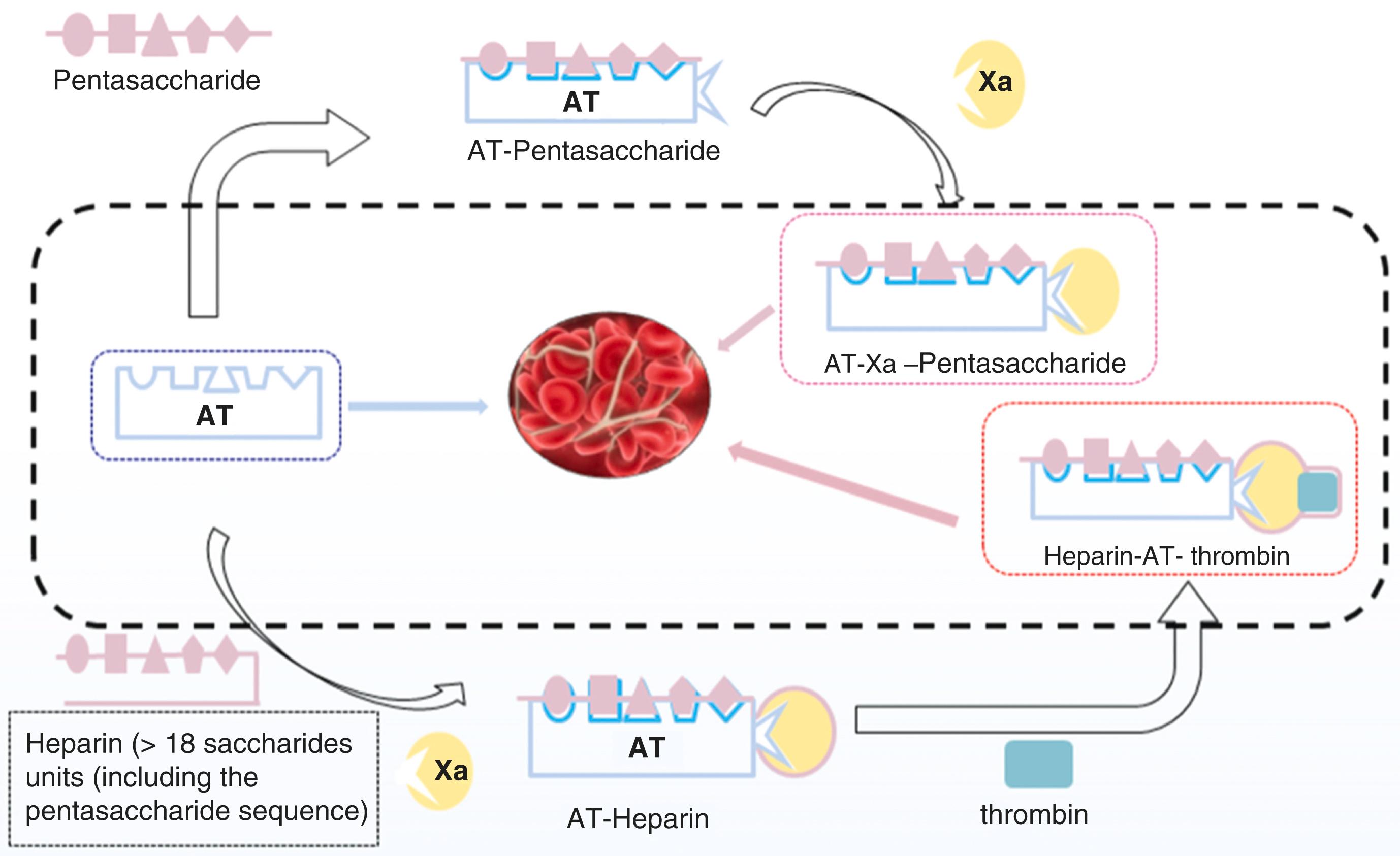
Heparin acts indirectly as an anticoagulant by binding to antithrombin III. , Given the significant heterogeneity with heparin, there exists variable affinity for antithrombin III. For example, heparins with repeating disaccharide trisulfate units have a low affinity for antithrombin III, and, therefore, have less of an anticoagulant effect. , Conversely, heparin fragments with a certain pentasaccharide sequence can bind to antithrombin III with high affinity ( Fig. 28.4 ). , , The anticoagulant properties of heparin arise through the indirect inhibition of coagulation factors, most notably thrombin and factor X, given their central roles in the common coagulation pathway (see Fig. 28.2 and Table 28.1 ). While heparin fragments can bind to antithrombin III, only chains with 18 units or more are able to augment the thrombin inhibition by antithrombin III ( Fig. 28.5 ). , Inhibition of thrombin by the heparin-antithrombin III complex prevents the formation of fibrin, but it also inhibits activation of factor V and factor VIII. ,
| Factor | Major Inhibitor | Acceleration of Inhibitory Activity by Heparin |
|---|---|---|
| XI | α 1 -Proteinase inhibitor | 40-fold |
| X | Antithrombin | 1000-fold |
| IX | Antithrombin | 10,000-fold |
| II | Antithrombin | 2000-fold |
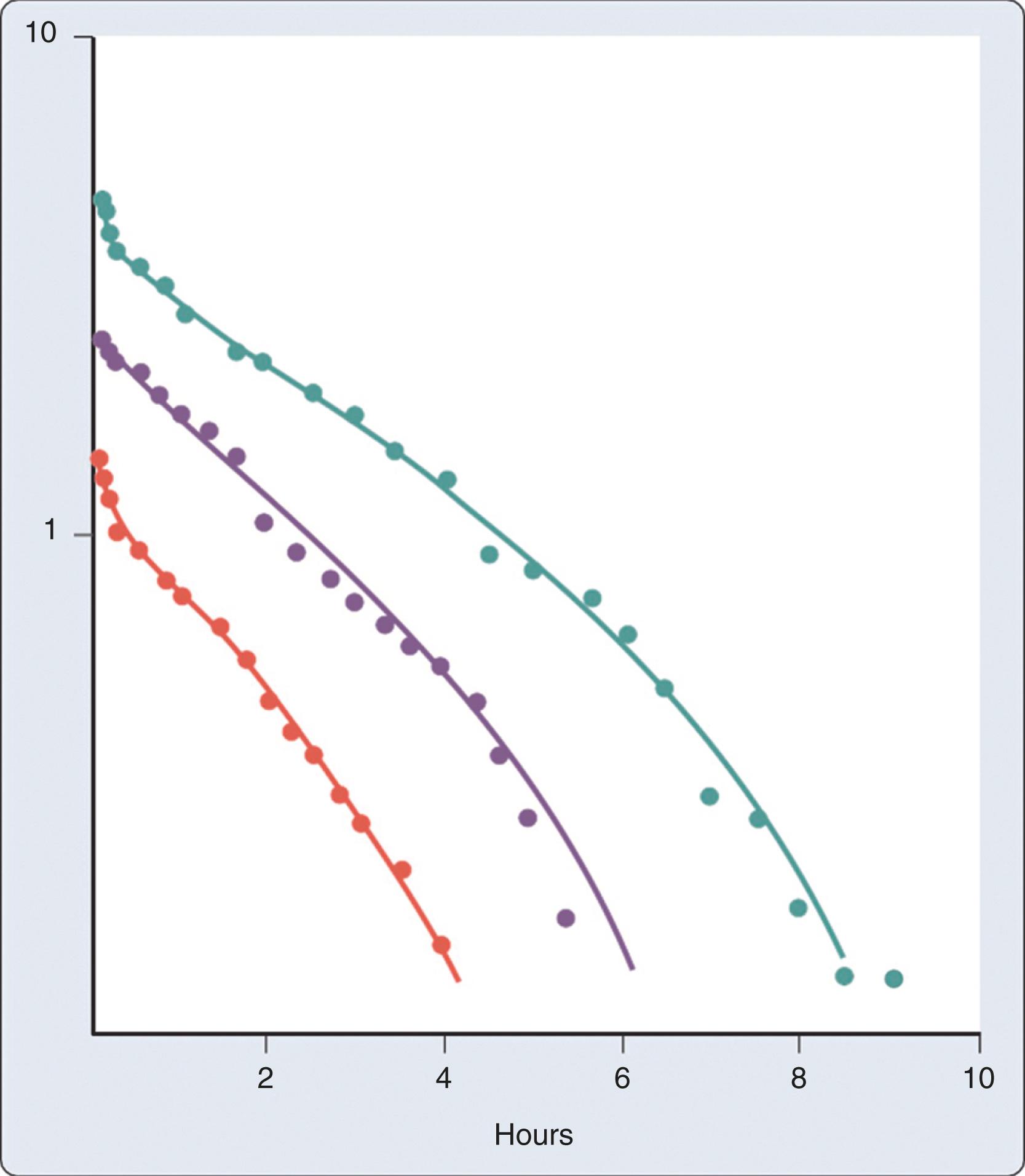
About a third of a given dose of UFH may have the pentasaccharide sequence required for augmentation of antithrombin III activity. The remaining heparin has minimal anticoagulant activity, although binding to nonspecific proteins may affect anticoagulant activity. The clearance of heparin is through a rapid saturable mechanism and slower first-order mechanisms ( Fig. 28.6 ). , At low doses, the saturable mechanism predominates and is likely due to binding to endothelial cell receptors and macrophages as part of the reticuloendothelial system. , At therapeutic doses, heparin is cleared by both a faster saturable mechanism and a slower, dose-independent renal mechanism. Finally, at very high doses of UFH, the slower, nonsaturable mechanism predominates and a nonlinear response curve is seen ( Fig. 28.6 ). , Therefore, the half-life of UFH is dose-dependent. Typically, the half-life of heparin is 30 to 90 minutes in healthy individuals, but can increase significantly with increasing doses. The presence of liver disease does not impact elimination. Similarly, dosing adjustments are not required in renal failure, but there may be an impact on clearance at high doses.
Monitoring for heparin effect can be achieved with an activated partial thromboplastin time (aPTT), activated clotting time, or anti–factor Xa heparin assay. , Monitoring is recommended given the variable therapeutic response among patients (see Chapter 13 ). The aPTT is routinely available and performs well for low-dose heparin anticoagulation, but can be limited by significant variability due to factors such as differing laboratory reagents, acute phase reactants, and high heparin doses. , , The anti–factor Xa assay is a functional assay that allows for measurement of inhibition of activated factor X by UFH. It provides a more direct measure of heparin activity and is used as a reference standard. ,
In the cardiac operating room and the cardiac catheterization laboratory, the activated clotting time (ACT) is the primary test for anticoagulation monitoring due to practicality. It serves as a point-of-care test that provides quick results with very low-volume blood samples. , , Depending on the test, various activators, such as celite, kaolin, or glass beads can be used to initiate contact activation. Given the large dose of heparin used for CPB (300–400 units/kg), the ACT test that produces maximally activated clot is recommended in order to more closely correlate with anti–factor Xa activity. The nonspecific nature of this test renders it vulnerable to variables such as hypothermia, platelet concentrations, and hemodilution. To prevent fibrin formation and to maintain a safe anticoagulation target, current guidelines recommend an ACT of 400 to 480 seconds. , ,
With continuous exposure to UFH, patients may develop tachyphylaxis, requiring increasing amounts of heparin to maintain therapeutic anticoagulation in a phenomenon known as heparin resistance. , , The incidence of heparin resistance may be as high as 10% to 20% depending on definitions. In cardiac patients requiring CPB, heparin resistance occurs when the ACT fails to be prolonged above 400 seconds after a dose of UFH in the 300 to 400 units/kg range. With the ubiquitous applications of heparin in clinical practice, heparin resistance during cardiac surgery is likely to remain a clinical challenge ( Box 28.2 ).
Heparin resistance
Protamine adverse reactions: hypotension, allergic responses, and pulmonary vasoconstriction
Heparin-induced thrombocytopenia
Heparin rebound
Heparin’s heterogeneity and variable potency
Antithrombin decrease
Although antithrombin III deficiency may mediate heparin resistance, there are multiple causative factors for a dampened sensitivity including elevated heparin-binding proteins that accompany the acute phase response (eg, fibrinogen; factor VIII), increased heparin clearance (eg, splenomegaly), and thrombocytosis. , The release of platelet factor 4, a known heparin-binding protein, is thought to play a role in heparin insensitivity associated with thrombocytosis. , The hypercoagulable state associated with COVID-19 infection has been associated with heparin resistance and appears to be multifactorial (see Chapter 4 ). , A further cause of heparin resistance is the presence of a factor Xa decoy such as andexanet alfa. , Andexanet alfa reverses the anticoagulant effects of direct activated factor X inhibitors, but also interacts with the heparin-antithrombin III complex to dampen the inhibitory effect of antithrombin II on the coagulation cascade. ,
Despite the multiple causes of heparin resistance, antithrombin III deficiency remains the most common. Congenital deficiencies have a prevalence in the range of 0.2% to 0.02% with qualitative and/or quantitative defects. Acquired deficiencies may result from multiple mechanisms including increased consumption, decreased production, hemodilution, and/or enhanced clearance. These mechanisms are apparent in clinical settings such as disseminated intravascular coagulation, cirrhosis, acute thrombosis, nephrotic syndrome, hemodialysis, CPB, ECMO, trauma, pregnancy, and/or oral contraceptive use. , , , While the ACT and the aPTT are commonly selected to measure heparin effect, chromogenic anti–factor Xa assays may have greater value in measuring heparin levels, especially with confounding factors that may impact standard assessments of anticoagulation.
The administration of additional heparin beyond the typical dose for CPB may overcome mild-to-moderate heparin resistance, but there is a ceiling effect with this approach when doses of 600 to 800 units/kg have been suggested. , , While fresh frozen plasma may prove adequate, current guidelines have recommended antithrombin III concentrates (purified human or recombinant formulations). , The dosing of antithrombin III can be titrated to the response of the ACT and may be more effective than additional heparin with an acceptable safety profile.
A further option in cases of heparin resistance is to replace heparin with an alternative anticoagulant such as the direct thrombin inhibitors argatroban and bivalirudin. , Another option to replace heparin is “platelet anesthesia” with a glycoprotein IIb/IIIa inhibitor such as tirofiban or a direct P2Y12 inhibitor such as cangrelor. , Although these options have been described, they are not as readily reversible as heparin.
The neutralization of heparin-induced anticoagulation remains the primary use of protamine that is derived either from animal sources or recombinant technology. Following intravenous injection, protamine, a polycation, forms large complexes with the negatively charged sulfate groups on heparin neutralizing its antithrombin effect. , Since protamine has poor efficacy in neutralizing activated factor X, it has limited utility for reversal of low-molecular-weight heparin. Given that porcine mucosal heparin and bovine lung heparin have varying levels of factor X inhibition, these derivatives may have different sensitivities to reversal by protamine. Although free protamine undergoes rapid clearance after administration, the removal of complexed protamine may involve endocytosis, protein binding, and/or proteolytic degradation.
Heparin reversal by protamine can be based on standard ratio dosing or titration that is guided by point-of-care testing with direct heparin concentration measurements or the ACT. Accurate protamine dosing is important to minimize bleeding. Standard ratio dosing can be based on either the total dose or the initial dose of heparin with ratios typically in the 1:1 range. , , Current consensus has recommended that this ratio not be exceeded to minimize bleeding complications, but the optimal ratio remains to be determined. , , , In contrast, limited evidence from small trials supports the current recommendation to use a select titration method for protamine dosing, with a preference for the heparin concentration method as compared to models based on the ACT (see Chapter 13 ). , , , ,
The principal protamine reactions are hypotension with rapid administration, allergic responses including anaphylaxis, and pulmonary vasoconstriction (see Box 28.2 ). Hypotension related to the speed of protamine administration may be due to endothelial release of nitric oxide and/or pharmacologic displacement of histamine from mast cells by the highly alkaline protamine, similar to the mechanism by which morphine, and alkaline antibiotics including vancomycin and clindamycin cause hypotension. This hypotensive reaction is minimized by slow administration of protamine over 5 to 10 minutes. ,
The allergic reactions to protamine involve release of vasoactive mediators resulting from antigen-antibody interaction. Anaphylactoid reactions include not only severe immediate hypersensitivity responses consistent with anaphylaxis but also other life-threatening idiosyncratic responses of nonimmunologic origin. , Risk factors for protamine reactions include diabetes, prior exposure to protamine, and vasectomies. , Previous protamine exposure during cardiovascular procedures has a risk of inducing protamine antibodies. , The management of these allergic responses to protamine typically includes titrated vasopressors.
The precipitation of pulmonary vasoconstriction by protamine may be due to complement activation, blockade of nitric oxide release, and direct pulmonary injury. , The duration of pulmonary hypertension may vary substantially from mild to severe, and may require reinstitution of CPB. During this type of pulmonary hypertensive crisis, heparin administration may reduce protamine-heparin complex size, and facilitate the return to bypass. Supportive therapy for right heart failure and pulmonary hypertension are typically required in this setting (see Chapters 8 , 19–21, and 26). , , A small randomized trial has also suggested that protamine administration via the ascending aorta may minimize hemodynamic instability. This possibility is a management consideration in patients with previous adverse reactions to protamine.
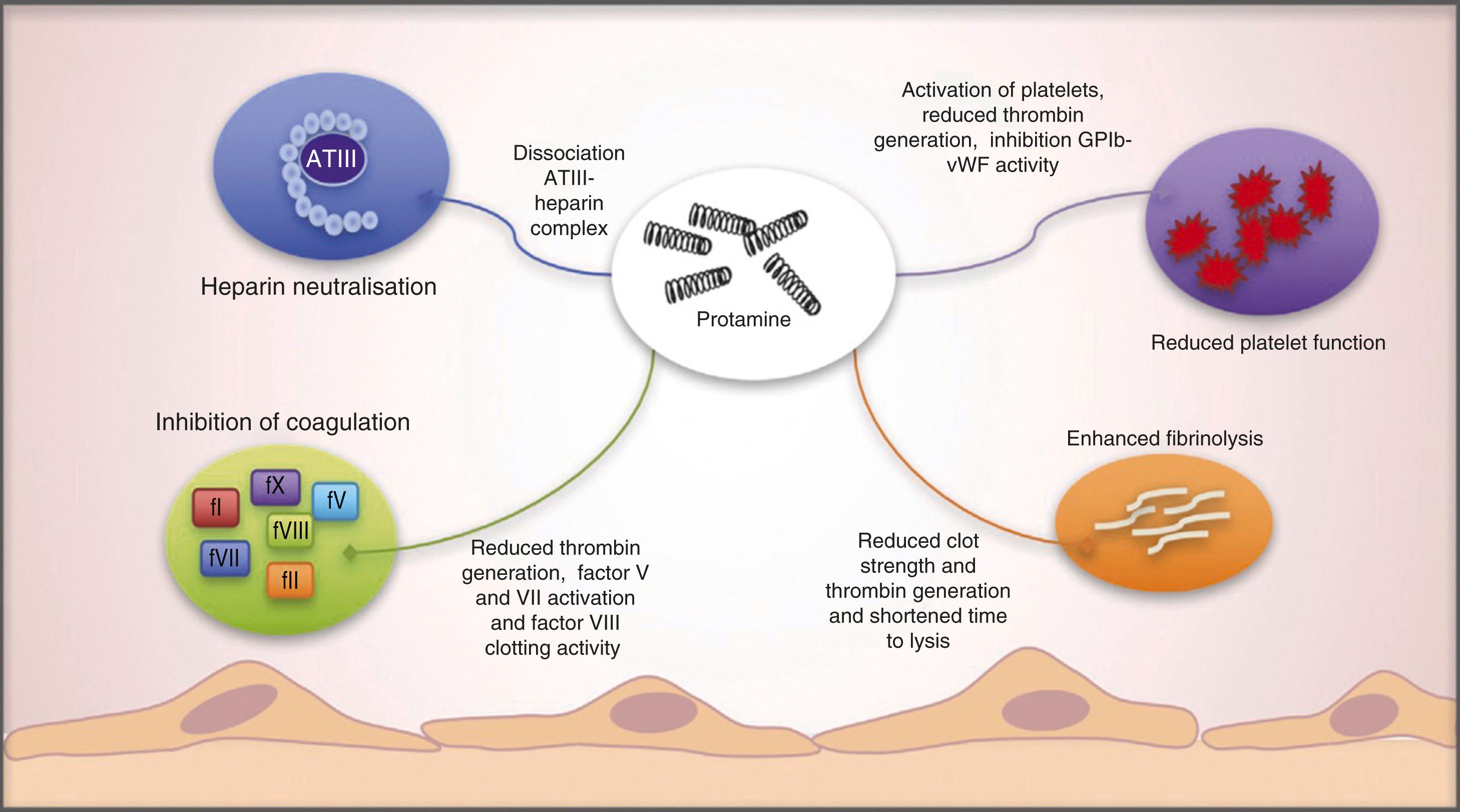
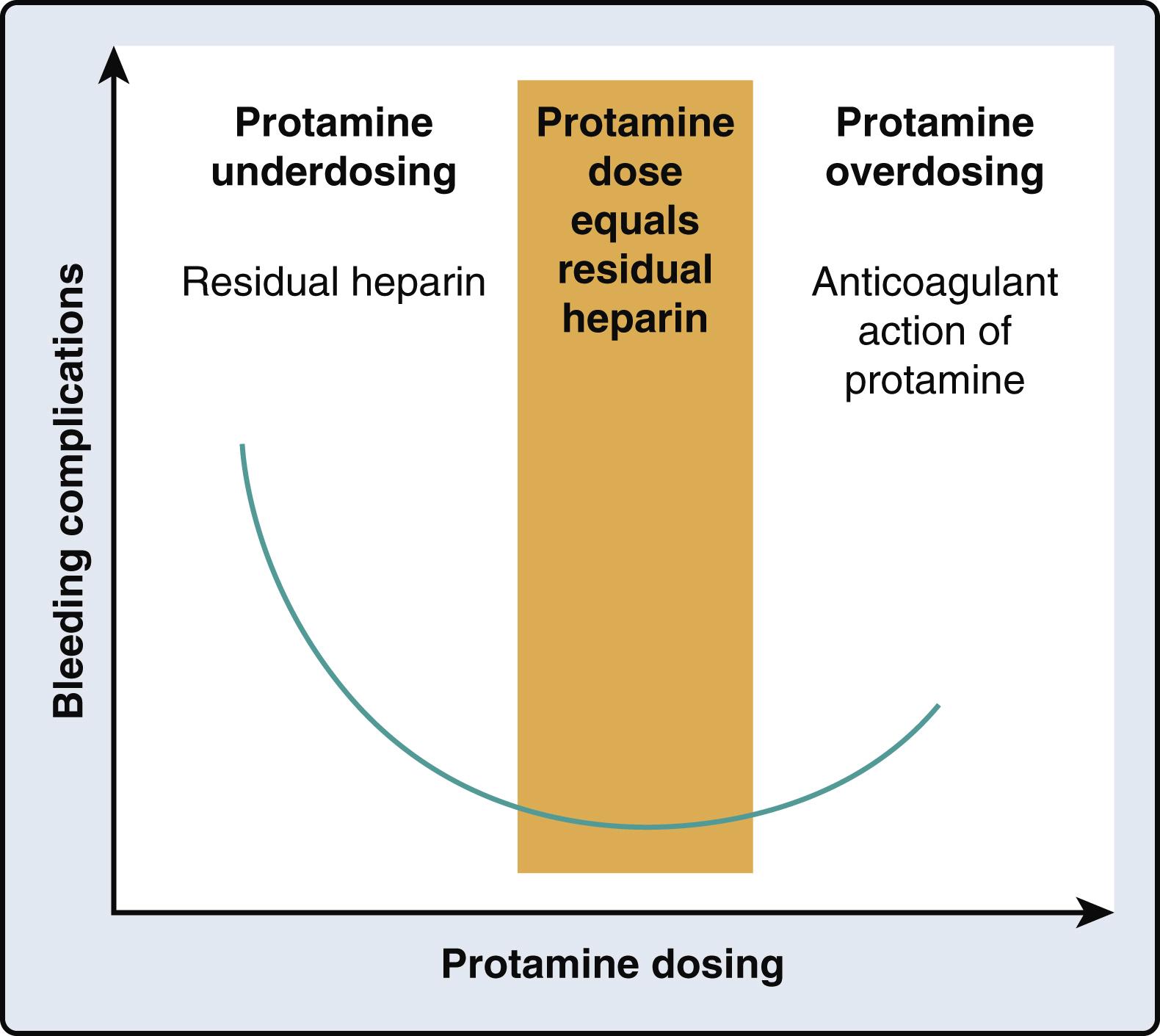
Although protamine can reverse the anticoagulant effects of heparin, it also can act as an anticoagulant via multiple effects including reducing platelet function, inhibiting the coagulation cascade, and enhancing fibrinolysis ( Fig. 28.7 ). These anticoagulant effects highlight the importance of precise titration of protamine to reverse heparin and to achieve hemostasis and minimize bleeding due to residual heparin or excess protamine ( Fig. 28.8 ). The cumulative side effects of protamine have prompted a search for clinical alternatives, although candidates such as hexadimethrine, recombinant platelet factor 4, and heparinase have been considered, their translation into clinical practice has not yet been realized.
After protamine neutralization of UFH after CPB, there is a risk of clinical bleeding associated with the reappearance of circulating heparin in a phenomenon known as “heparin rebound.” It is important to keep in mind that heparin rebound in the context of cardiac surgery refers to bleeding, while the same term may refer to thrombosis in the cardiology literature. Furthermore, a recent review has highlighted the possibility of protamine-associated coronary graft thrombosis that may be related to multiple mechanisms, including platelet activation. This phenomenon requires further investigation.
The pathogenesis of heparin rebound after CPB is likely due to the release of heparin from protamine-binding sites, and may be a complication of protamine underdosing. A further mechanism is that heparin administered during CPB may bind nonspecifically to plasma and platelet-derived proteins. Heparin at these sites remains bound, and therefore, does not form a complex with protamine. Over time, the bound heparin slowly dissociates, which again leads to free circulating heparin. , Given the short half-life of protamine of 5 to 10 minutes, recurrent anticoagulation can occur. As for the incidence and timing of heparin rebound, there is wide variability in the definitions, protamine dosing, and coagulation testing across studies. Given that the ACT lacks sensitivity to detect low heparin concentrations, alternatives such as viscoelastic testing may offer enhanced diagnostic accuracy in this setting. ,
Despite the occurrence of heparin rebound after CPB, its association with clinically important bleeding remains questionable. , When it does appear to aggravate clinical coagulopathy, a simple therapeutic approach is the administration of supplemental protamine to neutralize the remaining heparin. While the initial protamine dose to reverse heparin can be investigated further, it remains unclear if using models based on heparin clearance versus standard fixed ratio dosing (such as 1 mg protamine for every 100 units of UFH) would lead to prevention of heparin rebound. Furthermore, controversy has persisted about whether reversal should be based on total heparin dose or estimated remaining heparin in efforts to minimize the potential anticoagulant effects of excessive protamine. ,
While optimal protamine dosing strategies may correct incomplete heparin reversal, an ongoing challenge will be slow release of heparin from its nonspecific binding sites. A clinical solution may be a protamine infusion that persists into the postoperative period to dampen this heparin rebound and correct clinical coagulopathy after CPB. , A protamine infusion in the range of 25 mg/hour may have clinical benefit for as long as 24 hours after CPB. However, this approach has not been a consistent finding across studies due to multiple factors including differences in definitions and point-of-care coagulation testing practices. , ,
While the primary clinical indication of heparin is for its anticoagulant properties, it has effects beyond its anticoagulation effects as an agonist of antithrombin III. Many of the nonspecific substances that bind with heparin include chemokines, growth factors, adhesion molecules, cytotoxic peptides, and tissue destructive enzymes. , , These interactions may result in antiinflammatory effects, regulation of smooth muscle proliferation, antitumor effects, antiviral activity, and enhanced fibcrinolysis. The increased plasmin activity associated with heparin therapy may play a role in ongoing bleeding despite protamine neutralization. The diverse roles of heparin in biology are a focus for ongoing research that will likely advance understanding and enhance the impact of this important drug in clinical practice. ,
Heparin exposure can lead to direct platelet activation and increased platelet aggregation that may lead to thrombocytopenia. , The agglutinating effects on platelets can result in a transient thrombocytopenia that is typically mild and that tends to occur in the first few days after heparin exposure. , This non–immune-mediated form of thrombocytopenia is referred to as type 1 heparin-induced thrombocytopenia (HIT). This platelet response to heparin is common and benign. In fact, heparin therapy may continue in this setting with resolution of the thrombocytopenia.
In contrast with these predictable effects of heparin, some patients develop progressive and severe thrombocytopenia that may be accompanied by thrombosis. This syndrome may be called type II heparin-induced thrombocytopenia. The acronym “HITT” is often preferred for this immune-mediated and hypercoagulable response to heparin. , A platelet count in excess of 100,000/mm 3 may be consistent with HIT, since a decrease over 30% in the platelet count from a given baseline is often more suggestive for a diagnosis of HIT. This syndrome often develops 5 to 10 days after heparin exposure, although later presentations are possible due to the persistence of circulating antibodies.
The mechanism of thrombosis in HIT typically involves a heparin-dependent antibody, usually IgG, that triggers platelet aggregation in the presence of heparin. , The process begins with release of platelet factor 4 from platelets that binds to heparin on the platelet surface. Thereafter, antibodies then bind to this heparin complex to activate platelets via their FcγIIa receptors ( Fig. 28.9 ). , This results in the release of additional platelet factor 4, which further promotes thrombosis by binding to heparin-like molecules on the endothelium. The release of prothrombotic platelet-derived microparticles promotes thrombin generation and coagulation. The interaction of the heparin complexes with monocytes can also contribute to thrombotic risk through the production of tissue factor. This amplified process of platelet aggregation results in white thrombi that is specific to HITT. However, if thrombin generation is prominent due to platelet activation, then a fibrin clot may be the result.
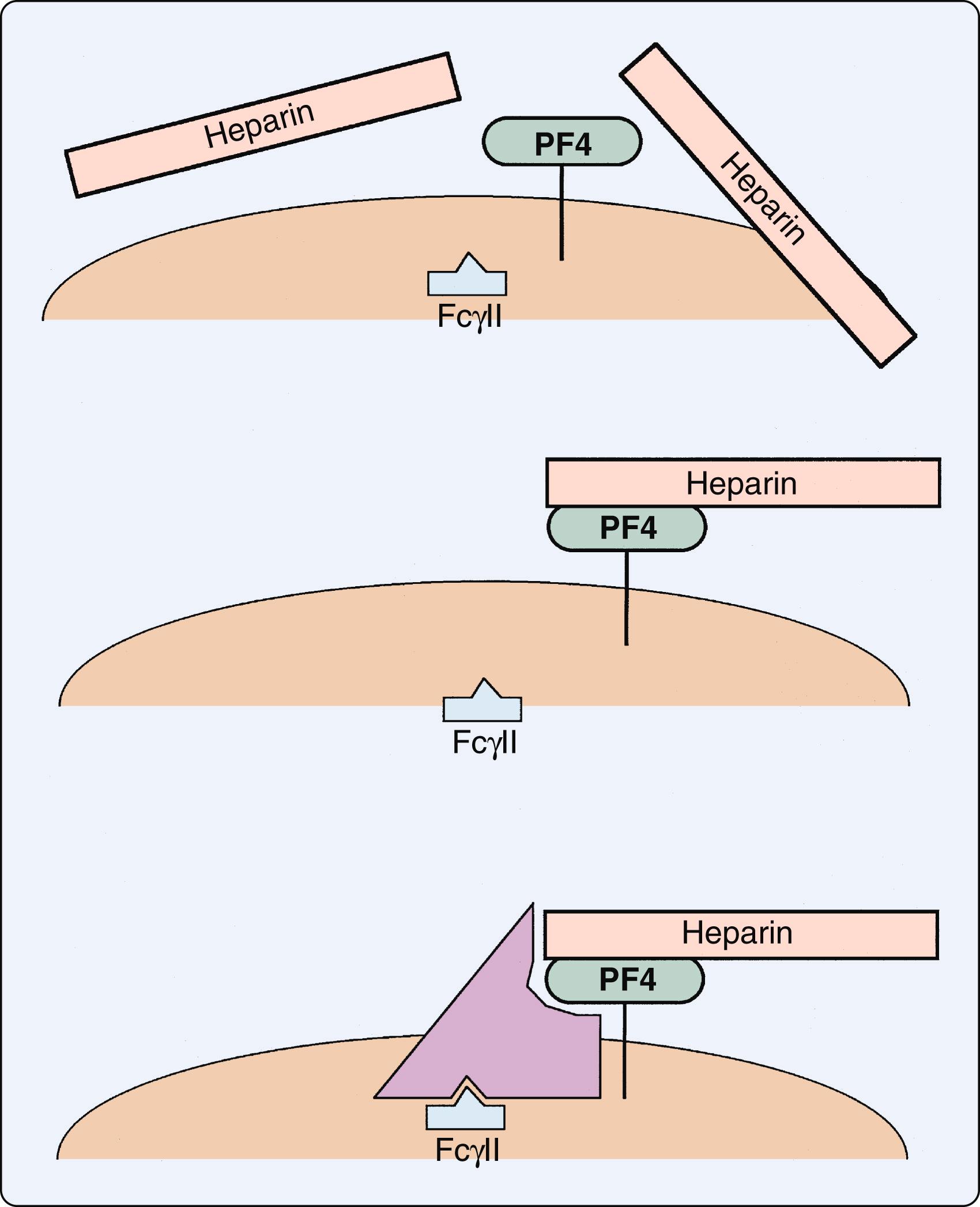
In the absence of endothelial injury, the responses to this antibody-antigen interaction are platelet consumption and thrombocytopenia. However, in the setting of disrupted endothelium due multiple possibilities including atherosclerotic plaque rupture, sepsis, and vascular procedures, an endothelial nidus is developed for platelet adhesion and subsequent activation. Released with platelet activation, platelet factor 4 binds to heparin locally, thus not only removing the inhibition of coagulation but also generating additional antigenic material. The resulting thrombosis can lead to loss of limb and/or life ( Fig. 28.10 ).
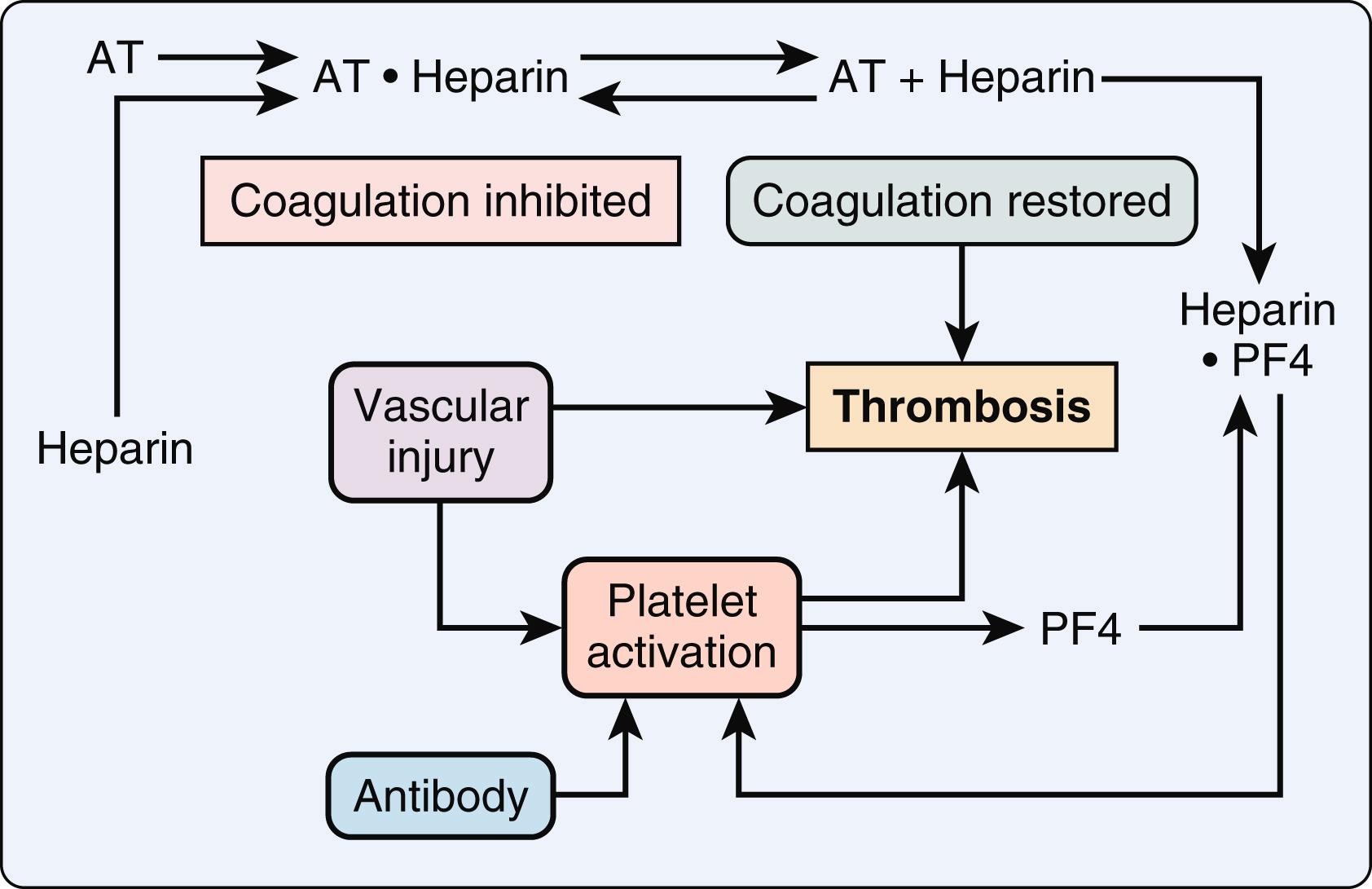
Current data suggest that HIT can be seen in 0.5% to 1% of patients exposed to UFH. In a review of COVID-19 cases, the incidence of HIT (0.8%) was found to be similar to non–COVID-19 data. Porcine heparin and low-molecular-weight heparin preparations have been associated with a lower risk of HIT. The risk of clinical thrombosis in HIT has been reported to be in the 10% to 20% range with a wide variation and an ongoing risk of thrombosis despite adequate therapy with an alternative anticoagulant. Thromboembolism may be arterial or venous in nature; although, arterial thrombosis involving the extremities is more often seen in cardiac surgery patients. In addition to deep venous thrombosis, pulmonary embolism, and limb ischemia, further clinical presentations of HIT include skin necrosis, venous gangrene, adrenal hemorrhage (due to adrenal vein occlusion), and cerebral vein thrombosis.
The diagnosis of HIT is considered when the platelet count decreases more than 30% to 50% about 5 to 10 days after heparin exposure. The development of thrombosis (with or without thrombocytopenia) can also be used as diagnostic criteria; in approximately 20% of patients, thrombosis occurs shortly before a decrease in platelet count. An absolute platelet count threshold cannot be utilized routinely given that some patients may experience a >50% decrease and still remain in the normal range (eg, a drop from 500,000/mm 3 to 200,000/mm 3 ).
The application of clinical scoring systems can have value in predicting the probability of a HIT diagnosis. , The 4T score has emerged as a preferred evaluation tool to estimate the probability of HIT. This scoring system takes into account the degree of thrombocytopenia, timing of onset, the presence of thrombosis or necrosis, and other possible causes of thrombocytopenia. , Each individual category is scored on a 0 to 2 scale, and cumulative totals classify the patient as either low (0–3), intermediate (4–5), or high risk (6–8) for HIT ( Table 28.2 ). A significant advantage of the 4T score comes from its high negative predictive value of 97% to 99%; when the score shows a low risk, it can rule out HIT. , The positive predictive values for intermediate and high 4T scores are 10% to 20% and 40% to 80%, respectively. , Therefore, additional laboratory testing has been recommended for patients with intermediate or high-risk scores. ,
| 4Ts | 2 Points | 1 Point | 0 Points |
|---|---|---|---|
| Thrombocytopenia | Platelet count fall >50% and nadir ≥20 k/μL | Platelet count fall 30%–50% or nadir 10–19 k/μL | Platelet count fall <30% or nadir <10 k/μL |
| Timing of drop in platelet count | Days 5–10, or day 1 if recent exposure | >Day 10 or unclear exposure | ≤Day 4 with no recent heparin exposure |
| Thrombosis | New thrombosis or anaphylactoid reaction after heparin bolus | Progressive or recurrent thrombosis | None |
| Other causes of thrombocytopenia | None | Possible | Definite |
Laboratory investigations can include immunologic and functional platelet-activation assays. The enzyme-linked immunosorbent assay is a common immunologic test for detecting heparin-PF4 antibodies. This test has a high negative predictive value due to its high degree of sensitivity (99%). The low positive predictive value for this test stems from its ability to detect clinically insignificant heparin-platelet 4 antibodies. In fact, a minority of heparin-treated patients develop HIT after a positive enzyme immunoassay. The specificity of an immunoassay test can be improved by specifically testing for the immunoglobulin G antibody. Furthermore, the degree of reactivity on an immunoassay test, or the optical density, can be useful in guiding treatment. Optical densities greater than 1.0 on these tests are more likely to be associated with a diagnosis of HIT, and values greater than 2.0 can be confirmatory, although the trigger values do vary across testing laboratories. , ,
To confirm the diagnosis of HIT after a positive immunoassay, a functional platelet-activation assay, is often warranted. These functional assays combine platelet-rich plasma with the patient’s plasma and heparin in order to measure platelet activation from the heparin-platelet factor 4-immunoglobulin G complex. The variations of this test include the choice of washed platelets (rather than platelet-rich plasma) and/or higher levels of heparin to enhance the overall specificity. In the heparin-induced platelet aggregation assay, platelet aggregation and changes in turbidity are examined with escalating heparin levels. The gold standard for functional tests remains the serotonin release assay that measures the sample’s radioactivity and percentage of serotonin release from platelets at both therapeutic and supra-therapeutic heparin. This test offers a high specificity (>95%) and sensitivity (>95%). , Thus a negative test makes HIT very unlikely, whereas a positive result makes HIT very likely.
Functional assays are not routinely available in all hospital laboratories. They are also time-consuming and costly. Therefore, immunoassays should be the first option when there is suspicion for HIT. The clinical picture, along with pretest probability based on the 4T score, should determine preemptive treatment and guide laboratory testing for confirmatory diagnosis, as outlined in recent guidelines.
In cases of HIT that are highly suspected or confirmed, the management includes immediate termination of all heparin exposure, including heparin-coated catheters. An alternative anticoagulant should be started whether or not clinical thrombosis has occurred. , While not a heparin agent, vitamin K antagonists like warfarin should also be avoided in the early stages of HIT due to the exhaustion of vitamin K–dependent proteins C and S that can increase the risk of venous limb gangrene. If warfarin was given prior to suspicion of HIT, reversal with vitamin K has been recommended. , The risk of thrombosis in HIT may be aggravated by platelet transfusion despite the level of thrombocytopenia. However, more recent data have suggested that if a significant bleeding risk exists in HIT with severe thrombocytopenia, especially if there is concomitant anticoagulation, admission to an intensive care unit is warranted. Current guidelines have recommended platelet transfusion as an option in active bleeding or for an invasive procedure with a high risk for bleeding. ,
Patients who develop HIT require anticoagulation with a nonheparin anticoagulant such as argatroban, bivalirudin, danaparoid, and fondaparinux to minimize the risk of thrombosis. Of the direct thrombin inhibitors, argatroban has been approved for the treatment of HIT, while off-label use of bivalirudin has been widely adopted. , Danaparoid is a low-molecular-weight heparinoid that is distinct from heparin, and it has been approval for the treatment of HIT outside of the United States. , Fondaparinux, an indirect inhibitor of factor Xa, has also been used successfully in HIT patients. Current guidelines also include direct oral anticoagulants; however, there is low certainty about the supporting evidence.
In the context of a patient with HIT presenting for cardiac surgery, clinical recommendations are typically based on the five phases of HIT ( Table 28.3 ). If possible, surgery should be delayed until the functional assay has recovered (subacute HIT phase B) or until the patient has remote HIT; both immunoassays and functional assays are negative. , In subacute HIT phase B and remote HIT, intraoperative anticoagulation can occur with heparin rather than an alternative anticoagulant. , In these settings, heparin would be limited to the intraoperative setting, and an alternative agent would be used postoperatively as needed along with continued clinical monitoring including tracking of the platelet count to assess for recurrence of HIT. ,
| Phase | Platelet Count | Functional Assay | Immunoassay |
|---|---|---|---|
| Suspected HIT | Decreased | ? | ? |
| Acute HIT | Decreased | + | + |
| Subacute HIT A | Normal | + | + |
| Subacute HIT B | Normal | – | + |
| Remote HIT | Normal | – | – |
The disappearance of antibodies to the heparin-platelet factor 4 complex antibodies may take as long as 50 to 85 days. , , If a patient with acute HIT or subacute HIT phase A requires urgent or emergent cardiac surgery, alternative anticoagulant strategies are required ( Box 28.3 ). The options include an alternative intraoperative anticoagulant to heparin, intraoperative heparin with plasmapheresis for removal of the hostile antibodies, or intraoperative heparin with a potent antiplatelet agent to prevent platelet activation. If one of the latter two options is utilized, heparin should again be limited to the intraoperative setting. The heparin alternatives will be reviewed in the following section. Plasmapheresis is an option to reduce circulating antibodies in HIT, and it has been employed with success in this context. , By decreasing antibody titers, plasmapheresis can decrease the risk of subsequent heparin exposure and decrease the potential risk of thrombosis postoperatively. An alternative to plasmapheresis is to produce platelet anesthesia with a powerful platelet blocker to prevent their activation in the presence of intraoperative heparin. Iloprost, a prostacyclin analog that inhibits platelet aggregation, has been successfully used in this setting for cardiac surgery, despite being a systemic vasodilator that can result in significant hypotension. Further options for intraoperative platelet anesthesia in this setting include glycoprotein IIb/IIIa blockers such as abciximab, or P2Y12 antagonists such as cangrelor. , ,
Alternative anticoagulant to heparin: argatroban, bivalirudin, or danaparoid
Heparin and plasmapheresis: plasmapheresis removes the hostile antibody complexes that lead to platelet activation and thrombosis
Heparin and platelet anesthesia: intravenous platelet blockers such as cangrelor prevent platelet activation that can precipitate thrombosis
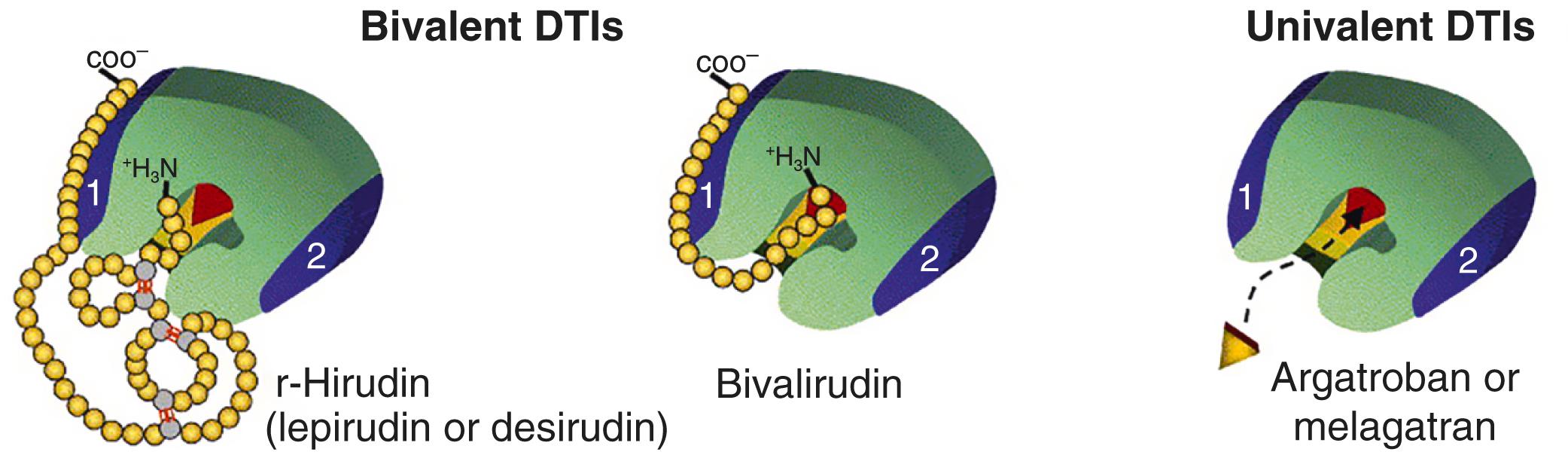
Thrombin has multiple roles in coagulation including the conversion of fibrinogen to fibrin and platelet activation. As outlined earlier, heparin is an indirect thrombin inhibitor through its effects on antithrombin III. Direct thrombin inhibitors allow for a more predictable anticoagulant response, as well as the ability to inhibit both circulating and clot-bound thrombin ( Table 28.4 ). , Their more reliable anticoagulation results from the lack of a required cofactor and the lack of plasma protein binding. Furthermore, they are also not associated with the immune response that can lead to HIT, thus explaining their role in patients with acute HIT who require cardiac surgery (see Table 28.3 ). Despite this advantage, the lack of a reversal agent can cause excessive bleeding after cardiac surgery. , The currently available parenteral options for direct thrombin inhibition include argatroban and bivalirudin.
| Argatroban | Bivalirudin | |
|---|---|---|
| Approved for treatment of HIT | Yes | No |
| Risk of HIT | No | No |
| Half-life | 40–50 min a | 25 min b |
| Metabolism/excretion | Hepatic | Serum proteases and renal |
| Dose adjustment in renal failure | No | Yes |
| Reversal agent | No | No |
Argatroban is a univalent direct thrombin inhibitor that reversibly binds to thrombin’s active site and that has been approved for anticoagulation in HIT. , While it is more prevalent in the intensive care unit setting, its application for CPB has been reported. Argatroban can produce immediate anticoagulation shortly after initiation of therapy and does not require dose adjustment in patients with renal failure. However, given its hepatic metabolism and its biliary excretion, significant reductions are required in the context of liver failure. , The half-life of argatroban is approximately 40 to 50 minutes, but it can increase to as long as 180 minutes in patients with abnormal hepatic function. Its anticoagulant activity can be monitored with the aPTT or the ACT. The target range for anticoagulation will depend on the indication and clinical setting. Argatroban also has a significant effect on the prothrombin time, suggesting anticoagulant effect beyond thrombin. , Although there is currently no available reversal agent for argatroban, recombinant activated factor VII may have a role. While argatroban has been successfully used for anticoagulation in CPB in selected patients, it has challenges in this setting, including the relatively long half-life, and lack of a reversal agent. These limitations explain the risk of excessive bleeding and have resulted in a weak recommendation for this agent as an anticoagulant for CPB. ,
Bivalirudin is a bivalent synthetic analog of hirudin with reversible inhibition of thrombin due to its cleavage by thrombin once it is bound ( Fig. 28.11 ). Its reversibility and platelet inhibitory effects explain its applications in percutaneous coronary interventions, CPB, and HIT (off-label application). , , , The clearance of bivalirudin is from both thrombin cleavage and from renal excretion. The half-life of bivalirudin is approximately 25 minutes in a patient with normal renal function. Its clearance decreases by 20% with a creatinine clearance below 60 mL/min, and up to 80% in renal failure requiring renal replacement therapy. , Its anticoagulant effect can be tracked with the aPTT or the ACT, with target ranges depending on the clinical context. Similar to argatroban, bivalirudin can cause elevations in the prothrombin time and there is currently no reversal agent for bivalirudin. , ,
Clinical trials have demonstrated the efficacy of bivalirudin for anticoagulation in cardiac surgery with and without CPB, including patients with HIT. Dosing protocols for bivalirudin depend on multiple factors including indication, clinical setting, and institutional protocol. , , Proposed regimens for CPB include a loading dose followed by an infusion titrated to the goal ACT with additional bolus doses as needed, including priming of the extracorporeal circuit. , , Clinical vigilance in this setting is essential as blood stasis triggers thrombin generation and inactivation of bivalirudin in areas such as the mediastinum, pleural cavities, and venous reservoir of the extracorporeal circuit. , , Consequently, blood stasis should be minimized where possible during the conduct of CPB with bivalirudin as the anticoagulant. Since there is no reversal agent, the infusion is typically terminated prior to separation from CPB with a time interval based on the estimated clearance of bivalirudin in the given clinical situation. , , Excess bivalirudin can also be removed after CPB with hemofiltration. , ,
Danaparoid is a low-molecular-weight heparinoid that is currently available outside the United States. Danaparoid consists of a combination of heparan sulfate (84%), dermatan sulfate (12%), and chondroitin sulfate (4%). , It is an antithrombin III–dependent inhibitor of activated factor X that can be administered parenterally with clinical monitoring via anti–factor X assays. , , , No reversal agent is available for danaparoid. Although this heparinoid has been a successful anticoagulant in HIT and CPB, its widespread application has been limited by significant individual variations in therapeutic response and the lack of a reversal agent. , ,
Fondaparinux is a synthetic heparinoid that increases the ability of antithrombin III to inhibit activated factor X. It has a pentasaccharide structure with primarily renal elimination resulting in a half-life of 15 to 17 hours. Its advantages include subcutaneous administration, and monitoring for effect is often not necessary. While typically indicated for deep vein thrombosis prophylaxis and/or treatment, it also has a therapeutic role in HIT, since it has minimal cross-reactivity with HIT antibodies. , Although it may have a role in the management of venous thrombosis after cardiac surgery, it does not have a role as an alternative anticoagulant for CPB. Furthermore, there is no reversal agent for fondaparinux.
A small percentage of UFH consists of low-molecular-weight fragments that inhibit activated factor X up to fourfold more than thrombin. The low-molecular-weight heparins such as dalteparin and enoxaparin have better bioavailability and longer half-lives as compared to UFH. , Although monitoring is not routine, the aPTT, PTT, and anti–factor X assays can track their anticoagulant activity in the management of thromboembolic disease. , There is a very low risk for the development of HIT with these agents. These anticoagulants are not indicated for CPB and only have partial reversal with protamine.
Bleeding during and after cardiac surgery can be due to patient factors, CPB-related factors, or preoperative anticoagulation ( Box 28.4 ).
Factor deficiencies
Platelet disorders
Liver disease
Renal disease
Hemodilution
Systemic inflammation
Hyperfibrinolysis
Loss of coagulation factors
Platelet dysfunction
Residual heparin/excessive protamine
Hypothermia
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here